Abstract
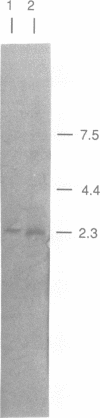
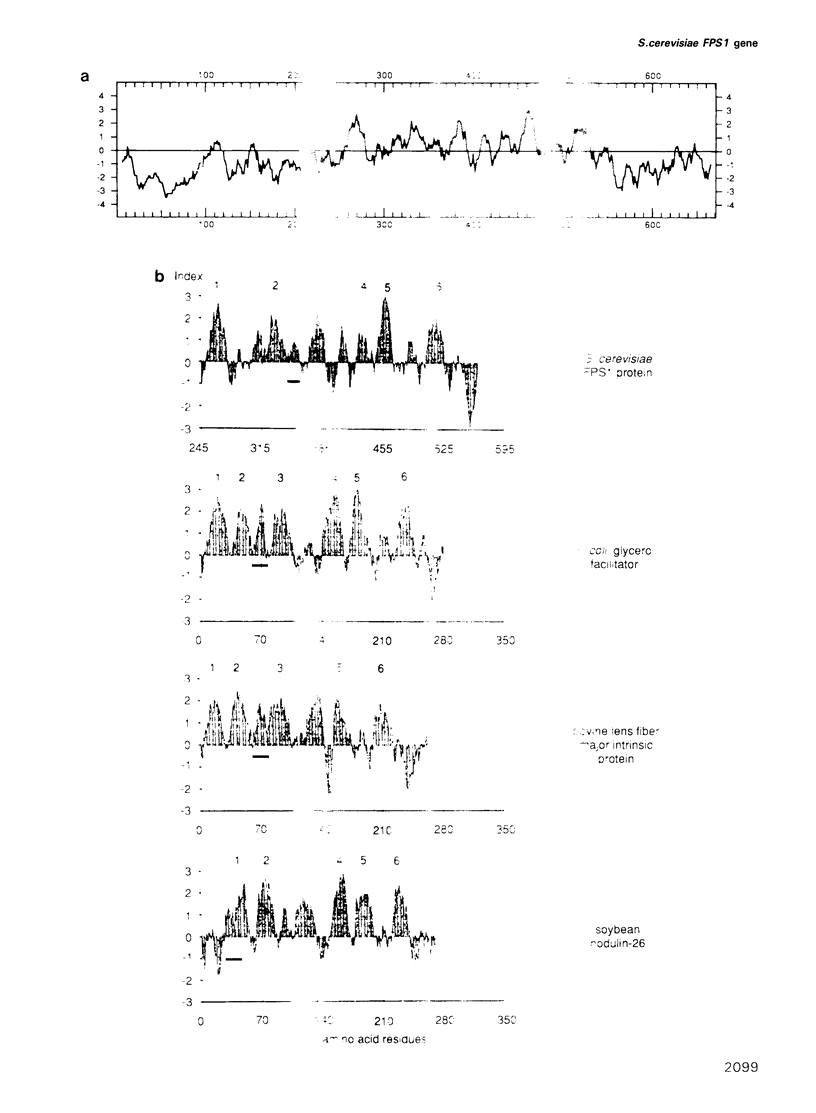
Recently a new family of membrane proteins comprising the bovine lens fibre major intrinsic protein, soybean nodulin-26 protein and the Escherichia coli glycerol facilitator has been described [M.E. Baker and M.H. Saier, Jr (1990) Cell, 60, 185-186]. These proteins have six putative membrane spanning domains and one (probably intracellular) intermembrane fragment is particularly well conserved. We have identified a new member of this family in the yeast Saccharomyces cerevisiae. It also possesses the six transmembrane domains and the highly conserved intermembrane sequence. In contrast to the other three proteins which are all approximately 280 amino acids long, the yeast protein has an N-terminal extension of approximately 250 amino acids, which contains a string of 17 asparagine residues and a C-terminal extension of approximately 150 amino acids. The gene, which we called FPS1 (for fdp1 suppressor), suppresses in single copy the growth defect on fermentable sugars of the yeast fdp1 mutant but it is not allelic to FDP1. The deficiency of the fdp1 mutant in glucose-induced RAS-mediated cAMP signalling and in rapid glucose-induced changes in the activity of certain enzymes was not restored. Deletion of FPS1 does not cause any of the phenotypic deficiencies of the fdp1 mutant.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argüelles J. C., Mbonyi K., Van Aelst L., Vanhalewyn M., Jans A. W., Thevelein J. M. Absence of glucose-induced cAMP signaling in the Saccharomyces cerevisiae mutants cat1 and cat3 which are deficient in derepression of glucose-repressible proteins. Arch Microbiol. 1990;154(2):199–205. doi: 10.1007/BF00423333. [DOI] [PubMed] [Google Scholar]
- Baker M. E., Saier M. H., Jr A common ancestor for bovine lens fiber major intrinsic protein, soybean nodulin-26 protein, and E. coli glycerol facilitator. Cell. 1990 Jan 26;60(2):185–186. doi: 10.1016/0092-8674(90)90731-s. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Henderson P. J. Homologies between sugar transporters from eukaryotes and prokaryotes. Annu Rev Physiol. 1989;51:459–471. doi: 10.1146/annurev.ph.51.030189.002331. [DOI] [PubMed] [Google Scholar]
- Bañuelos M., Fraenkel D. G. Saccharomyces carlsbergensis fdp mutant and futile cycling of fructose 6-phosphate. Mol Cell Biol. 1982 Aug;2(8):921–929. doi: 10.1128/mcb.2.8.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Beullens M., Mbonyi K., Geerts L., Gladines D., Detremerie K., Jans A. W., Thevelein J. M. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1988 Feb 15;172(1):227–231. doi: 10.1111/j.1432-1033.1988.tb13877.x. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Neigeborn L., Carlson M., Fraenkel D. G. The SNF3 gene is required for high-affinity glucose transport in Saccharomyces cerevisiae. J Bacteriol. 1987 Apr;169(4):1656–1662. doi: 10.1128/jb.169.4.1656-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Marshall-Carlson L., Carlson M. The yeast SNF3 gene encodes a glucose transporter homologous to the mammalian protein. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2130–2134. doi: 10.1073/pnas.85.7.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlab R., Oliveira D. E., Panek A. D. Investigation of the relationship between sst1 and fdp mutations in yeast and their effect on trehalose synthesis. Braz J Med Biol Res. 1985;18(4):447–454. [PubMed] [Google Scholar]
- Cheng Q., Michels C. A. The maltose permease encoded by the MAL61 gene of Saccharomyces cerevisiae exhibits both sequence and structural homology to other sugar transporters. Genetics. 1989 Nov;123(3):477–484. doi: 10.1093/genetics/123.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76(2):132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leucine-zipper motif update. Nature. 1989 Jul 13;340(6229):103–104. doi: 10.1038/340103a0. [DOI] [PubMed] [Google Scholar]
- Mazón M. J., Gancedo J. M., Gancedo C. Phosphorylation and inactivation of yeast fructose-bisphosphatase in vivo by glucose and by proton ionophores. A possible role for cAMP. Eur J Biochem. 1982 Oct;127(3):605–608. doi: 10.1111/j.1432-1033.1982.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Mbonyi K., Beullens M., Detremerie K., Geerts L., Thevelein J. M. Requirement of one functional RAS gene and inability of an oncogenic ras variant to mediate the glucose-induced cyclic AMP signal in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1988 Aug;8(8):3051–3057. doi: 10.1128/mcb.8.8.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonyi K., van Aelst L., Argüelles J. C., Jans A. W., Thevelein J. M. Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1990 Sep;10(9):4518–4523. doi: 10.1128/mcb.10.9.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder T., Küntzel H. Glucose-induced cAMP signaling in Saccharomyces cerevisiae is mediated by the CDC25 protein. FEBS Lett. 1989 Jan 2;242(2):341–345. doi: 10.1016/0014-5793(89)80498-3. [DOI] [PubMed] [Google Scholar]
- Rose M., Entian K. D., Hofmann L., Vogel R. F., Mecke D. Irreversible inactivation of Saccharomyces cerevisiae fructose-1,6-bisphosphatase independent of protein phosphorylation at Ser11. FEBS Lett. 1988 Dec 5;241(1-2):55–59. doi: 10.1016/0014-5793(88)81030-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamhart D. H., Van Den Heijkant M. P., Van De Poll K. W. Inactivation of fructose diphosphatase by sucrose in yeast. J Bacteriol. 1977 Apr;130(1):526–528. doi: 10.1128/jb.130.1.526-528.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengstag C., Hinnen A. The sequence of the Saccharomyces cerevisiae gene PHO2 codes for a regulatory protein with unusual aminoacid composition. Nucleic Acids Res. 1987 Jan 12;15(1):233–246. doi: 10.1093/nar/15.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkutnicka K., Tschopp J. F., Andrews L., Cirillo V. P. Sequence and structure of the yeast galactose transporter. J Bacteriol. 1989 Aug;171(8):4486–4493. doi: 10.1128/jb.171.8.4486-4493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., den Hollander J. A., Jans A. W. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: intracellular pH and the effect of membrane depolarizing compounds. J Gen Microbiol. 1987 Aug;133(8):2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Wigler M. SCH9, a gene of Saccharomyces cerevisiae that encodes a protein distinct from, but functionally and structurally related to, cAMP-dependent protein kinase catalytic subunits. Genes Dev. 1988 May;2(5):517–527. doi: 10.1101/gad.2.5.517. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Boy-Marcotte E., Camonis J. H., Thevelein J. M., Jacquet M. The C-terminal part of the CDC25 gene product plays a key role in signal transduction in the glucose-induced modulation of cAMP level in Saccharomyces cerevisiae. Eur J Biochem. 1990 Nov 13;193(3):675–680. doi: 10.1111/j.1432-1033.1990.tb19386.x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- van Aelst L., Jans A. W., Thevelein J. M. Involvement of the CDC25 gene product in the signal transmission pathway of the glucose-induced RAS-mediated cAMP signal in the yeast Saccharomyces cerevisiae. J Gen Microbiol. 1991 Feb;137(2):341–349. doi: 10.1099/00221287-137-2-341. [DOI] [PubMed] [Google Scholar]
- van de Poll K. W., Kerkenaar A., Schamhart D. H. Isolation of a regulatory mutant of fructose-1,6-diphosphatase in Saccharomyces carlsbergensis. J Bacteriol. 1974 Mar;117(3):965–970. doi: 10.1128/jb.117.3.965-970.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poll K. W., Schamhart D. H. Characterization of a regulatory mutant of fructose 1,6-bisphosphatase in Saccharomyces carlsbergensis. Mol Gen Genet. 1977 Jul 7;154(1):61–66. doi: 10.1007/BF00265577. [DOI] [PubMed] [Google Scholar]