Abstract
Objective
To identify distinct behavioral and cognitive profiles associated with ADHD in Turner syndrome (TS), relative to idiopathic ADHD and neurotypical controls, in order to elucidate X-linked influences contributing to ADHD.
Methods
We used a multilevel-model approach to compare 49 girls with TS to 37 neurotypical females, aged 5–12, on established measures of behavior (BASC-2) and neurocognitive function (NEPSY). We further compared girls with TS to BASC-2 and NEPSY age-matched reference data obtained from children with idiopathic ADHD.
Results
Within the TS group, 51% scored at or above the “at-risk” range for ADHD-associated behaviors on the BASC-2 (TS/+ADHD). The BASC-2 behavioral profile in this TS/+ADHD-subgroup was comparable to a reference group of boys with ADHD with respect to attentional problems and hyperactivity. However, the TS/+ADHD-subgroup had significantly higher hyperactivity scores relative to a reference sample of girls with ADHD (p=0.016). The behavioral profile in TS was associated with significantly lower attention and executive function scores on the NEPSY relative to neurotypical controls (p=0.015); but was comparable to scores from a reference sample of children with idiopathic ADHD. Deficits in attention and executive function were not observed in girls with TS having low levels of ADHD-associated behavior (TS/−ADHD).
Conclusions
ADHD-associated behavioral and cognitive problems in TS are prevalent and comparable in severity to those found in children with idiopathic ADHD. The ADHD phenotype in TS also appears relatively independent of cognitive features typically associated with TS, like visuospatial weaknesses. These findings suggest that X-linked haploinsufficiency and downstream biological effects contribute to increased risk for ADHD.
Introduction
Sex differences in the manifestation of psychiatric disorders, including Attention Deficit Hyperactivity Disorder (ADHD), are among the most prominent findings in psychiatry (Holden, 2005). For ADHD, sexual dimorphism is observed in prevalence, clinical manifestation, and course and in the comorbidities of the disorder. For example, ADHD manifestations differ between males and females (e.g., higher levels of inattentive type and lower hyperactive/impulsive behavior disorders in females) (Biederman et al., 1999, Biederman et al., 2002). This might result in sex-based referral biases and lead to underdiagnosis and undertreatment of females. This, in turn, could result in an increased risk for psychological morbidity and academic problems in females with ADHD, thereby increasing the public health burden due to the effects of undertreated ADHD on academic and occupational performance. In males, ADHD is prevalent and associated with distinct comorbid psychiatric disorders such as conduct and oppositional defiant disorders. In females, ADHD is associated with depressive and anxiety disorders (McGough et al., 2005). Thus, ADHD is associated with significant lifetime comorbidity in males and females, yet the topography of the disorder is different in each of the sexes.
The sexual dimorphism in ADHD suggests involvement of biological mechanisms related to the sex chromosomes including number of X chromosomes and X-linked gene haploinsufficiency (Davies, 2014). As idiopathic ADHD is a highly heterogeneous disorder, elucidating these putative contributory mechanisms can be a challenging task. Traditional methods comparing males and females with idiopathic ADHD to controls have yielded limited information. This is likely due to the fact that ADHD subtypes, comorbidities and developmental effects significantly increase symptom heterogeneity, thereby affecting our ability to detect differences between the sexes. Moreover, research focused on underlying neural substrates using neuroimaging of individuals with idiopathic ADHD (iADHD) is usually underpowered to detect sex effects (Davies, 2014).
A promising strategy for advancing our understanding of ADHD is to identify tractable models for this behaviorally defined disorder. A model that potentially incorporates several of the factors noted above is Turner syndrome (TS), a common genetic condition caused by absence of most or all of one of two X chromosomes (Stochholm et al., 2006). TS is associated with significantly increased risk for ADHD in the context of normal overall intellectual function. Approximately 25% of girls and adolescents with TS meet the DSM-IV diagnostic criteria for ADHD (Russell et al., 2006). Individuals diagnosed with ADHD tend to have associated deficits in executive function (EF) (Willcutt et al., 2005), visuospatial working memory (Martinussen et al., 2005) and social cognition (Cadesky et al., 2000). Individuals with TS also suffer from specific deficits in EF and social cognition, and a global impairment in visuospatial abilities (Lepage et al., 2011, Romans et al., 1998, Ross et al., 2002) including associated visuospatial working memory (Ross, Stefanatos, 2002).
As the genetic cause for TS is X-monosomy, this model allows us to make inferences about the potential contribution of X-linked biological mechanisms to attention problems and hyperactivity. In typically developing females, inactivation of one of the X chromosomes occurs such that females will have a similar level of X chromosome expression for most genes relative to males. However, some of the genes on the inactivated X chromosome escape the inactivation process. For these genes, two copies are expressed in typically developing females, whereas only one of these genes is expressed in males and in females with X-monosomy (Arnold, 2004). Thus, TS is a unique human condition with X-linked genetic haploinsufficiency but without Y-linked genes affecting the phenotype. Accordingly, the study of attention problems and hyperactivity in this syndrome can potentially advance our understanding of sexual dimorphism in ADHD.
The primary goal of this study was to delineate the behavioral and cognitive profiles associated with ADHD in TS relative to age-matched children with iADHD and age and IQ-matched neurotypical female controls. This novel approach allows for the identification of distinct behavioral and cognitive profiles associated with ADHD in TS relative to iADHD, which will help elucidate putative X chromosome linked biological mechanisms that contribute to this common behavioral disorder. To accomplish this goal we: (1) examined behavioral characteristics associated with ADHD, and (2) assessed neurocognitive features that may be specifically tied to the ADHD phenotype in TS, relative to iADHD and neurotypical controls. Given that X-monosomy occurs naturally in males, and as a genetic anomaly associated with TS, we hypothesized that our TS group with high levels of ADHD behaviors would demonstrate a behavioral profile comparable to boys with iADHD. We also predicted that girls with TS would demonstrate specific neurocognitive deficits in attention and EF compared to neurotypical controls, and that these deficits would be comparable to an iADHD group. Furthermore, given the association between ADHD and EF deficits in clinical populations of children with iADHD (Martinussen, Hayden, 2005), we expected that neuropsychological impairment in attention and EF abilities would be specific to girls with TS who had high levels of ADHD behaviors, while weaknesses in visuospatial and sensorimotor abilities would be present all girls with TS.
Methods
Study design
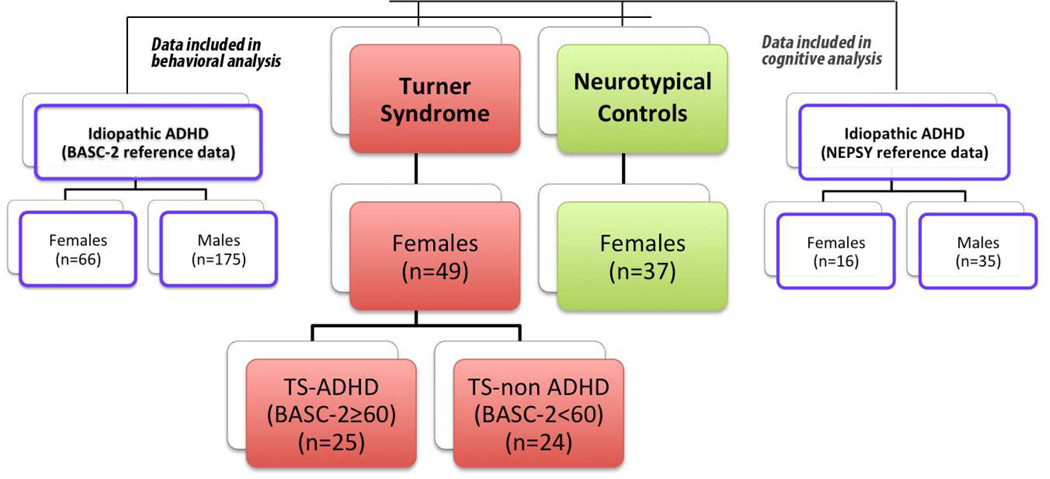
This study included four groups: girls with TS; neurotypical controls; BASC-2 reference data collected from children diagnosed with iADHD (BASC-2/iADHD) and NEPSY reference data collected from children diagnosed with iADHD (NEPSY/iADHD) (Fig. 1). To accomplish the study goals, the following comparisons were made:
Behavioral features of the entire TS group to age-, sex- and verbal IQ-matched healthy (i.e., neurotypical) controls, and to children with iADHD (BASC-2/iADHD);
Cognitive features of the entire TS group to neurotypical controls and to children with iADHD (NEPSY/iADHD) group;
Behavioral features of the TS subgroup with more severe ADHD-associated behaviors to children with iADHD (BASC-2/iADHD);
Cognitive features of the TS subgroup with more severe ADHD-associated behaviors to age-matched children with iADHD (NEPSY/iADHD);
Cognitive features of the TS subgroup with more severe ADHD-associated behaviors to the TS subgroup with less severe ADHD-associated behaviors.
Figure 1.
Participants and groups (Turner, neurotypical and reference idiopathic ADHD). BASC-2 = Behavior Assessment System for Children-Second Edition; NEPSY = NEuroPSYchological assessment.
Participants
We collected data from 49 girls with TS and 37 typically developing girls enrolled in a study focused on brain-behavior associations in young girls with TS. Exclusion criteria for both groups included premature birth (gestational age under 34 weeks), low birth weight (less than 2000g), known diagnosis of a major psychiatric or current neurological disorder including seizures. X-monosomy was established through standard karyotype analysis of at least 20 cells, which allows exclusion of 11% mosaicism or greater with a 0.90 confidence (Hook, 1977). Girls with TS exhibiting mosaic or uncommon structural karyotypes were excluded. Parental origin of the X chromosome was determined by comparison of amplification patterns of four polymorphic markers located exclusively on the X chromosome and one marker in the pseudo-autosomal region between the proband and mother (for details see past publication (Lepage et al., 2012)). Participants with TS were recruited with the assistance of the Turner Syndrome Society of the United States and the Turner Syndrome Foundation, a local network of physicians, and advertisement on the Stanford University School of Medicine website. Control participants were recruited through local print media and parent networks.
To compare girls with TS to children with iADHD on behavioral measures we used mean raw scores for the Attention Problems and Hyperactivity scales listed in the BASC-2 manual (Reynolds R. C., 2004) from children diagnosed with iADHD (BASC-2/iADHD). These mean Attention Problems and Hyperactivity were obtained on a sample of 241 children formally diagnosed with iADHD. To compare girls with TS to children with iADHD on neurocognitive measures, we used standardization data from the NEPSY of 51 children formally diagnosed with iADHD (NEPSY/iADHD).
The local Institutional Review Board of the Stanford University School of Medicine approved this study and informed written consent was obtained from a legal guardian for all participants, as well as written assent from participants over 7 years of age.
Behavioral assessment
We used the Behavior Assessment System for Children-Second Edition Parent Rating Scale (BASC-2) (Reynolds R. C., 2004) to evaluate clinical and adaptive aspects of behavior. Among other purposes, the BASC-2 was developed to assist in differential diagnosis of DSM-IV-TR disorders. For the clinical scales, on which high scores represent more problematic behaviors, T scores between 60 and 69 are considered “at-risk”. We used a BASC-2 T score of 60 or above in the Attention Problems or Hyperactivity scales as a cut-off score for defining a group of girls with TS higher ADHD-associated behaviors (TS/+ADHD, n=25). The remainder of the TS group was considered to be within the normative range for ADHD symptoms (TS/−ADHD). Although the age in our TS sample ranges from 5.1–12.3 years, we only analyzed data behavioral from participants with TS who completed the child form (6–11) to allow for questionnaire consistency between participants, and to permit comparison to the BASC reference sample of individuals with iADHD. Forty of the 49 girls with TS were within the 6–11 age rages of the iADHD comparison group.
Neuropsychological and Cognitive Assessments
All participants were assessed using cognitive assessments appropriate for their age, either the WIPPSI-III (Wechsler, 2002) or the WISC-IV (Wechsler, 2003). A developmental neuropsychological assessment was obtained using NEuroPSYchological (NEPSY®)(Korkman M, 1998). The NEPSY comprises tasks in five domains: Attention and Executive Functions, Language, Sensorimotor Functions, Visuospatial Processing and Memory and Learning. Test administrators followed standard procedures as outlined in the published product manuals; all cognitive/neuropsychological variables were age-normed scaled scores. As the NEPSY sample included only children with full-scale intelligence quotient (FSIQ) above 80, we also restricted our sample of girls with TS to only include individuals with FSIQ ≥80. Given the uneven profile of IQ in TS, with performance IQ (PIQ) more affected by TS than verbal IQ (VIQ), VIQ is considered to better represent mental age than FSIQ in girls with TS (Hong et al., 2009, Ross et al., 2004, Rovet, 1993). Thus TS, neurotypical controls were matched on VIQ.
Data Analysis
We performed statistical analyses using The R Project for Statistical Computing (R) (http://www.r-project.org). Unpaired t-tests were used to compare age and IQ scores between TS, neurotypical and NEPSY/iADHD reference samples. We used the Wilcoxon rank-sum test for non-normally distributed data (only FSIQ). Difference in parent education level was assessed using Chi-square tests. To compare the TS and neurotypical groups on BASC-2 scores and to compare NEPSY scores between TS, neurotypical and NEPSY/iADHD reference samples, we used a multilevel model approach to accommodate nested data (nmle package in R) (Aarts et al., 2014). Specifically, we used a multilevel model to estimate the fixed effects of Diagnosis, BASC-2/NEPSY domain type and interactions between these terms on BASC-2 and NEPSY total scores. A random intercept modeled the variance dependent on the BASC-2/NEPSY domain type nested within each participant. Chi-square likelihoods ratio were used to compare the fit of the model after each variable was added. To test fixed effects in the model (Diagnosis, BASC-2/NEPSY domain type), we used ANOVA. Our domains of interest were Attention Problems and Hyperactivity for the BASC-2 and all five domains of the NEPSY. Since only mean scores and SD were available for the BASC-2/iADHD, unpaired t-tests were used to compare the TS participants to this group. All p-values were corrected for multiple comparisons using either Tukey contrasts for multiple comparisons of means (multcomp package in R) for the multilevel models and Bonferroni for the unpaired t-tests. BASC-2/iADHD sample size (n=241, female=66) enabled us to contrast the TS group with either females or males with iADHD. The sample size for females with iADHD in the NEPSY reference sample (n=51, female=16) did not allow us to separately contrast the TS group to females with ADHD.
Results
Demographic and Cognitive Measures
There were no significant differences in age and parent’s education between the TS, neurotypical, and NEPSY/iADHD groups. In TS, FSIQ is not considered a reliable representation of overall intellectual ability due to visual–spatial weaknesses often causing a significant discrepancy between VIQ and PIQ (Hong, Scaletta Kent, 2009). As expected, we found a discrepancy of 11 points between VIQ (106.1±11.97) and PIQ (95.1±10.73) in the TS group. Thus, girls with TS and neurotypical girls were group-matched on VIQ (Table 1). The VIQ and PIQ scores for the NEPSY/iADHD group were not available to us. Despite lower PIQ scores in the TS group, FSIQ scores were within the normal range for all three groups and, as expected, the neurotypical group and NEPSY/iADHD group scored significantly higher than the TS group for FSIQ.
| Turner Syndrome |
Neurotypical | Idiopathic ADHD |
Idiopathic ADHD |
p value† | p value†† |
p value††† |
|
|---|---|---|---|---|---|---|---|
| Number of participants | 49 | 37 | 51 | 241 | -- | -- | -- |
| Sex (n) | Female (49) | Female (37) | Female (16) | Female (66) | -- | -- | -- |
| Parent Education | |||||||
| ≤ 11years (%) | 0 | 2.9 | 4.1 | -- | NS | NS | -- |
| 12–15 years (%) | 63.3 | 64.7 | 57.1 | -- | NS | NS | -- |
| ≥16 (%) | 35.7 | 32.4 | 38.8 | -- | NS | NS | -- |
| GH | 40, U=1 | -- | -- | -- | -- | -- | |
| Estrogen | None | -- | -- | ||||
| Stimulants | 1, U=3 | -- | -- | ||||
| Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | ||||
| Age | 8.6(2.15) | 9.2(2.27) | 9.3(1.9) | 8.9(1.60)¥ | NS | NS | NS |
| FSIQ | 96.3(10.11) | 112.1(10.45) | 104.4(13.5) | -- | P<0.001 | 0.002 | -- |
| PIQ | 95.1(10.73) | 112.2(10.51) | -- | -- | P<0.001 | -- | -- |
| VIQ | 106.1(11.97) | 110.6(15.01) | -- | -- | NS | -- | -- |
Note: FSIQ, Full-scale intelligence quotient; PIQ, Performance intelligence quotient; VIQ, Verbal intelligence quotient; NS not significant.
NEPSY- NEuroPSYchological assessment,
BASC-2- Behavior Assessment System for Children-Second Edition,
Neurotypical vs. Turner Syndrome,
Idiopathic ADHD (NEPSY) vs. Turner Syndrome,
Idiopathic ADHD(BASC-2) vs. Turner Syndrome,
Calculated mean and SD from Table 11.13 in the BASC-2 manual: representation of the sample by age.
Behavioral measures of attention deficit hyperactive disorder symptoms
For either Attention Problems or Hyperactivity in the TS group, 25 girls (51.0%) scored at or over ≥60 and, among these participants, 12 (24.5%) scored at or above the clinically significant range (≥70). In the control group 5 girls (13.9%) scored within the at-risk range and none scored within the clinical range.
The mean T-score on the BASC-2 Attention Problems scale was 54.5±10.5 for the TS group vs. 46.7±7.4, for the neurotypical controls, and the mean T-score on the BASC-2 Hyperactivity scale was 58.7±14.2 for the TS group vs. 46.3±8.2 for neurotypical controls. The multilevel model analysis yielded a main effect for Diagnosis (TS vs. neurotypical controls), F(1,82)=23.9, p<0.0001, such that the average BASC-2 score was significantly higher for girls with TS than controls. The main effect of BASC-2 domains was not significant F(1,83)=2.66, p=0.107. However, the interaction effect was significant F(1,89)=4.06, p=0.0472. Tukey post hoc tests revealed that the mean for the TS group was significantly greater than that of the neurotypical group for both the Attention Problems (z=3.34, p=0.004) and Hyperactivity scales (z=5.34, p<0.001) (Table S1).
We next tested whether BASC-2 behavioral measures of ADHD differ between girls with TS and reference scores for BSAC-2/iADHD. The mean raw score from the overall sample of girls with TS was significantly lower than the reference scores on the Attention Problems (TS 8.1±3.8 vs. iADHD 11.1±4.2, p<0.00001, cohen’s d= −0.75) and Hyperactivity (TS 11.9±6.8 vs. iADHD 14.8±6.6, p<0.03, cohen’s d= −0.43) scales. To assess the utility of an ADHD diagnosis in TS, we tested whether BASC-2 behavioral measures of inattention and hyperactivity differ between TS/+ADHD and reference scores for BSAC-2/iADHD. No differences between groups were found for the Attention Problems (t=−1.06, p=0.30) and Hyperactivity (t=1.60, p=0.12) scales (Table S1).
We next tested whether BASC-2 behavioral measures of ADHD differ between TS/+ADHD and reference scores (BSAC-2/iADHD) for only boys or only girls with ADHD. We found no differences between TS/+ADHD and males with BSAC-2/iADHD for Attention Problems and Hyperactivity. We found significant differences between TS/+ADHD and girls with ADHD such that girls with TS/+ADHD had higher Hyperactivity scores (mean 16.6±5.09) than did girls with BSAC-2/iADHD (mean 13.3±6.41, t=2.50, p=0.016, cohen’s d= 0.57). No difference between the TS/+ADHD group and girls with BSAC-2/iADHD was identified for the Attention Problems scale.
We next tested whether the profile of comorbid psychiatric symptoms differed between BSAC-2/iADHD and TS/+ADHD groups. We found that overall the behavioral profile of the two groups was similar except for Conduct Behavior and Depression domains in the girls with BSAC-2/iADHD vs. TS/+ADHD and Conduct Behavior and Social Skills in the boys with BSAC-2/iADHD vs. TS/+ADHD. Specifically, girls and boys with BSAC-2/iADHD scored higher than the TS/+ADHD risk group for Conduct Behavior (8.97±5.36 vs. 6.18±3.14 p= 0.004 Cohen’s d = 0.64 for girls, and 9.18±5.09 vs. 6.18±3.14 p= 0.005 Cohen’s d=0.71, for boys). Girls in the BSAC-2/iADHD group scored higher on the Depression domain than TS/+ADHD (11.73±7.43 vs. 8.41±6.13, p=0.043, Cohen’s d = 0.48) and boys in the BSAC-2/iADHD group scored lower on the Social Skills domain than TS/+ADHD (10.87±4.14 vs. 14.64±4.99, p=0.032, Cohen’s d = −0.82.) Hence, in girls with TS at risk for ADHD, there is some specificity to attention problems and hyperactivity beyond the comorbidity profile typically found in children with iADHD.
Cognitive features of Turner syndrome, neurotypical controls, and idiopathic ADHD/NEPSY
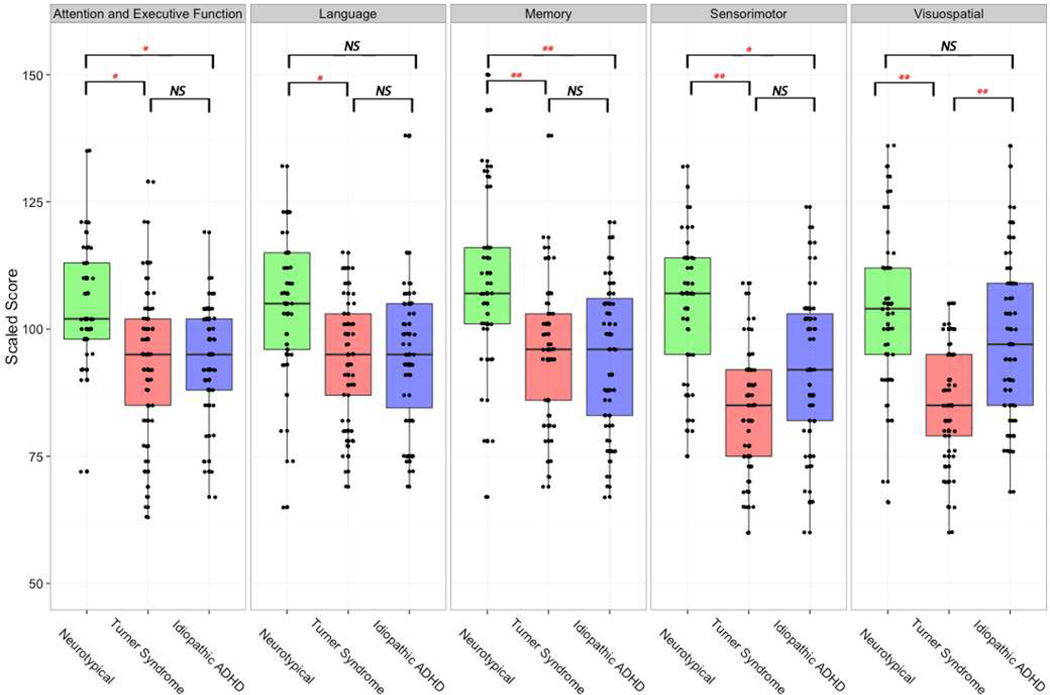
The multilevel model yielded a main effect for Diagnosis (TS vs. neurotypical vs. iADHD/NEPSY), F(2,134)=8.4, p=0.0004, such that the average NEPSY total score was significantly lower for girls with TS (mean 90.6±13.60) and iADHD/NEPSY (94.8±14.52) than for controls (mean 105.4±15.16). The main effect of NEPSY domains was not significant F(4,535)=1.8, p=0.12 but the interaction effect between Diagnosis and NEPSY-domains was significant F(8,535)=3.3, p=0.0012. Tukey post hoc tests revealed that the TS group differed from the iADHD/NEPSY group only on the Visuospatial domain (z=−4.21, p<0.01) (Figure 2). The model-adjusted mean for the TS group was significantly lower than that of the neurotypical group for all NEPSY-domains: Attention and Executive functions (z=−3.74, p=0.015), Language (z=−3.51, p=0.034), Memory (z=−4.69, p<0.01), Sensorimotor (z=−6.57, p<0.01) and Visuospatial (z=−5.81, p<0.01) domains. The model adjusted mean of the iADHD group was significantly lower than that of the neurotypical group for Attention and Executive functions (z=−3.52, p=0.031), Memory (z=−5.01, p<0.01), Sensorimotor (z=−3.73, p=0.015) domains and approached significance for the Language (z=3.32, p=0.06) domain (Figure 2).
Figure 2.
The neurocognitive profile for neurotypical, idiopathic ADHD and Turner syndrome groups across the five NEPSY (NEuroPSYchological assessment) domains. NS, not significant; * p<0.05; **p<0.001.
We also compared the three groups on subtests comprising the Attention and Executive Function domain and found no differences between the TS and iADHD/NEPSY groups. Compared to neurotypical controls, the TS group scored significantly lower on the Auditory Attention and Response Set subtest (z=−3.53, p=0.0128) and Visual Attention (z=−3.398, p=0.0193) subtest and the iADHD group scored lower on the Visual Attention (z=−4.5.03, p<0.01) subtest.
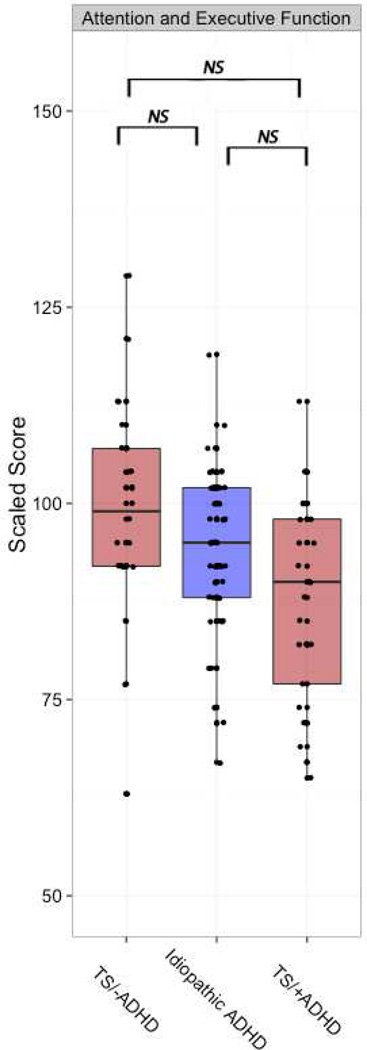
We next tested whether NEPSY domain scores differ between TS/−ADHD, TS/+ADHD and reference scores for iADHD/NEPSY. The multilevel model yielded a main effect for Diagnosis (TS/−ADHD vs. TS/+ADHD vs. iADHD/NEPSY), F(2,97)=4.49, p=0.014. The main effect of NEPSY domains was significant F(4,387)=8.3, p<0.0001 and the interaction effect between Diagnosis and NEPSY-domains was significant F(8,387)=3.7, p=0.0003. Tukey post hoc tests revealed that both TS/−ADHD and TS/+ADHD groups differed from the iADHD/NEPSY group only on the Visuospatial domain (z=−3.74, p=0.039 and z=−3.99, p<0.01, respectively) (Table S1). No significant differences in Attention and Executive Function domain scores were found between the groups. Post hoc exploration of the data showed that the average score for the TS/−ADHD group was around the mean standardized score of 100, while the iADHD group scored below the mean standard score but above the TS/−ADHD group, whose scores were the lowest among the three groups (Figure 3).
Figure 3.
Attention and Executive Function (NEPSY-NEuroPSYchological assessment) scores for TS/− ADHD, TS/+ADHD and idiopathic ADHD groups.
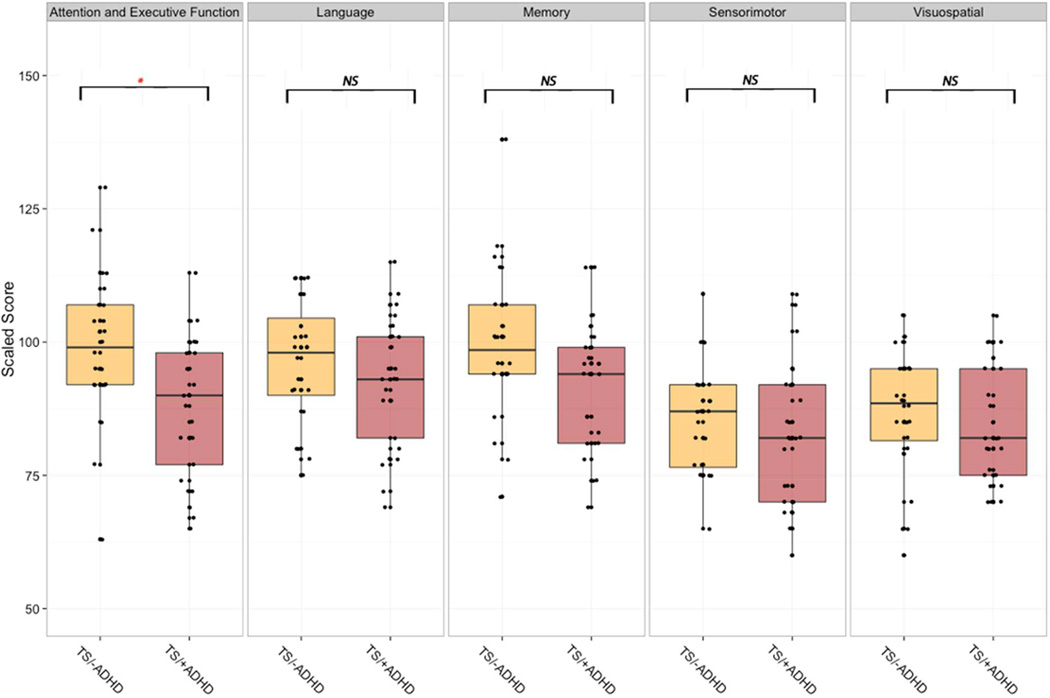
As an exploratory step, we compared the NEPSY cognitive domains across the 25 girls (age 8.3±1.92) with TS/+ADHD to 24 girls (age 8.9±2.36, p=0.30) with TS below the at −risk cutoff for ADHD. No differences were found in age (8.3±1.9 vs. 8.9±2.4, p=0.30), FSIQ (94.0±9.3 vs. 98.6±10.5, p=0.11) or parent education level (Chi^2=0.10, p=0.75). The group differed only on the Attention and Executive Function domain such that girls with TS/+ADHD (87.8±13.18) scored significantly lower than TS subgroup without ADHD symptoms (99.17±13.77, p=0.02) (Figure 4). Comparing the groups on subtests within the Attention and Executive Function domain, we found that girls with TS/+ADHD scored significantly lower than girls with TS/−ADHD on the Auditory Attention and Response Set subtest (mean 7.4±3.14 vs. 10.0±1.59, p<0.001). No such differences were found for the Tower and Visual Attention subtests.
Figure 4.
The neurocognitive profile for Turner syndrome subgroup with ADHD-associated behaviors within the normative range (TS/−ADHD) and girls with Turner syndrome with higher ADHDassociated behaviors (TS/+ADHD) across the five NEPSY (NEuroPSYchological assessment) domains. NS, not significant; * p<0.05; **p<0.001.
Imprinting
Within the TS group, there was no significant association between X-chromosome parent of origin and TS/+ADHD or TS/−ADHD (X2 = 1.01, df=1, p=0.31).
Discussion
In the current study, we sought to elucidate behavioral and cognitive profiles associated with Turner syndrome due to X-monosomy in order to investigate possible X-linked factors contributing to, and sexual dimorphism associated with, ADHD. Our findings of a distinctive profile of ADHD symptoms that include a high rate of hyperactivity symptoms in girls with TS are in line with earlier findings (Rovet, 1993, Russell, Wallis, 2006). However, our results significantly extend these previous findings by comparing a TS group to age- and VIQ-matched neurotypical controls and to males and females with iADHD. In particular, we observed that the ADHD behavioral profile in TS was comparable to the profile of males with iADHD. Moreover, TS status was associated with neurocognitive deficits in attention and executive function, similar to that observed in children with iADHD. ADHD symptoms in TS appeared to be related to EF deficits but discrete from cognitive features typically associated with TS such as visuospatial weaknesses.
The rates reported here of girls with TS and clinically significant ADHD symptoms (24%) match the rates reported in another sample of girls with TS (27 with X-monosomy and 23 with mosaic or other karyotypes) (Russell, Wallis, 2006). However, to our knowledge, this is the first case control study reporting high rates of ADHD symptomatology in a sample consisting only of young girls with X-monosomy relative to a control group. We further specify this observation by finding significantly higher scores of hyperactivity in girls with TS/+ADHD compared to girls with iADHD, but not compared to boys with iADHD. In this study we were able to match the groups of girls with TS and neurortypical controls on VIQ and control for possible confounders related to the association between low IQ and ADHD symptoms (Neece et al., 2013). Thus, our results suggest a specific effect of TS on attention and hyperactivity as well as EF beyond the differences that might be accounted for by visual-spatial weaknesses in TS. Nevertheless, we were not able to match our data from children with iADHD on VIQ. These data would have enabled us to account for cognitive differences that might occur between girls with TS and controls.
The cognitive-behavioral profile we describe in girls with TS may stem from haploinsufficiency of gene expression associated with the X chromosome. For example, the MAOA (Biederman et al., 2008, Rommelse et al., 2008), HTR2C (Li et al., 2006, Xu et al., 2009) and STS (Trent et al., 2013) genes associated with hyperactivity and impulsivity are located within X chromosome regions that are not subject to X-inactivation. Hypothetically, this mechanism might expose females with TS to higher risk for developing an ADHD symptom profile more often seen in male populations. However, the extent to which these genes escape inactivation in typical females (46,XX) is variable (Carrel and Willard, 2005, Peeters et al., 2014). Loss of function mutations of the STS gene in humans is associated with ADHD symptoms (Kent et al., 2008) and STS gene polymorphism has been suggested to influence ADHD risk and symptom profile (Brookes et al., 2010, Stergiakouli et al., 2011). Moreover, the STS gene is located within Xp22.3 region, a region implicated in the neurocognitive phenotype of TS (Zinn et al., 2007). As suggested in males with ADHD (Brookes, Hawi, 2010, Stergiakouli, Langley, 2011) due to hemizygosity of X-linked genes, functional polymorphisms for these genes might also influence the severity and profile of ADHD behaviors in girls with TS. Though speculative, such an effect might result in a bimodal distribution of ADHD symptoms in TS, as suggested by the distribution of BASC-2 Attention Problems scores in our sample of girls with TS (Figure S1).
With respect to cognition, our results indicate a clear neurocognitive profile in TS group and in children with iADHD. Specifically, these two groups scored lower in attention and executive function, memory and sensorimotor skills compared to neurotypical controls (Figure 2, Table S1). We found no differences between the entire TS group and children with iADHD when examining the subtests comprising the NEPSY Attention and Executive function domain. However, these groups significantly differed on the NEPSY Visuospatial domain. These and previous findings (Lepage, Dunkin, 2011) indicate that in TS, visuospatial dysfunction is segregated, at least in part, from deficits in attention and executive function.
Using the multilevel model approach, no differences were found between the TS/−ADHD, TS/+ADHD, and iADHD groups except for lower scores in the visuospatial domain for both TS groups. Thus, we could not prove that the TS/+ADHD accounted for the results of the NEPSY comparisons between the iADHD and the entire TS group. The lack of statistical significant differences may stem from smaller sample size in our TS subgroups (dividing the TS group into TS/+ADHD and TS/−ADHD) and multiple comparisons (three groups and five domains). An exploratory analysis of only the TS/+ADHD and the TS/−ADHD results indicate a distinct, non-IQ dependent, neurocognitive profile in a subgroup of young girls with TS/+ADHD compared to TS/−ADHD. This distinct profile includes, in addition to expected weaknesses in the visuospatial (Lepage, Dunkin, 2011, Murphy et al., 1994, Romans, Stefanatos, 1998, Ross et al., 1995) and sensorimotor (Nijhuis-Van der Sanden et al., 2004, Nijhuis-Van der Sanden et al., 2003, Ross et al., 1996) domains, deficits in attention and EF (Lepage, Dunkin, 2011). These findings suggest a strong influence of X-monosomy on attention and executive functions in a subgroup of girls with TS.
The results presented have clinical significance. First, the findings present a clear need for comprehensive clinical evaluation of ADHD in young girls with TS. As ADHD can have significant effects on the academic and social development of children, and is associated with significant psychiatric comorbidities (Biederman, Mick, 2002), careful monitoring of ADHD symptoms could have a significant effect on the mental health of girls with TS. Second, since visuospatial abilities and executive functions contribute to academic performance, the observations of pervasive deficits in visuospatial abilities in TS (Downey et al., 1991, Ross et al., 2003, Simon et al., 2008) might overshadow specific deficits in EF (Lepage, Dunkin, 2011). This might lead clinicians to attribute academic problems primarily to deficits in visuospatial cognition without considering other neuropsychological domains that could be associated with ADHD symptoms. Finally, no study to date, to the best of our knowledge, has carefully examined the effects of targeted interventions (such as stimulants) on these symptoms in TS. Such studies are of clinical importance as up to 70% of the girls with TS suffer from congenital heart anomalies and the majority suffer from short stature, two conditions that affect clinical decision-making regarding stimulant administration.
Beyond the important implications of this study with respect to clinical evaluation and treatment of young girls with TS, these findings implicate the use of TS as a unique, tractable model to better understand ADHD. Specifically, this model has the potential to provide new insights into the influence of X chromosome gene expression and downstream biology on ADHD symptomatology. TS is an established disease with a known genetic basis that affects cognition and behavior associated with ADHD. Moreover, individuals with TS usually present with overall IQ within the normal range (Ross, Stefanatos, 2002), thus permitting an examination of ADHD in TS irrespective of IQ. This is imperative given the association between low IQ scores and ADHD symptoms found in iADHD populations and in children with other neurogenetic syndromes (Green et al., 2009, Neece, Baker, 2013). Thus, TS presents an opportunity to examine behaviors and cognitive profiles associated with ADHD in the context of a known genetic risk factor associated with the X chromosome.
Possible limitations of the current study include potential demographic differences between our sample of girls with TS and the reference samples from the BASC-2 and NEPSY. For example, there are no data provided for parental level of education in the BASC-2 ADHD sample. However, for the reference samples of the NEPSY, we were able to overcome this limitation and groups were matched for level of education as well as age. Further, it is difficult to determine if the clinical criteria used to establish an ADHD diagnosis in the BASC-2 and NEPSY reference samples were compatible. Thus, our overall conclusions regarding differences between girls with TS and children with iADHD should be considered in light of possible differences between our two reference data groups of children with iADHD. Nevertheless, utilizing the available reference data enables a more comprehensive view of the behavioral and neurocognitive profile of females with TS who have ADHD symptoms. In addition, behavioral data from the school (e.g. BASC-2 teacher rating scale) would have contributed to a more comprehensive assessment of behavior in the TS and neurotypical control cohorts. Finally, this study focuses on attention problems and hyperactivity within a narrow age range of prepubertal children. This approach to overcomes potential confounders that relate to the inclusion of a wide age range (e.g. children and adolescents) in a cross-sectional study. The nature of these data did not allow for the investigation of the developmental trajectories of attention problems and hyperactivity as girls with TS start puberty. Further longitudinal study of ADHD-related behaviors would allow for such investigations.
In conclusion, we found high rates (51%) of ADHD-associated behaviors and specific cognitive deficits in TS. We were able to show that these behavioral and cognitive profiles are comparable to behavioral and cognitive profiles found in groups of children with iADHD, and that these profiles are, at least in part, independent of other TS-associated cognitive features (i.e. visuospatial abilities). Since TS is caused by X-monosomy, these findings suggests a putative role of X-linked genes and associated downstream biology in ADHD. Overall, our findings indicate that TS may be a unique, tractable model for understanding X-linked genetic and neurobiological mechanisms contributing to ADHD.
Supplementary Material
Figure S1 Distribution plot of Attention Problems measured on the BASC-2. One-sample Kolmogorov-Smirnov test for normal distribution, D=0.188, p=0.064.
Acknowledgements
The authors would like to thank Pearson Inc for providing us with standardization data from the NEPSY. The Turner Syndrome Society and the Turner Syndrome Foundation made this work possible. The authors would like to sincerely thank all of the families who kindly volunteered to participate.
Role of Funding Source
This work was supported by grants from the NICHD (HD049653), NIMH (MH099630), and the Chain of Love and Sharon Levine Foundations to A.L.R. T.G. was supported by a grant from the Gazit-Globe Post-Doctoral Fellowship Award, D.S.H. was supported by funding from the NIMH (MH092170). Dr. Reiss is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation. The funding sources mentioned above had no role in the study design; in the collection, analysis and interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentation: AACAP 61th Annual Meeting, San Diego, CA, US, October 2014
Author contribution:
Study concept and design: Green, Bade Shrestha, Pennington, Hong and Reiss.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Green, Bade Shrestha, Chromik and Rutledge
Critical revision of the manuscript for important intellectual content: Green, Pennington, Hong and Reiss
Statistical analysis: Green, Rutledge and Pennington
Administrative, technical, or material support: Green, Bade Shrestha, Rutledge and Chromik
Study supervision: Green, Hong and Reiss
Conflict of interests: T.G., S.B.S, L.C.C., K.R., B.P. D.S.H. and A.L.R. have nothing to declare.
References
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 2014;17:491–496. doi: 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nature reviews Neuroscience. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Williamson S, Wilens TE, Spencer TJ, et al. Clinical correlates of ADHD in females: findings from a large group of girls ascertained from pediatric and psychiatric referral sources. J Am Acad Child Adolesc Psychiatry. 1999;38:966–975. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Biederman J, Kim JW, Doyle AE, Mick E, Fagerness J, Smoller JW, et al. Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1511–1518. doi: 10.1002/ajmg.b.30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry. 2002;159:36–42. doi: 10.1176/appi.ajp.159.1.36. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Hawi Z, Park J, Scott S, Gill M, Kent L. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1417–1424. doi: 10.1002/ajmg.b.31120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadesky EB, Mota VL, Schachar RJ. Beyond words: how do children with ADHD and/or conduct problems process nonverbal information about affect? J Am Acad Child Adolesc Psychiatry. 2000;39:1160–1167. doi: 10.1097/00004583-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Davies W. Sex differences in attention Deficit Hyperactivity Disorder: candidate genetic and endocrine mechanisms. Frontiers in neuroendocrinology. 2014;35:331–346. doi: 10.1016/j.yfrne.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Downey J, Elkin EJ, Ehrhardt AA, Meyer-Bahlburg HF, Bell JJ, Morishima A. Cognitive ability and everyday functioning in women with Turner syndrome. Journal of learning disabilities. 1991;24:32–39. doi: 10.1177/002221949102400107. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Holden C. Sex and the suffering brain. Science. 2005;308:1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EB. Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use. Am J Hum Genet. 1977;29:94–97. [PMC free article] [PubMed] [Google Scholar]
- Kent L, Emerton J, Bhadravathi V, Weisblatt E, Pasco G, Willatt LR, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519–524. doi: 10.1136/jmg.2008.057729. [DOI] [PubMed] [Google Scholar]
- Korkman MKU, Kemp SL. NEPSY: a developmental neuropsychological assessment. San Antonio, TX: The Psychological Corp; 1998. NEPSY® - Standardization data from the NEPSY®, Copyright © 2007 NCS Pearson, Inc. Used with permission. All rights reserved. [Google Scholar]
- Lepage JF, Dunkin B, Hong DS, Reiss AL. Contribution of executive functions to visuospatial difficulties in prepubertal girls with Turner syndrome. Developmental neuropsychology. 2011;36:988–1002. doi: 10.1080/87565641.2011.584356. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Hong DS, Hallmayer J, Reiss AL. Genomic imprinting effects on cognitive and social abilities in prepubertal girls with Turner syndrome. J Clin Endocrinol Metab. 2012;97:E460–E464. doi: 10.1210/jc.2011-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Y, Zhou R, Zhang H, Yang L, Wang B, et al. Association between polymorphisms in serotonin 2C receptor gene and attention-deficit/hyperactivity disorder in Han Chinese subjects. Neuroscience letters. 2006;407:107–111. doi: 10.1016/j.neulet.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- McGough JJ, Smalley SL, McCracken JT, Yang M, Del'Homme M, Lynn DE, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621–1627. doi: 10.1176/appi.ajp.162.9.1621. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Allen G, Haxby JV, Largay KA, Daly E, White BJ, et al. The effects of sex steroids, and the X chromosome, on female brain function: a study of the neuropsychology of adult Turner syndrome. Neuropsychologia. 1994;32:1309–1323. doi: 10.1016/0028-3932(94)00065-4. [DOI] [PubMed] [Google Scholar]
- Neece CL, Baker BL, Crnic K, Blacher J. Examining the validity of ADHD as a diagnosis for adolescents with intellectual disabilities: clinical presentation. J Abnorm Child Psychol. 2013;41:597–612. doi: 10.1007/s10802-012-9698-4. [DOI] [PubMed] [Google Scholar]
- Nijhuis-Van der Sanden MW, Eling PA, Van Asseldonk EH, Van Galen GP. Decreased movement speed in girls with turner syndrome: a problem in motor planning or muscle initiation? Journal of clinical and experimental neuropsychology. 2004;26:795–816. doi: 10.1080/13803390490509394. [DOI] [PubMed] [Google Scholar]
- Nijhuis-Van der Sanden MW, Van Asseldonk EH, Eling PA, Van Galen GP. Slow motor performance in girls with Turner Syndrome is not related to increased neuromotor noise. Motor control. 2003;7:111–133. doi: 10.1123/mcj.7.2.111. [DOI] [PubMed] [Google Scholar]
- Peeters SB, Cotton AM, Brown CJ. Variable escape from X-chromosome inactivation: identifying factors that tip the scales towards expression. BioEssays : news and reviews in molecular, cellular and developmental biology. 2014;36:746–756. doi: 10.1002/bies.201400032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RCKRW. Behavior Assessment System for Children-Second Edition Manual. 2ed ed. 4201 Woodland Road, Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. Transition to young adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. Am J Med Genet. 1998;79:140–147. [PubMed] [Google Scholar]
- Rommelse NN, Altink ME, Arias-Vasquez A, Buschgens CJ, Fliers E, Faraone SV, et al. Differential association between MAOA, ADHD and neuropsychological functioning in boys and girls. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1524–1530. doi: 10.1002/ajmg.b.30845. [DOI] [PubMed] [Google Scholar]
- Ross JL, Kushner H, Roeltgen DP. Developmental changes in motor function in girls with Turner syndrome. Pediatric neurology. 1996;15:317–322. doi: 10.1016/s0887-8994(96)00227-5. [DOI] [PubMed] [Google Scholar]
- Ross JL, Roeltgen D, Stefanatos GA, Feuillan P, Kushner H, Bondy C, et al. Androgen-responsive aspects of cognition in girls with Turner syndrome. J Clin Endocrinol Metab. 2003;88:292–296. doi: 10.1210/jc.2002-021000. [DOI] [PubMed] [Google Scholar]
- Ross JL, Stefanatos G, Roeltgen D, Kushner H, Cutler GB., Jr Ullrich-Turner syndrome: neurodevelopmental changes from childhood through adolescence. Am J Med Genet. 1995;58:74–82. doi: 10.1002/ajmg.1320580115. [DOI] [PubMed] [Google Scholar]
- Ross JL, Stefanatos GA, Kushner H, Bondy C, Nelson L, Zinn A, et al. The effect of genetic differences and ovarian failure: intact cognitive function in adult women with premature ovarian failure versus turner syndrome. J Clin Endocrinol Metab. 2004;89:1817–1822. doi: 10.1210/jc.2003-031463. [DOI] [PubMed] [Google Scholar]
- Ross JL, Stefanatos GA, Kushner H, Zinn A, Bondy C, Roeltgen D. Persistent cognitive deficits in adult women with Turner syndrome. Neurology. 2002;58:218–225. doi: 10.1212/wnl.58.2.218. [DOI] [PubMed] [Google Scholar]
- Rovet JF. The psychoeducational characteristics of children with Turner syndrome. Journal of learning disabilities. 1993;26:333–341. doi: 10.1177/002221949302600506. [DOI] [PubMed] [Google Scholar]
- Russell HF, Wallis D, Mazzocco MM, Moshang T, Zackai E, Zinn AR, et al. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol. 2006;31:945–955. doi: 10.1093/jpepsy/jsj106. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Takarae Y, DeBoer T, McDonald-McGinn DM, Zackai EH, Ross JL. Overlapping numerical cognition impairments in children with chromosome 22q11.2 deletion or Turner syndromes. Neuropsychologia. 2008;46:82–94. doi: 10.1016/j.neuropsychologia.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E, Langley K, Williams H, Walters J, Williams NM, Suren S, et al. Steroid sulfatase is a potential modifier of cognition in Attention Deficit Hyperactivity Disorder. Genes, brain, and behavior. 2011 doi: 10.1111/j.1601-183X.2010.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- 40.Trent S, Dean R, Veit B, Cassano T, Bedse G, Ojarikre OA, et al. Biological mechanisms associated with increased perseveration and hyperactivity in a genetic mouse model of neurodevelopmental disorder. Psychoneuroendocrinology. 2013;38:1370–1380. doi: 10.1016/j.psyneuen.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Prescholl and Primary Scale of Intelligence, 3th ed. (WIPPSI-III) : Technical and interactive manual. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, 4th ed (WISC-IV).: Technical and interactive manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Xu X, Brookes K, Sun B, Ilott N, Asherson P. Investigation of the serotonin 2C receptor gene in attention deficit hyperactivity disorder in UK samples. BMC research notes. 2009;2:71. doi: 10.1186/1756-0500-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn AR, Roeltgen D, Stefanatos G, Ramos P, Elder FF, Kushner H, et al. A Turner syndrome neurocognitive phenotype maps to Xp22.3. Behav Brain Funct. 2007;3:24. doi: 10.1186/1744-9081-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Distribution plot of Attention Problems measured on the BASC-2. One-sample Kolmogorov-Smirnov test for normal distribution, D=0.188, p=0.064.