Abstract
Against the backdrop of late 20th century declines in heart disease mortality in the United States, race-specific rates diverged because of slower declines among blacks compared with whites. To characterize the temporal dynamics of emerging black-white racial disparities in heart disease mortality, we decomposed race-sex–specific trends in an age-period-cohort (APC) analysis of US mortality data for all diseases of the heart among adults aged ≥35 years from 1973 to 2010. The black-white gap was largest among adults aged 35–59 years (rate ratios ranged from 1.2 to 2.7 for men and from 2.3 to 4.0 for women) and widened with successive birth cohorts, particularly for men. APC model estimates suggested strong independent trends across generations (“cohort effects”) but only modest period changes. Among men, cohort-specific black-white racial differences emerged in the 1920–1960 birth cohorts. The apparent strength of the cohort trends raises questions about life-course inequalities in the social and health environments experienced by blacks and whites which could have affected their biomedical and behavioral risk factors for heart disease. The APC results suggest that the genesis of racial disparities is neither static nor restricted to a single time scale such as age or period, and they support the importance of equity in life-course exposures for reducing racial disparities in heart disease.
Keywords: age-period-cohort models, blacks, health status disparities, heart diseases, United States, whites
Editor's note:An invited commentary on this article appears on page 313, and the authors’ response appears on page 318.
The dramatic reduction in heart disease mortality in the United States—a 60% decline between 1950 and 1999—ranks among the Centers for Disease Control and Prevention's top 10 greatest public health achievements of the 20th century (1). All major racial and ethnic groups in the United States experienced declines, but the rate of decline for whites was relatively consistent and steep, while it has been slower for blacks, resulting in a widening relative gap between blacks and whites (2, 3). In 2010, the racial gap in heart disease mortality accounted for more than a quarter of the black-white life expectancy disparity (4).
In order to further develop sound hypotheses regarding the conditions contributing to the widening gap in heart disease mortality between blacks and whites, it is important to examine the dimensions of these trends from multiple angles. For example, are the growing racial disparities a function of differential rates by age group, differential exposures to contemporaneous period effects, or differential cohort experiences? It is not uncommon to attribute population-wide heart disease mortality declines in the United States to a combination of risk factor reduction and implementation of new effective secondary and tertiary medical and surgical interventions (5). However, age-period-cohort (APC) analyses, which decompose trends into component dimensions of time, suggest that the overall declines may be attributable primarily to generational cohort improvements (6)—a pattern mirrored in some other countries (7–9).
While APC analyses have been used in sex-stratified studies, this analytical approach has not (to our knowledge) been used to examine the emergence and persistence of racial disparities in heart disease mortality. Such results would have important implications for better understanding the determinants of the widening disparities and would have implications for prevention efforts. For instance, large period trends in emerging racial disparities would point to the relative importance of equitable access to advances in medical technology and health services as drivers of disparities, whereas a larger cohort trend would be consistent with racial differences in exposures during critical periods of early life or cumulatively across the life course.
To more fully characterize the temporal dynamics of the late 20th century emergence of black-white racial disparities in heart disease mortality, we carried out APC analysis of race- and sex-specific rates of heart disease mortality for black and white adults in the United States between 1973 and 2010.
METHODS
Data sources
Annual numbers of US heart disease deaths among people aged 35 years or older were obtained from the National Center for Health Statistics (10). We analyzed data from the period 1973–2010, as it represented the longest span of complete rather than sampled data. Deaths were defined according to the International Classification of Diseases (ICD) definition for “all diseases of the heart,” which included the following codes: for the Eighth Revision of the ICD (1968–1978), codes 390–398, 402, 404, and 410–429; for the Ninth Revision (1979–1998), codes 390–398, 402, and 404–429; and for the Tenth Revision (1999–present), codes I00–I09, I11, I13, and I20–I51. This inclusive definition had the benefit of comparability ratios between ICD revisions that were approximately 1, indicating that temporal changes in the ICD codes introduced minimal bias into the study and no adjustments for ICD coding changes were necessary (11, 12).
Heart disease deaths and US Census Bureau midyear population estimates for persons aged ≥35 years (13) were aggregated by year of death (1973–2010), age at death, sex, and race (white or black). Year of birth was calculated as year of death minus age. Age, period, and cohort time scales were grouped into 5-year categories. Information on Hispanic ethnicity was not collected in the United States nationally on death records prior to 1999, and therefore data for Hispanics were not analyzed separately in this study.
Descriptive analysis
Data were arranged separately by race and sex into contingency tables permitting summarization in several ways. Age-specific rates can be compared along columns, period-specific rates can be compared across rows, and the experience of a birth cohort can be tracked along the diagonal as each group ages up to a subsequent period. Age × period and age × cohort plots of these rates permit visualization of trends. Age-specific plots of black-white mortality rate ratios visualize variation in the magnitude of the black-white disparity by age and cohort.
Statistical analysis
Formal testing of independent age, period, and cohort patterns was carried out using Poisson regression. We compared parameters and Akaike's Information Criterion values for a nested series of 1-, 2-, and 3-factor models including age alone, age + period, age + cohort, and age + period + cohort in order to assess whether additional time dimensions had independent associations with death rates and whether additional parameters significantly improved model fit. Reported race- and sex-specific estimates were derived from a 3-factor model with interactions between each time scale and race-sex group membership.
The challenges involved in fitting the full 3-factor APC model have been widely discussed in the statistical literature (14, 15). Fitting of such models is hindered by the inherent collinearity in the predictors: cohort + age = period (16). Several methods have been proposed to address this parameter identification problem, but none is without limitations. Following common practice, our primary analysis was a Poisson regression that constrained 2 parameters in 1 time scale (period categories 1973–1977 and 1978–1982) to be zero in order to make other parameters estimable (17). The final model specification is more fully described in the Web Appendix (available at http://aje.oxfordjournals.org/).
The primary criticism of the constraint approach to estimating APC models is that parameter estimates may be sensitive to the arbitrary choice of constraints (14, 15, 18). Therefore, we implemented 2 alternative approaches—the intrinsic estimator and the median polish—as sensitivity analyses. The intrinsic estimator is also a constraint-based Poisson regression, but the constraint is imposed via a principal-components analysis in which the design matrix of age, period, and cohort indicators is weighted to adjust for linear dependency (19, 20). The median polish is a nonparametric means of characterizing cohort patterns as a nonlinear departure from the additive contributions of age and period (21, 22). Further details about the intrinsic estimator and the median polish are provided in the Web Appendix.
Descriptive and regression analyses were implemented in R 3.0 (R Foundation for Statistical Computing, Vienna, Austria), and the intrinsic estimator modeling was carried out using the apc_ie macro in Stata 12 (StataCorp LP, College Station, Texas) (20).
RESULTS
We analyzed 23.2 million deaths from all diseases of the heart among black and white adults in the United States, with individuals and populations at risk categorized into 11 age categories, 8 period categories, and 18 cohort categories. In 1973, white men had the highest age-adjusted rate of heart disease death, followed closely by black men (Table 1, Figure 1). Women had lower rates than men, but unlike men, in the early 1970s black women experienced higher mortality rates than white women. Heart disease mortality rates decreased for all groups between 1973 and 2010, but whites—particularly white men—saw steeper and more consistent declines than did blacks. Among men, black and white rates crossed over in the late 1970s as the rate of improvement for black men slowed between 1980 and 1990, producing an increasing black-white gap. Black women also experienced slower rates of decline than white women. The disparities produced by these differential rates of decline are plotted in Figure 2, where the age-adjusted racial gap for both men and women increases until the early 2000s and then declines slightly.
Table 1.
Rates of Heart Disease Mortality in the United States, by Age, Period, Race, and Sex, 1973–2010
| Race-Sex and Age Group, years |
Heart Disease Mortality per 100,000 Persons |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1973–1977 | 1978–1982 | 1983–1987 | 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | 2008–2010 | |
| White men | ||||||||
| 35–39 | 47.9 | 41.4 | 35.7 | 27.5 | 27.3 | 24.9 | 24.1 | 22.5 |
| 40–44 | 117.8 | 95.9 | 81.1 | 62.6 | 57.6 | 53.3 | 50.7 | 45.2 |
| 45–49 | 240.3 | 201.3 | 162.3 | 128.5 | 113.9 | 99.3 | 93.0 | 86.0 |
| 50–54 | 419.9 | 358.2 | 303.5 | 230.6 | 205.6 | 169.4 | 155.0 | 144.4 |
| 55–59 | 695.4 | 595.9 | 509.2 | 404.0 | 340.3 | 278.7 | 234.6 | 217.3 |
| 60–64 | 1,107.6 | 944.0 | 818.9 | 654.2 | 567.2 | 445.6 | 360.6 | 315.7 |
| 65–69 | 1,663.7 | 1,462.6 | 1,262.7 | 1,014.1 | 869.7 | 695.6 | 530.2 | 453.1 |
| 70–74 | 2,452.0 | 2,220.7 | 1,992.2 | 1,582.1 | 1,350.5 | 1,102.4 | 835.5 | 695.5 |
| 75–79 | 3,673.4 | 3,321.2 | 3,018.8 | 2,461.2 | 2,132.1 | 1,783.3 | 1,385.9 | 1,153.9 |
| 80–84 | 5,455.2 | 5,080.5 | 4,700.9 | 3,993.3 | 3,549.9 | 3,030.7 | 2,443.9 | 2,043.0 |
| ≥85 | 6,014.1 | 5,510.8 | 5,029.3 | 4,490.8 | 4,249.9 | 3,801.1 | 3,203.6 | 2,744.2 |
| Black men | ||||||||
| 35–39 | 104.4 | 93.3 | 85.0 | 74.8 | 63.3 | 54.2 | 53.4 | 50.3 |
| 40–44 | 197.5 | 186.5 | 172.4 | 142.8 | 126.8 | 104.9 | 92.4 | 83.4 |
| 45–49 | 370.4 | 335.9 | 292.9 | 271.7 | 241.5 | 194.3 | 172.0 | 145.1 |
| 50–54 | 596.6 | 549.0 | 510.2 | 439.4 | 406.8 | 325.7 | 295.4 | 245.4 |
| 55–59 | 854.0 | 844.5 | 757.4 | 698.3 | 618.1 | 529.9 | 441.1 | 385.5 |
| 60–64 | 1,253.9 | 1,185.6 | 1,184.6 | 1,034.9 | 929.8 | 769.7 | 647.3 | 550.7 |
| 65–69 | 1,663.3 | 1,585.0 | 1,570.3 | 1,459.1 | 1,247.6 | 1,066.4 | 870.6 | 774.8 |
| 70–74 | 2,482.7 | 2,229.8 | 2,277.9 | 2,033.3 | 1,905.8 | 1,570.2 | 1,254.7 | 1,046.7 |
| 75–79 | 3,138.6 | 2,993.9 | 2,949.2 | 2,786.4 | 2,502.5 | 2,233.7 | 1,847.4 | 1,566.1 |
| 80–84 | 4,310.6 | 4,483.4 | 4,548.5 | 4,065.4 | 3,770.1 | 3,274.0 | 2,784.9 | 2,340.7 |
| ≥85 | 3,748.9 | 3,813.8 | 3,785.7 | 3,805.0 | 3,529.0 | 3,277.0 | 2,906.4 | 2,506.0 |
| White women | ||||||||
| 35–39 | 13.3 | 11.8 | 10.0 | 8.4 | 9.6 | 10.2 | 9.8 | 9.5 |
| 40–44 | 28.8 | 25.5 | 21.5 | 16.5 | 17.1 | 17.7 | 18.7 | 17.6 |
| 45–49 | 55.4 | 50.5 | 43.9 | 34.9 | 32.9 | 30.1 | 32.0 | 31.5 |
| 50–54 | 105.4 | 95.4 | 87.8 | 70.7 | 64.4 | 55.6 | 50.8 | 50.2 |
| 55–59 | 198.6 | 179.0 | 163.8 | 139.2 | 122.3 | 104.0 | 85.1 | 76.1 |
| 60–64 | 370.0 | 336.2 | 308.3 | 255.0 | 230.5 | 189.3 | 151.7 | 129.3 |
| 65–69 | 648.8 | 592.5 | 540.6 | 443.0 | 390.4 | 331.7 | 252.8 | 211.1 |
| 70–74 | 1,168.7 | 1,054.0 | 962.6 | 791.9 | 690.7 | 577.8 | 453.6 | 368.2 |
| 75–79 | 2,112.8 | 1,866.3 | 1,693.0 | 1,413.2 | 1,236.3 | 1,049.1 | 831.5 | 691.1 |
| 80–84 | 3,668.0 | 3,395.8 | 3,093.0 | 2,637.7 | 2,343.5 | 2,025.0 | 1,632.0 | 1,343.4 |
| ≥85 | 4,381.1 | 3,974.1 | 3,568.3 | 3,120.7 | 2,864.0 | 2,532.3 | 2,087.0 | 1,753.8 |
| Black women | ||||||||
| 35–39 | 49.9 | 41.5 | 36.1 | 33.5 | 34.3 | 30.0 | 27.6 | 24.5 |
| 40–44 | 97.4 | 82.7 | 73.3 | 66.9 | 63.6 | 59.1 | 51.3 | 46.6 |
| 45–49 | 178.5 | 153.3 | 132.2 | 123.2 | 113.9 | 100.9 | 88.7 | 78.9 |
| 50–54 | 295.4 | 260.1 | 239.8 | 215.0 | 193.6 | 160.0 | 144.5 | 126.9 |
| 55–59 | 455.3 | 419.8 | 398.8 | 357.0 | 320.1 | 267.0 | 213.7 | 186.7 |
| 60–64 | 725.1 | 660.9 | 645.1 | 584.4 | 504.5 | 422.7 | 332.3 | 279.3 |
| 65–69 | 1,043.1 | 954.1 | 934.8 | 879.9 | 761.6 | 647.2 | 493.6 | 401.7 |
| 70–74 | 1,751.7 | 1,484.3 | 1,460.9 | 1,289.3 | 1,221.6 | 1,001.8 | 762.1 | 618.3 |
| 75–79 | 2,272.8 | 2,177.2 | 2,098.7 | 1,941.1 | 1,743.4 | 1,574.9 | 1,219.7 | 999.6 |
| 80–84 | 3,237.2 | 3,418.4 | 3,447.3 | 3,062.2 | 2,811.5 | 2,444.9 | 2,033.7 | 1,662.6 |
| ≥85 | 2,920.8 | 2,854.9 | 2,921.5 | 2,946.6 | 2,671.1 | 2,462.9 | 2,091.5 | 1,734.0 |
Figure 1.
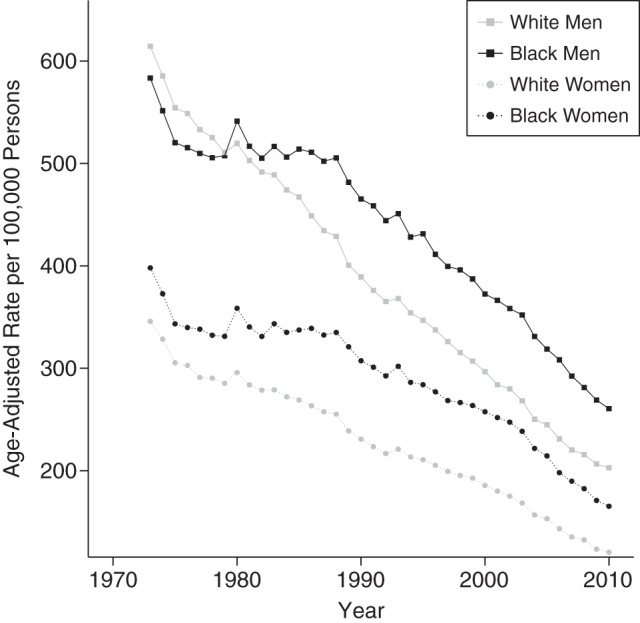
Age-adjusted rates of heart disease mortality per 100,000 persons, by race and sex, United States, 1973–2010. Rates were age-adjusted to the 2000 US Standard Population.
Figure 2.
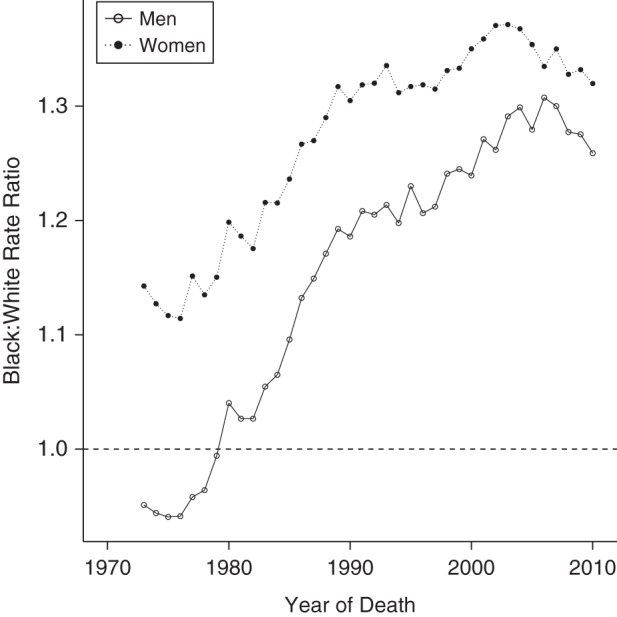
Age-adjusted black-white heart disease mortality rate ratios, by sex, United States, 1973–2010. Rates were age-adjusted to the 2000 US Standard Population.
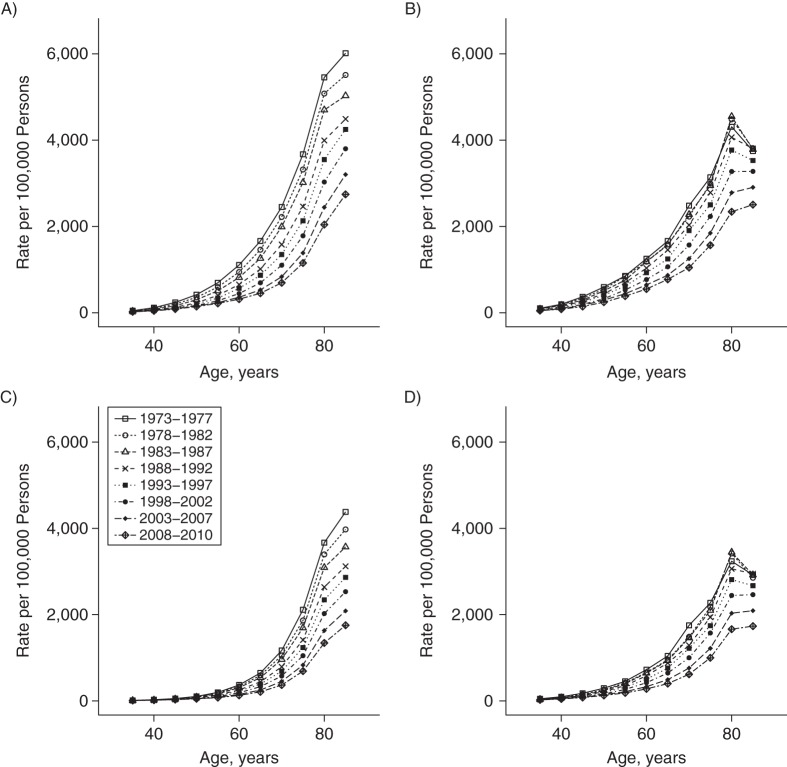
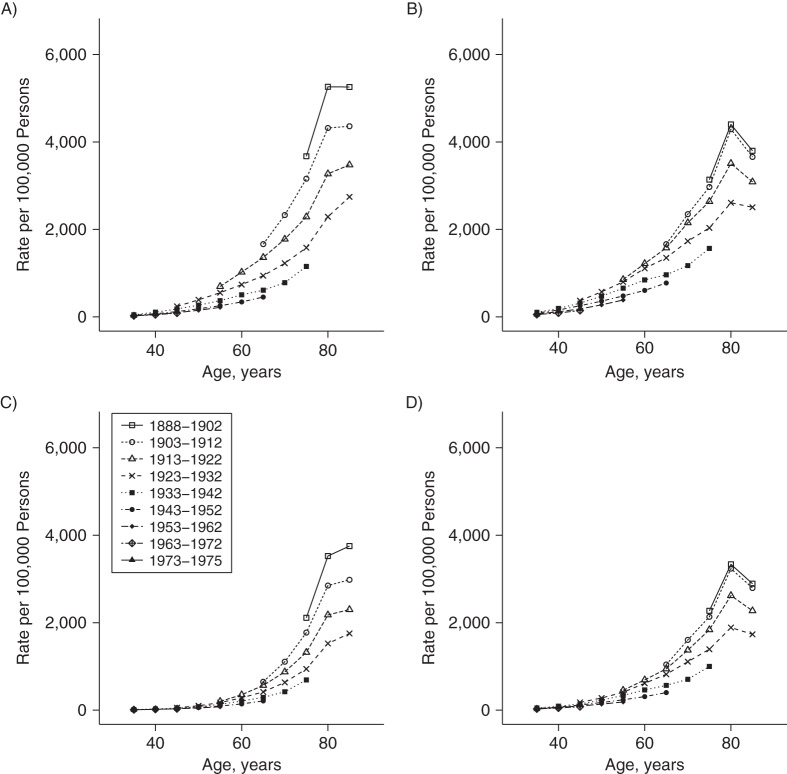
The race- and sex-specific age trends by period and cohort are displayed in Figures 3 and 4. The decline in heart disease mortality across successive periods is apparent for all groups, but the relative compression of the period-specific lines for black men and women (Figures 3B and 3D, respectively) illustrates the smaller improvement for blacks in comparison with whites. Figure 4 displays the longitudinal experience of specific birth cohorts across their observed life courses. Each generation experienced lower rates than the generation preceding it, although the cohort lines were most spread out for white men (Figure 4A) and most compressed for black women (Figure 4D).
Figure 3.
Age-specific heart disease mortality rates for 5-year periods between 1973 and 2010, by sex and race, United States. A) White men; B) black men; C) white women; D) black women.
Figure 4.
Age-specific heart disease mortality rates for 10-year birth cohorts born between 1888 and 1975, by sex and race, United States. A) White men; B) black men; C) white women; D) black women. Each line connects rates for a common 10-year birth cohort at each observed age.
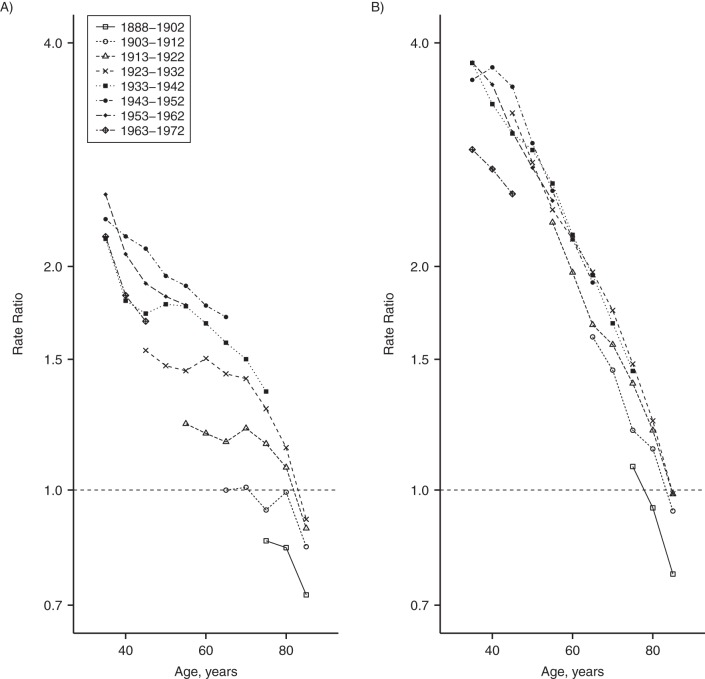
In order to examine the evolution of racial disparities across generations, in Figure 5 we graphed the black-white heart disease mortality rate ratio for birth cohorts (rate ratios and 95% confidence intervals in Web Table 1). For each birth cohort, the magnitude of the racial gap is inversely related to age, with large disparities (black-white rate ratios of 1.2–2.7 for men and 2.3–4.0 for women) among adults aged 35–59 years and little-to-no disparity at the oldest ages. Comparing one generation with the next, patterns vary by sex. Among women, the age-specific disparities are relatively consistent across generations (Figure 5B). However, among men, the black-white gap has grown with each successive generation, with the possible exception of men born in the 1950s and 1960s, for whom disparities were similar to or even slightly smaller than those of preceding cohorts (Figure 5A).
Figure 5.
Black-white heart disease mortality rate ratios among men (A) and women (B) in the United States, by age and birth cohort, 1973–2010. Each line connects rate ratios for a common 10-year birth cohort at each observed age. Rate ratios with 95% confidence intervals are shown in Web Table 1.
Table 2 shows the Akaike's Information Criterion model fit statistics from 1-, 2-, and 3-factor Poisson models. For each sex-race group separately and for comprehensive models with race-sex interaction terms, each time scale was independently associated with heart disease death rates, and the 3-factor constrained model had the best fit, as evidenced by the smaller fit statistic.
Table 2.
Model Fit Statistics for 1-, 2-, and 3-Factor Age-Period-Cohort Models of Racial Disparities in Heart Disease Mortality, United States, 1973–2010
| Model | Degrees of Freedom | Akaike's Information Criterion |
||||
|---|---|---|---|---|---|---|
| Whites |
Blacks |
Race × Sex Interactiona |
||||
| Men | Women | Men | Women | |||
| Intercept only | 87 | 18,811,486 | 19,164,309 | 1,640,338 | 1,871,111 | 41,487,245 |
| Age, years | 77 | 1,466,678 | 876,091 | 85,073 | 81,906 | 2,509,749 |
| Age-period | 71 | 53,774 | 18,063 | 4,455 | 5,109 | 101,333 |
| Age-cohort | 60 | 9,383 | 10,547 | 3,966 | 4,658 | 28,554 |
| Age-period-cohort (constrained) | 54 | 6,646 | 4,983 | 1,706 | 1,647 | 14,982 |
a The “intercept only” model in the “Race × Sex Interaction” column included race and sex; other models included race × sex × time interactions, where “time” was age, period, or cohort as indicated.
In 3-factor interaction models, there was significant statistical interaction of each time scale (age, period, and cohort) by race and sex (P < 0.001 for all tests; data not shown). We summarized results from the interaction model in 2 complementary ways. In Table 3 and Web Figure 1, within-group contrasts are reported separately for each race-sex group. Controlling for period and cohort, the patterns of heart disease mortality rate ratios by age (referent group, age 65–70 years) are similar across race-sex groups, with the exception of white women, who have lower relative risks at the younger ages but higher relative risks at the oldest ages. Within-race and -sex group cohort patterns are characterized by declining relative rates with successive generations born from the late 19th century to the mid-20th century, with a slowdown in change for the latter generations of the 20th century (referent birth cohort, 1913–1922). Black women and white women have similar relative cohort patterns, but among men, blacks have more modest relative generational improvements than whites. The period trends—conditional on age and cohort—are modest, with little race or sex difference.
Table 3.
Race- and Sex-Specific Rate Ratios for Heart Disease Mortality From a 3-Factor Constrained Age-Period-Cohort Model, United States, 1973–2010
| Time Scale | White Men |
Black Men |
White Women |
Black Women |
||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Age, years | ||||||||
| 35–39 | 0.08 | 0.08, 0.08 | 0.09 | 0.09, 0.10 | 0.04 | 0.04, 0.04 | 0.09 | 0.08, 0.09 |
| 40–44 | 0.16 | 0.16, 0.16 | 0.17 | 0.16, 0.17 | 0.08 | 0.08, 0.08 | 0.16 | 0.15, 0.16 |
| 45–49 | 0.27 | 0.27, 0.28 | 0.28 | 0.27, 0.29 | 0.14 | 0.14, 0.14 | 0.26 | 0.25, 0.26 |
| 50–54 | 0.41 | 0.41, 0.42 | 0.43 | 0.42, 0.44 | 0.24 | 0.24, 0.24 | 0.38 | 0.37, 0.39 |
| 55–59 | 0.58 | 0.57, 0.58 | 0.59 | 0.58, 0.60 | 0.40 | 0.40, 0.40 | 0.55 | 0.54, 0.55 |
| 60–64 | 0.77 | 0.77, 0.78 | 0.80 | 0.79, 0.81 | 0.65 | 0.65, 0.66 | 0.76 | 0.76, 0.77 |
| 65–69 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 70–74 | 1.32 | 1.31, 1.32 | 1.34 | 1.33, 1.35 | 1.58 | 1.57, 1.58 | 1.38 | 1.37, 1.40 |
| 75–79 | 1.77 | 1.76, 1.78 | 1.70 | 1.68, 1.73 | 2.51 | 2.50, 2.53 | 1.83 | 1.80, 1.86 |
| 80–84 | 2.51 | 2.49, 2.52 | 2.39 | 2.34, 2.44 | 4.18 | 4.14, 4.21 | 2.65 | 2.59, 2.72 |
| ≥85 | 2.62 | 2.60, 2.64 | 2.15 | 2.09, 2.21 | 4.56 | 4.51, 4.60 | 2.32 | 2.26, 2.40 |
| Period | ||||||||
| Pre-1983 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1983–1987 | 1.01 | 1.01, 1.02 | 1.04 | 1.02, 1.05 | 1.00 | 1.00, 1.01 | 1.06 | 1.05, 1.08 |
| 1988–1992 | 0.96 | 0.95, 0.96 | 1.01 | 0.99, 1.03 | 0.95 | 0.94, 0.95 | 1.08 | 1.06, 1.11 |
| 1993–1997 | 0.98 | 0.97, 0.99 | 0.99 | 0.96, 1.01 | 0.95 | 0.94, 0.96 | 1.10 | 1.07, 1.13 |
| 1998–2002 | 0.97 | 0.96, 0.98 | 0.92 | 0.89, 0.94 | 0.92 | 0.91, 0.93 | 1.07 | 1.04, 1.11 |
| 2003–2007 | 0.93 | 0.92, 0.94 | 0.85 | 0.82, 0.88 | 0.85 | 0.83, 0.86 | 0.98 | 0.94, 1.02 |
| 2008–2010 | 0.94 | 0.93, 0.96 | 0.80 | 0.76, 0.83 | 0.80 | 0.79, 0.81 | 0.91 | 0.87, 0.96 |
| Cohort | ||||||||
| 1888–1892 | 1.57 | 1.55, 1.58 | 1.08 | 1.04, 1.12 | 1.61 | 1.59, 1.63 | 1.29 | 1.24, 1.34 |
| 1893–1897 | 1.47 | 1.45, 1.48 | 1.11 | 1.08, 1.15 | 1.47 | 1.45, 1.48 | 1.26 | 1.22, 1.30 |
| 1898–1902 | 1.37 | 1.36, 1.38 | 1.13 | 1.10, 1.16 | 1.36 | 1.34, 1.37 | 1.27 | 1.24, 1.30 |
| 1903–1907 | 1.27 | 1.26, 1.27 | 1.12 | 1.10, 1.14 | 1.23 | 1.23, 1.24 | 1.25 | 1.22, 1.27 |
| 1908–1912 | 1.14 | 1.14, 1.15 | 1.04 | 1.03, 1.05 | 1.12 | 1.11, 1.12 | 1.09 | 1.08, 1.10 |
| 1913–1917 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1918–1922 | 0.85 | 0.85, 0.85 | 0.93 | 0.92, 0.94 | 0.89 | 0.88, 0.89 | 0.90 | 0.89, 0.90 |
| 1923–1927 | 0.72 | 0.71, 0.72 | 0.89 | 0.87, 0.90 | 0.78 | 0.77, 0.78 | 0.82 | 0.81, 0.83 |
| 1928–1932 | 0.59 | 0.59, 0.60 | 0.79 | 0.77, 0.81 | 0.67 | 0.67, 0.68 | 0.71 | 0.69, 0.72 |
| 1933–1937 | 0.49 | 0.48, 0.49 | 0.72 | 0.70, 0.74 | 0.59 | 0.59, 0.60 | 0.61 | 0.59, 0.63 |
| 1938–1942 | 0.40 | 0.39, 0.40 | 0.64 | 0.62, 0.67 | 0.51 | 0.51, 0.52 | 0.53 | 0.51, 0.55 |
| 1943–1947 | 0.34 | 0.33, 0.34 | 0.60 | 0.58, 0.63 | 0.46 | 0.45, 0.47 | 0.46 | 0.44, 0.48 |
| 1948–1952 | 0.29 | 0.29, 0.30 | 0.53 | 0.51, 0.56 | 0.42 | 0.41, 0.42 | 0.41 | 0.39, 0.43 |
| 1953–1957 | 0.26 | 0.26, 0.27 | 0.49 | 0.47, 0.52 | 0.40 | 0.39, 0.41 | 0.38 | 0.36, 0.41 |
| 1958–1962 | 0.25 | 0.24, 0.25 | 0.44 | 0.41, 0.47 | 0.44 | 0.43, 0.45 | 0.37 | 0.34, 0.39 |
| 1963–1967 | 0.23 | 0.22, 0.23 | 0.40 | 0.37, 0.43 | 0.47 | 0.46, 0.49 | 0.34 | 0.32, 0.37 |
| 1968–1972 | 0.21 | 0.21, 0.22 | 0.40 | 0.37, 0.43 | 0.48 | 0.47, 0.50 | 0.33 | 0.30, 0.36 |
| 1973–1975 | 0.20 | 0.20, 0.21 | 0.41 | 0.38, 0.46 | 0.50 | 0.47, 0.53 | 0.31 | 0.28, 0.35 |
Abbreviations: CI, confidence interval; RR, rate ratio.
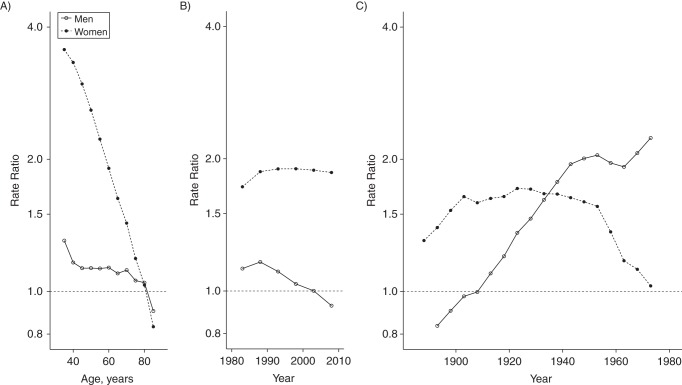
A complementary perspective on the results for interaction of race-sex groups with each of the 3 time scales is to focus on the contrast of rates between blacks and whites within each time category. Figure 6 and Web Table 2 depict such black-white contrasts from the same 3-factor constrained Poisson interaction model, showing important differences by sex. This disparities perspective suggests that among women, racial disparities vary most on the age scale, where black-white gaps are largest for young women but decline to equality by older ages (Figure 6A). Period-specific black-white rate ratios among women increased from approximately 1.6 to 1.9 between 1973 and 1983 and then plateaued through 2010 (Figure 6B). For women, black-white rate ratios within each generational cohort were relatively constant at approximately 1.6 from 1900 to around 1960, when they began to decline (Figure 6C).
Figure 6.
Black-white heart disease mortality rate ratios on age (A), period (B), and cohort (C) time scales from a 3-factor constrained Poisson model, by sex, United States, 1973–2010. The model was fitted by constraining the first 2 period categories (1973–1977 and 1978–1982) to be zero. Rate ratios represent the contrast of black mortality with white mortality (referent) for each time value and sex. See Web Table 2 for point estimates and 95% confidence intervals.
For men, age-specific disparities were more consistent, but generational cohort differences changed dramatically. Conditional on age and period, black men born before 1913 had lower heart disease mortality than white men of the same generations, but from approximately 1917 to the late 1950s, the black-white gap grew until black men experienced approximately twice the heart disease mortality as white men of the same birth cohort. The black-white disparities among men along the period time scale were small and declined from the late 1980s to 2010.
As a sensitivity analysis, we analyzed the same data with 2 alternative methods. The general patterns of the intrinsic estimator results (Web Table 3 and Web Figure 2) were consistent with the Poisson constraint-based model. Age was the dominant time scale, followed by birth cohort, with modest period trends. Racial differences in cohort patterns emerged for early 20th century cohorts of both sexes but declined by 1960. The median polish detected cohort trends as a departure from additive age and period associations and was consistent with greater racial differences in cohort trends among men than among women, particularly for mid-20th century cohorts (Web Table 4 and Web Figure 3).
DISCUSSION
Enthusiasm about the dramatic overall reductions in heart disease mortality in the United States since 1970 is dampened somewhat by the emergence and persistence of black-white racial disparities. To more fully describe the temporal dynamics of this disparity, we applied an APC analysis to race- and sex-specific trends. Three patterns were evident. First, the magnitude of racial disparities in heart disease mortality varied substantially by age, particularly among women, with the largest disparities being observed among the youngest adults. Second, the change in period-specific disparities between 1973 and 2010 was modest for both men and women. Finally, cohort trends indicative of risk differences between generations were notable, with growing black-white disparities, particularly for men born between 1920 and 1960, suggesting exposures with impacts over the life course that slowed the rate of decline for black men relative to white men born during the mid-20th century.
The observation that racial disparities in heart disease mortality are greatest among young-to-middle-aged adults is not novel (23) but is important given the years of life lost and the impact on life expectancy gaps (24). Common use of age-adjusted summary rates for surveillance purposes masks this important source of disparity (25). Such patterns could arise from racial differences in age-specific risk factor profiles or age at onset of comorbidity. For example, there are racial differences in early-onset hypertension among blacks, as well as lower rates of hypertension control (26–29). The “John Henryism” hypothesis of stress among low-income blacks resulting from high-effort coping—trying to advance economically in the face of structural discrimination—has been posited as one explanation for earlier and more aggressive hypertension among young blacks (30, 31). Whether through a stress pathway or some other pathway, there is also growing evidence for contributions of residential segregation, neighborhood deprivation, and unhealthy built environments to racial disparities in cardiovascular disease risk factors and outcomes, and these processes particularly impact young and middle-aged adults (32–36). The smaller sex gap among blacks as compared with whites generally, along with differences in risk factor burden and secondary prevention, particularly among black women, probably explain some of the sex differences in age-specific disparities (37, 38). APC analyses typically treat the age dimension as a purely biological aspect of disease risk, but in the case of age-varying racial disparities, age may reflect social as well as biological factors.
The results observed on the period time scale were most notable for their modest contribution to race-sex–specific heart disease mortality trends. Independent period contributions are those which similarly affect all age groups during a given period of time. For instance, the advent of a medical or surgical intervention which enhanced survival across the age spectrum would produce a period-specific decline in mortality. As much as 50% of overall declines in heart disease mortality have been attributed to medical and technological advancements made since 1980 (5). The modest declines in racial disparities among men since the early 1990s may reflect some reduction in the racial gap in health care among men, but persistent period-specific disparities among women suggest that the intersection of sex and race complicates interpretation (39). Regardless, it is not apparent from our analysis that a differential distribution of medical or technological advances along the period time scale is a primary driver of observed trends in racial disparities.
The dominance of cohort contributions over period contributions to heart disease mortality in the general population has been previously reported in the United States (6), but the identification in our study of racial and sex differences in the magnitude and timing of cohort changes is a novel finding, as far as we know. Cohort trends—with the connotation of life-course and generational differences—point toward exposure histories that are unique to specific generations due to the social systems and environments into which they were born or in which they experienced critical life-stage benchmarks (21).
The cohort patterns described do not arise simply from a generational difference in risk for a single older age group. Instead differences are seen across the age spectrum, producing patterns that can be understood through the lens of life-course processes for chronic disease etiology (40, 41). Evidence for cohort differences in accumulated exposure to major risk factors comes in part from other APC analyses. An APC analysis of midlife smoking prevalence found that smoking peaked for both black and white birth cohorts born between 1925 and 1935, while subsequent generations had lower smoking prevalences, with similar rates of decline by race and sex (42–44). Therefore, population shifts in smoking patterns correlate roughly with overall cohort improvements in heart disease death rates but probably do not explain racial differences in improvement. In a separate APC analysis of systolic and diastolic blood pressure, there was little independent cohort effect on trends, but for every generation blacks had higher mean blood pressure than whites, and the racial gap widened over time, particularly for men born after 1920 (45). Thus, changes in blood pressure probably do not explain the overall cohort patterns in heart disease for all race-sex groups, but they may partially explain the racial gap in improvement among men born midcentury.
While the distribution of well-established cardiovascular disease risk factors among individuals is important, the presence of both strong cohort patterns and racial disparities in heart disease mortality raises questions about the “causes of causes,” including social determinants of health (46, 47). The 20th century brought broad improvements in nutrition, health technology, and socioeconomic status, but diffusion of such benefits has been socially patterned, thereby giving rise to health disparities (48). Blacks in the United States have a higher prevalence of heart disease than blacks in the Caribbean, independent of socioeconomic status and health behaviors (49), and efforts to reduce socioeconomic barriers to advanced treatment for heart disease by providing universal access to health care have thus far not reduced racial disparities (50). Together these findings raise questions about other aspects of the sociohistorical context in which racial disparities arise in the United States. For example, Krieger et al. (51) reported an association of discriminatory “Jim Crow” laws in the southern United States with cohort and period trends for black premature mortality but not white premature mortality. The sex differences in both age and cohort trends in racial disparities suggest that changing social and health environments may affect the life-course health of men and women differently.
APC decomposition of surveillance data has several limitations. APC models do not describe causal mechanics of disease incidence, but instead provide an exploratory descriptive tool for examining population health patterns. No statistical method fully solves the fundamental nonidentifiability problem of full APC modeling, but the relative consistency in patterns between the constrained Poisson, intrinsic estimator, and median polish approaches lends cautious support to the current findings. Our choice to examine all diseases of the heart may have masked differences in age, period, or cohort patterns of subtypes of heart disease mortality, but it offered the advantage of reduced bias from time-varying misclassification over several revisions of the ICD. Because information on ethnicity was not routinely recorded on US death records until 1999, we could not further separate race and Hispanic ethnicity for the study period. While Hispanic whites have lower rates of heart disease than non-Hispanic whites, the trends are virtually identical (Web Figure 4).
In conclusion, this APC analysis identified modest period contributions but larger age and cohort contributions to race- and sex-specific trends in heart disease mortality. Racial disparities among men may arise from differences in birth cohorts born in the mid-20th century, whereas disparities for women may be a combination of age-specific advantages for white women and modest period-specific disadvantages for black women. These findings point to the significance of a large racial disparity in heart disease mortality at younger ages and the importance of life-course determinants of this racial disparity in heart disease mortality. In addition to ensuring equitable access to secondary prevention for heart disease mortality, further investigations aimed at identifying levers for reduction of black-white disparities in heart disease mortality should consider racial differences in early life and cumulative exposures which have slowed improvements in the burden of heart disease mortality among black Americans compared with white Americans.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division for Heart Disease and Stroke Prevention, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia (Michael R. Kramer, Amy L. Valderrama, Michele L. Casper); and Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Michael R. Kramer).
M.R.K. was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award K01HD074726 and by an appointment to the Research Participation Program of the Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Centers for Disease Control and Prevention. Decline in deaths from heart disease and stroke—United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;4830:649–656. [PubMed] [Google Scholar]
- 2.Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the National Conference on Cardiovascular Disease Prevention. Circulation. 2000;10225:3137–3147. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF, Mehari A, Curry B, et al. Racial and geographic variation in coronary heart disease mortality trends. BMC Public Health. 2012;12:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochanek KD, Arias E, Anderson RN. How did cause of death contribute to racial differences in life expectancy in the United States in 2010? NCHS Data Brief. 2013;125:1–8. [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;35623:2388–2398. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y. Trends in U.S. adult chronic disease mortality, 1960–1999: age, period, and cohort variations. Demography. 2008;452:387–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HA, Park H. Trends in ischemic heart disease mortality in Korea, 1985–2009: an age-period-cohort analysis. J Prev Med Public Health. 2012;455:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma E, Iso H, Takahashi H, et al. Age-period-cohort analysis of mortality due to ischemic heart disease in Japan, 1955 to 2000. Circ J. 2008;726:966–972. [DOI] [PubMed] [Google Scholar]
- 9.Peltonen M, Asplund K. Age-period-cohort effects on ischaemic heart disease mortality in Sweden from 1969 to 1993, and forecasts up to 2003. Eur Heart J. 1997;188:1307–1312. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics, Centers for Disease Control and Prevention. CDC WONDER. Underlying Cause of Death Database. Detailed Multiple Cause of Death Data—All County, 1973–2010. (Compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program) Hyattsville, MD: National Center for Health Statistics; 2015. [Google Scholar]
- 11.Anderson RN, Miniño AM, Hoyert DL, et al. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;492:1–32. [PubMed] [Google Scholar]
- 12.Klebba A, Scott J. Estimates of selected comparability ratios based on dual coding of 1976 death certificates by the Eighth and Ninth Revisions of the International Classification of Diseases. Mon Vital Stat Rep. 1980;2811:1–19. [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute. SEER*Stat Database: Incidence — SEER 9 Regs Research Data, Nov 2012 Sub (1973–2010) — <Single Ages to 85+, Katrina/Rita Adjustment> Linked to County Attributes — Total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research. (Based on the November 2012 submission) Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 14.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: age-period-cohort models. Stat Med. 1987;64:469–481. [DOI] [PubMed] [Google Scholar]
- 15.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age-period and age-cohort models. Stat Med. 1987;64:449–467. [DOI] [PubMed] [Google Scholar]
- 16.Kupper LL, Janis JM, Karmous A, et al. Statistical age-period-cohort analysis: a review and critique. J Chronic Dis. 1985;3810:811–830. [DOI] [PubMed] [Google Scholar]
- 17.Mason WM, Feinberg SE. Cohort Analysis in Social Research: Beyond the Identification Problem. New York, NY: Springer-Verlag New York; 1985. [Google Scholar]
- 18.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. [DOI] [PubMed] [Google Scholar]
- 19.Tu YK, Krämer N, Lee WC. Addressing the identification problem in age-period-cohort analysis: a tutorial on the use of partial least squares and principal components analysis. Epidemiology. 2012;234:583–593. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Fu WJ, Land KC. A methodological comparison of age-period-cohort models: the intrinsic estimator and conventional generalized linear models. Sociol Methodol. 2004;341:75–110. [Google Scholar]
- 21.Keyes KM, Utz RL, Robinson W, et al. What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc Sci Med. 2010;707:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahpar C, Li G. Homicide mortality in the United States, 1935–1994: age, period, and cohort effects. Am J Epidemiol. 1999;15011:1213–1222. [DOI] [PubMed] [Google Scholar]
- 23.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff. 2007;261:38–48. [DOI] [PubMed] [Google Scholar]
- 24.Harper S, Lynch J, Burris S, et al. Trends in the black-white life expectancy gap in the United States, 1983–2003. JAMA. 2007;29711:1224–1232. [DOI] [PubMed] [Google Scholar]
- 25.Cooper RS. Coronary heart disease burden among persons of African origin. In: Marmot M, Elliot P, eds. Coronary Heart Disease Epidemiology: From Aetiology to Public Health. New York, NY: Oxford University Press; 2005:118–134. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Racial/ethnic disparities in the awareness, treatment, and control of hypertension—United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2013;6218:351–355. [PMC free article] [PubMed] [Google Scholar]
- 27.Downie DL, Schmid D, Plescia MG, et al. Racial disparities in blood pressure control and treatment differences in a Medicaid population, North Carolina, 2005–2006. Prev Chronic Dis. 2011;83:A55. [PMC free article] [PubMed] [Google Scholar]
- 28.Gadegbeku CA, Lea JP, Jamerson KA. Update on disparities in the pathophysiology and management of hypertension: focus on African Americans. Med Clin North Am. 2005;895:921–933. [DOI] [PubMed] [Google Scholar]
- 29.Cooper RS, Liao Y, Rotimi C. Is hypertension more severe among U.S. blacks, or is severe hypertension more common? Ann Epidemiol. 1996;63:173–180. [DOI] [PubMed] [Google Scholar]
- 30.James SA, Keenan NL, Strogatz DS, et al. Socioeconomic status, John Henryism, and blood pressure in black adults. The Pitt County Study. Am J Epidemiol. 1992;1351:59–67. [DOI] [PubMed] [Google Scholar]
- 31.James SA, Strogatz DS, Wing SB, et al. Socioeconomic status, John Henryism, and hypertension in blacks and whites. Am J Epidemiol. 1987;1264:664–673. [DOI] [PubMed] [Google Scholar]
- 32.Greer S, Casper M, Kramer M, et al. Racial residential segregation and stroke mortality in Atlanta. Ethn Dis. 2011;214:437–443. [PubMed] [Google Scholar]
- 33.Greer S, Kramer MR, Cook-Smith JN, et al. Metropolitan racial residential segregation and cardiovascular mortality: exploring pathways. J Urban Health. 2014;913:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer MR, Hogue CR. Is segregation bad for your health? Epidemiol Rev. 2009;311:178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kershaw KN, Diez Roux AV, Burgard SA, et al. Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol. 2011;1745:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mujahid MS, Diez Roux AV, Cooper RC, et al. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;242:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho JE, Paultre F, Mosca L. The gender gap in coronary heart disease mortality: is there a difference between blacks and whites? J Womens Health (Larchmt). 2005;142:117–127. [DOI] [PubMed] [Google Scholar]
- 38.Leifheit-Limson EC, Spertus JA, Reid KJ, et al. Prevalence of traditional cardiac risk factors and secondary prevention among patients hospitalized for acute myocardial infarction (AMI): variation by age, sex, and race. J Womens Health (Larchmt). 2013;228:659–666. [DOI] [PubMed] [Google Scholar]
- 39.Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;3537:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuh D, Ben-Shlomo Y, Lynch J, et al. Life course epidemiology. J Epidemiol Community Health. 2003;5710:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. [DOI] [PubMed] [Google Scholar]
- 42.Anderson CM, Burns DM, Dodd KW, et al. Chapter 2: birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Anal. 2012;32(suppl 1):S14–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health. 1996;862:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. The changing influence of gender and race. JAMA. 1989;2611:49–55. [PubMed] [Google Scholar]
- 45.Finch BK, Beck AN, Lin S, et al. Temporal racial trends in cardiovascular health: cohort and period trends and explanations. Presented at the Population Association of America 2012 Annual Meeting, San Francisco, California, May 3–5, 2012. [Google Scholar]
- 46.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;397:887–903. [DOI] [PubMed] [Google Scholar]
- 47.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(1 suppl):S28–S40. [DOI] [PubMed] [Google Scholar]
- 48.Chang VW, Lauderdale DS. Fundamental cause theory, technological innovation, and health disparities: the case of cholesterol in the era of statins. J Health Soc Behav. 2009;503:245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H, Kershaw KN, Hicken MT, et al. Cardiovascular disease among black Americans: comparisons between the U.S. Virgin Islands and the 50 U.S. states. Public Health Rep. 2013;1283:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albert MA, Ayanian JZ, Silbaugh TS, et al. Early results of Massachusetts healthcare reform on racial, ethnic, and socioeconomic disparities in cardiovascular care. Circulation. 2014;12924:2528–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krieger N, Chen JT, Coull BA, et al. Jim Crow and premature mortality among the US black and white population, 1960–2009: an age-period-cohort analysis. Epidemiology. 2014;254:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.