Abstract
We have previously shown that agonists selective for the cannabinoid receptor 2 (CB2), including O-1966, inhibit the Mixed Lymphocyte Reaction (MLR), an in vitro correlate of organ graft rejection, predominantly through effects on T-cells. Current studies explored the mechanism of this immunosuppression by O-1966 using mouse spleen cells. Treatment with O-1966 dose-relatedly decreased levels of the active nuclear forms of the transcription factors NF-κB and NFAT in wild-type T-cells, but not T-cells from CB2 knockout (CB2R k/o) mice. Additionally, a gene expression profile of purified T-cells from MLR cultures generated using a PCR T-cell activation array showed that O-1966 decreased mRNA expression of CD40 ligand and CyclinD3, and increased mRNA expression of Src-like-adaptor 2 (SLA2), Suppressor of Cytokine Signaling 5 (SOCS5), and IL-10. The increase in IL-10 was confirmed by measuring IL-10 protein levels in MLR culture supernatants. Further, an increase in the percentage of regulatory T-cells (Tregs) was observed in MLR cultures. Pretreatment with anti-IL-10 resulted in a partial reversal of the inhibition of proliferation and blocked the increase of Tregs. Additionally, O-1966 treatment caused a dose-related decrease in the expression of CD4 in MLR cultures from wild-type, but not CB2R k/o, mice. These data support the potential of CB2-selective agonists as useful therapeutic agents to prolong graft survival in transplant patients, and strengthens their potential as a new class of immunosuppressive agents with broader applicability.
Keywords: Cannabinoids, Cannabinoid Receptor 2, Transplantation, Immunosuppression, T-reg cells, IL-10
Introduction
It has been well established that cannabinoids can modulate the function of the immune system. Cannabinoids mediate their actions on the immune system through two identified cannabinoid receptors, designated CB1 and CB2. CB1 is highly expressed on neurons in the central nervous system (Galiegue et al. 1995;Herkenham et al. 1991;Matsuda et al. 1990) and to a lesser extent on cells of the immune system and testes (Daaka et al. 1996;Galiegue et al. 1995;Waksman et al. 1999). CB2 is primarily expressed on cells of the immune system (Galiegue et al. 1995;Munro et al. 1993), including activated microglia, (Murikinati et al. 2010) and sparsely on neurons (Gong et al. 2006), and thus has emerged as a possible target for immunomodulation. There is now a significant body of research showing that activation of CB2 largely suppresses the action of leukocytes (Basu and Dittel 2011). The CB2 receptor has been found to be important in the attenuation of several inflammatory and autoimmune disease models in rodents, including Experimental Autoimmune Encephalitis (EAE), which is a mouse model of multiple sclerosis, (Maresz et al. 2007;Zhang et al. 2009c), ischemic/perfusion injury following an induced stroke (Ni et al. 2004;Zhang et al. 2007;Zhang et al. 2009a), inflammatory bowel disease (Storr et al. 2008;Storr et al. 2009), Crohn's disease (Wright et al. 2008), inflammatory autoimmune diabetes (Li et al. 2001), spinal cord injury (Baty et al. 2008), sepsis (Tschöp et al. 2009), autoimmune uveoretinitis (Xu et al. 2007), osteoporosis (Ofek et al. 2006) and systemic sclerosis (Servettaz et al. 2010). In the present studies, a CB2-selective agonist was tested as a potential therapeutic treatment to prevent graft rejection and graft-versus-host disease using the widely accepted Mixed Lymphocyte Reaction (MLR) as an in vitro correlate of the immune reactivity to a transplant. This application of CB2-selective agonists has been suggested (Nagarkatti et al. 2010), but has not been previously tested experimentally, with the exception of a report from our laboratory (Robinson et al. 2013). Rejection of grafts is primarily mediated by alloreactive T-cells (Heeger 2003). Therefore, most current maintenance protocols for immunosuppressive anti-rejection therapies use compounds that inhibit T-cells, including the calcineurin inhibitors, tacrolimus (FK506) and cyclosporine (Anonymous 2012). However, these medications have serious side effects, such as nephrotoxicity, post-transplant diabetes mellitus, hypertension, neurotoxicity, and hyperlipidemia (Jose 2007). Improved therapeutic agents with decreased toxicity are needed for use alone or in combination with existing therapies given at reduced doses.
T-cells have been reported to be sensitive to inhibition by CB2-selective cannabinoid agonists under several experimental conditions, as evidenced by decreased production of cytokines (IL-2 and IFN-γ), by inhibition of migration of T-cells to inflammatory stimuli, and by inhibition of proliferation of T-cells (Börner et al. 2009;Cencioni et al. 2010;Coopman et al. 2007;Ghosh et al. 2006;Maresz et al. 2007;Robinson et al. 2013). Previous work from our laboratory demonstrated that the CB2-selective agonists JWH-015 and O-1966 inhibited the murine MLR (Robinson et al. 2013). The inhibition was via the CB2 receptor, as shown using cannabinoid receptor selective antagonists and spleen cells of mice with a genetic deficiency in this receptor (CB2R k/o). Additional results showed that these agonists acted primarily on CD3+ T-cells, rather than on CD11b+ accessory cells, as exposure of CD3+cells to these compounds completely inhibited their action in a reconstituted MLR, while exposure of CD11b+ cells resulted in partial suppression. Further, proliferation of purified T- cells by anti-CD3 and anti-CD28 antibodies was inhibited. T-cell function was decreased by CB2-selective agonists, as an ELISA of MLR culture supernatants revealed IL-2 release was significantly reduced in the cannabinoid treated cells. These effects were not due to the induction of apoptosis, as cultures treated with the CB2 agonists did not exhibit increased levels of caspases or fragmented DNA measured by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay.
Current studies explored mechanisms by which the CB2-selective agonist O-1966 suppresses T-cells in the MLR. It was found that this cannabinoid increased Treg cells and IL-10 in MLR cultures, and down-regulated CD40 ligand, CyclinD3, and the transcription factors, NF-κB and NFAT, as well as decreasing CD4 expression. Together, these data show that a CB2-selective agonist can suppress T-cells by inhibiting certain parameters of their activation, while promoting an immunosuppressive Treg phenotype, and thus support the potential of this class of compounds as useful therapeutic agents to prolong graft survival in transplant patients.
Materials and Methods
Mice
Six week-old, specific pathogen-free C3HeB/FeJ and C57BL/6J female mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Founder CB2 receptor deficient (CB2R k/o) mice, on a C57BL/6J background (Buckley et al. 2000) were obtained from the National Institutes of Health (Bethesda, MD) and bred in the Animal Core of the Center for Substance Abuse Research, P30 Center for Excellence, at Temple University School of Medicine Central Animal Facility. All animal experiments were performed in accordance with the guidelines of the Temple University Animal Care and Use Committee.
Compounds
O-1966 (CB2-selective agonist) was a generous gift from Anu Mahadevan (Organix, Woburn, MA). O-1966 was supplied as crystals and stored at -20°C and dissolved in absolute ethanol before each use. This solution was added drop-wise to the culture medium, RPMI-1640, used for the assay to obtain the desired concentration.
One-way Mixed Lymphocyte Reaction (MLR)
Mice were sacrificed and their spleens aseptically removed. Single cell suspensions were obtained by passing spleens through nylon mesh bags (Sefar Inc., Depew, NY) in RPMI-1640 with 5% fetal bovine serum (FBS) containing 50 μM 2-mercaptoethanol (2-Me), and 100 U/ml penicillin and streptomycin sulfate. All reagents were purchased from Gibco Life Technologies (Carlsbad, CA), with the exception of FBS, which was purchased from HyClone Laboratories (Logan, UT). Red blood cells were lysed by hypotonic shock for 10 seconds with sterile water. Responder spleen cells from C57BL/6 mice were resuspended in RPMI with 10% FBS, 50 μM 2-Me, and 100 U/ml penicillin and streptomycin sulfate. Splenocytes from C3HeB/FeJ were similarly prepared to serve as the stimulator cells, but they were inactivated by treatment with 50 μg/ml of mitomycin C for 20 min at 37°C. Both responder and stimulator cells were resuspended to the desired concentration using a Beckman Coulter Z1 Dual Cell and Particle Counter (Beckman Coulter Inc., Indianapolis, IN). Responder cells (8 × 105) and stimulator cells (8 × 105) were co-cultured in 200 μl in 96 well plates for 48 h at 37°C in 5% CO2. In wells where it was desired, cannabinoid was added to responder cells 3 h prior to mixing with stimulator cells. After a 48 h incubation period, cultures were pulsed with 1 μCi/well [3H]-thymidine and harvested 18 h later onto glass fiber filters (Packard, Downers Grove, IL) using a Packard multichannel harvester, and placed in vials in liquid scintillation solution (Cytoscint, MP-Biomedical, Irvine, CA). [3H]-thymidine incorporation on the filters was measured using a Packard 1900 TR liquid scintillation counter. Data were corrected for background by subtraction of [3H]-thymidine incorporation in the absence of stimulator cells. Results are expressed as a Suppression Index (SI), where untreated spleen cells are given a value of 1.00 (100%), and responses of cultures receiving treatment with cannabinoids are calculated as:
Cell Viability
Cell viability was assessed using cell cultures that were run in parallel with each experimental MLR. Viability was measured by flow cytometry using the LIVE/DEAD® Fixable Dead Cell Stain Kit from Molecular Probes, Inc. (Eugene, OR). 1×106 cells from cultures were resuspended in 1 ml PBS and incubated for 30 min at room temperature with 1 μl Dead Cell Stain. Cells were washed twice, resuspended in staining buffer and analyzed using an LSRII (BD Biosciences, San Jose, CA) and analyzed with FACSDiva™ software (BD Biosciences).
Fluorescence Activated Cell Sorting (FACS)
Splenocytes were resuspended in staining buffer (PBS containing 1% BSA, Sigma, St. Louis, MO). Cells were incubated with 1 μg/106 cells 2.4G2 antibody specific for Fcγ III/II receptor (BioLegend, San Diego, CA) at 4°C for 5 minutes to prevent nonspecific binding. Cells were then stained with 1 μl/106 cells LIVE/DEAD® Dead Cell Stain (Molecular Probes, Inc) and incubated for 30 min on ice with 1 μl Dead Cell Stain. For positive selections, cells were incubated with 0.5 μg/106 cells of fluorophore-conjugated anti-mouse CD3ε, CD4, or CD8 (BioLegend) for 30 min on ice. Cells were then washed twice with sorting buffer: PBS containing 0.1% BSA (Sigma), resuspended in sorting buffer to a concentration of 4 × 107 cells/ml and sorted using the FACSAria™ system (BD Biosciences). For negative selection of T-cells, cells were prepared as above, but incubated with 0.5 μg/106 cells of fluorophore-conjugated rat anti-mouse CD11b (BioLegend) and 0.5 μg/106 cells of fluorophore-conjugated rat anti-mouse CD45R (B220) (Molecular Probes) for 30 min on ice, sorted with the FACSAria™ system (BD Biosciences), and collecting the double negative population.
In Vitro T-cell Activation
96 well microplates were coated with 0.5 μg/well of LEAF™ Purified anti-mouse CD3ε (BioLegend) and incubated for 2h at 37°C. Wells were washed to remove unbound antibody. C57BL/6J splenocytes were sorted by flow cytometry as described above and CD3+ T-cells were negatively selected. 2×105 cells were added to each well and incubated at 37°C for 30 min. Following incubation, 0.4 μg soluble LEAF™ Purified anti-mouse CD28 (BioLegend) was added to each well for a final concentration of 2 μg/mL. Cultures were incubated for 48 hr at 37°C, pulsed with 1μCi/well [3H]-thymidine, and harvested 18 hr later, and radioactive uptake measured by liquid scintillation counting using a Packard 1900 TR liquid scintillation counter.
Transcription Factor Analysis
Splenocytes from CB2R k/o mice or wild-type mice were treated for 3 h with O-1966 before activation with anti-CD3 and anti-CD28 antibodies, and then incubated for 18 hours. 8.8×106 cells per treatment group were harvested and nuclear protein was extracted using a Nuclear Extract Kit (Active Motif, Carlsbad, CA), following the provided protocol. Protein levels were quantified using the Bradford reagent (Sigma) and absorbance read on a POLARstar Omega microplate reader (BMG LABTECH, Offenburg, Germany). Protein levels were adjusted to 2.5 μg/μl and stored at -80°C until use. Levels of activated NFAT were measured using a TransAM™ NFATc1 Transcription Factor Assay Kit (Active Motif) according to manufacturer's protocol. Briefly, 20 pmol (in 2 μl) of oligonucleotide containing the consensus sequence for NFAT and 50 μl containing 5 μg of nuclear extract were used in the assay in the provided 96-well assay plate. 100 μl mouse anti-NFATc1 antibody was added to the bound transcription factor followed by the addition of 100 μl of anti-mouse horse radish peroxidase (HRP)-conjugated antibody and the provided reagents for a colorimetric reaction. The optical density was determined using a POLARstar Omega microplate reader (BMG LABTECH). NF-κB levels were measured using a TransAM™ Flexi NFκB p50 Transcription Factor Assay Kit (Active Motif) according to manufacturer's protocol. The protocol was the same as described above, except using 1 pmol (in 1 μl) of biotinylated oligonucleotide containing the consensus sequence for NFκB combined with 50 μl containing 5 μg of nuclear extract. Bound transcription factor was detected with 100 μl rabbit anti- NFκB p50 and 100 μl anti-rabbit HRP-conjugated antibody.
mRNA Expression Analysis
Cells were harvested from the MLR 18 hr into culture and total RNA was extracted using an RNeasy® Mini Kit (Qiagen, Valencia, CA) according to the provided protocol. RNA concentration and purity was checked with a NanoDrop2000 (Thermo Fisher Scientific, Waltham, MA). RNA was then reverse transcribed to cDNA using the RT2 First Strand Kit (Qiagen) following the provided protocol. cDNA was then analyzed using the RT2 Profiler PCR Array for Mouse T-cell and B-cell Activation (Qiagen) on the Mastercycler ep realplex2 (Eppendorf, Hamburg, Germany). The changes in expression of several genes that showed ≥ 4-fold changes were confirmed by individual quantitative PCR (qPCR) with gene-specific primers (200 nM forward primer and 200 nM reverse primer) (Invitrogen, Grand Island, NY) and 10 μl Power SYBR® Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) on the Mastercycler ep Realplex2 (Eppendorf). The relative quantification of experimental genes in comparison to the reference gene, β-Actin, was determined. The relative expression ratio was calculated based on the qPCR efficiency and the crossing points for the experimental genes and β-Actin transcripts.
Flow Cytometry
MLR cultures were harvested at various time points and washed with staining buffer, (PBS containing 1% BSA, Sigma, St. Louis, MO). 1×106 cells in 1 ml of PBS were added to Falcon™ polystyrene round-bottom tubes (BD Biosciences) and stained with 1 μl of LIVE/DEAD® Dead Cell Stain (Molecular Probes, Inc) for 30 min on ice. The cells were washed twice with staining buffer and resuspended in 50 μl of staining buffer. To prevent nonspecific binding, the cells were incubated with 1 μg of 2.4G2 antibody specific for Fcγ III/II receptor (BioLegend) at 4°C for 5 minutes. To determine the number of Treg cells, suspensions were then incubated with 0.5 μg of fluorophore conjugated rat anti-mouse CD3ε (BioLegend), rat anti-mouse CD4 (BioLegend), or isotype control for 30 min on ice, washed twice with staining buffer and resuspended in PBS with 2% (w/v) paraformaldehyde (Sigma) on ice for 15 min. The cells were washed 3 times with PBS and resuspended in 1 ml PBS with 0.5% (v/v) Tween 20 (Sigma), washed 3 times with staining buffer and resusupended in 100 μl staining buffer containing 0.5 μg rat anti-mouse Foxp3 or isotype control (BioLegend) at room temperature for 30 min. The cells were washed 3 times with staining buffer, resuspended in 400 μl staining buffer, and analyzed immediately on the LSRII cytometer (BD Biosciences) equipped with 488 nm, 405 nm, 640 nm and 355 nm lasers, and analyzed using FACSDiva software (BD Biosciences) and post-analyzed with FlowJo (Tree Star, Inc., Ashland, OR). Compensation for spectrum overlaps between fluorochromes was performed using FACSDiva software (or Flowjo software). To determine which cells were secreting IL-10, separate MLR cultures, after 48 hrs incubation, were treated with GolgiStop® Protein Transport Inhibitor containing monensin (BD Biosciences) for at least 4 hrs at 37°C before harvesting. Cells were then harvested and washed in staining buffer, and stained with 1μl of eFluor 780 Fixable Viability Stain (eBioscience) for 30 min at 4°C, then stained for surface markers with eFluor 450 labeled anti-mouse CD3ε, PE-Cy7 labeled anti-mouse CD45R (eBioscience, San Jose, CA), and BV605 labeled anti-mouse CD11b (BioLegend), as above. After washing in staining buffer, cells were fixed in 4% paraformaldehyde solution (Sigma Chemical Co.) for 20 min at 4°C, and washed 2× in staining buffer. Cells were then resuspended in BD Perm/Wash® buffer (BD Bioscience) for 15 minutes, pelleted by centrifugation, and resuspended in 50 μl of Perm/Wash® buffer. Cells were then stained with APC labeled anti-mouse IL-10 (BD Biosciences) for 30 min at 4°C in the dark, and washed 2× with Perm/Wash® buffer. Cells were resuspended in 400 μl staining buffer for flow cytometry using the LSRII and software as described above.
ELISA
IL-10 levels in the MLR culture supernatant were determined using the Ready-Set-Go!® reagent set (eBioscience, San Diego, CA). Costar® 96 well flat bottom high affinity protein binding microplates (Corning Inc. Life Sciences, Tewksbury, MA) were coated overnight at 4°C with capture antibody specific for mouse IL-10. The rest of the assay and incubations were performed at room temperature. After 24 h in culture, the MLR supernatant was harvested and 100 μl/well was added into the prepared microplate and incubated for 2 h. After incubation, the wells were washed 5 times with wash buffer, PBS with 0.05% (v/v) of Tween-20 (Sigma), to remove unbound antigen. Then 100 μl/well of capture biotin-conjugated antibody against mouse IL-10 was added and incubated for 1 h. The wells were then washed 5 times to remove unbound antibody and an Avidin-horseradish peroxidase (HRP) solution was added and incubated for 30 min. The wells were washed 5 times and 100 μl/well of Tetramethylbenzidine (TMB) substrate solution was added and incubated for 15 min, followed by addition of 50 μl/well of dilute hydrochloric acid stop solution. The optical density was determined using a POLARstar Omega microplate reader (BMG LABTECH, Offenburg, Germany).
Statistics
Two-sample (independent or paired) t-test was employed to compare two treatment groups or post vs. pre-treatment within one sample. In cases with more than two treatment groups, ANOVA was utilized for between group comparisons, where multiple testing p-values were adjusted using the Dunnett method. Normality assumption of the study endpoints was empirically examined and transformations such as log or square root were explored if evidence from the data did not support normality. The two-way ANOVA was also employed to test differences in outcome measurements across different groups when there were two treatment conditions involved as well as possible interactions between them. A p-value of 0.05 or less was considered to be statistically significant. SAS© software (version 9.3, Cary, NC) was used for all the data analyses reported here.
Results
A CB2-selective agonist alters gene expression in T-cells
Previously, we have shown that the CB2-selective agonist, O-1966, directly inhibits the proliferation of T-cells in the MLR and of purified T-cells activated by anti-CD3/CD28 antibodies (Robinson et al. 2013). To examine a possible mechanism of this suppression, nuclear levels of the transcription factors NF-κB and NFAT were measured in the presence or absence of O-1966. Splenocytes from wild-type C57BL/6 mice or CB2 receptor knockout (CB2R k/o) mice were sorted by flow cytometry and T-cells were negatively selected (CD11b-B220-). The T-cells were treated for 3 hours with O-1966 or ethanol vehicle and then activated with anti-CD3 and anti-CD28 antibodies and incubated for 18 h. The cells were then harvested and nuclear proteins extracted. Levels of activated NF-κB and NFAT that were able to bind to their target promoters were measured using the TransAM® transcription factor ELISA kits for NF-κB p50 and NFATc1. Figure 1 shows that treatment of T-cells from wild-type mice with O-1966 significantly decreased levels of both transcription factors in a dose-related manner, with suppression observed at concentrations of the cannabinoid of 16 and 32 μM for NF-κB, and of 8 to 32 μM for NFAT, as compared to ethanol vehicle controls. Suppression of neither transcription factor was observed in cultures containing T-cells from CB2R k/o mice. Identical cultures run in parallel, were harvested at 18 h and tested to measure cell viability using a Live/Dead® dead cell stain. No difference was observed in the number of dead cells between control and cannabinoid treated groups (data not shown).
Figure 1.
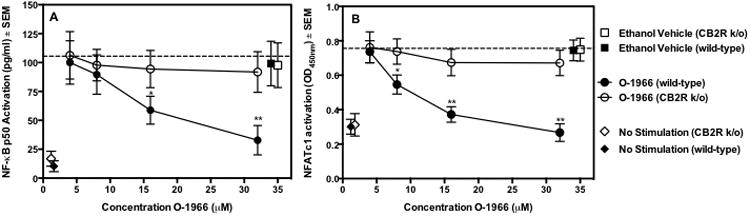
O-1966 decreases levels of activated nuclear forms of NFκB and NFAT in T-cells. Splenocytes from CB2R k/o mice (open symbols) or wild-type mice (closed symbols) were treated for 3 h with O-1966 (WT: ●, k/o:○) or ethanol vehicle (WT: ■, k/o: □) and then added to a plate coated with 25 μg anti-CD3 antibody/well and soluble anti-CD28 antibody (0.4 μg/well) or left unstimulated (WT: ◆, k/o: ◇). The cultures were incubated for 18 h and cultures were harvested and nuclear proteins extracted. Levels of activated nuclear NFκB (Panel A) and NFAT (Panel B) were measured using a TransAM® Transcription Factor ELISA. Data are the mean ± S.E.M. of 3 separate experiments with triplicate wells for each treatment. *p < 0.01, **p < 0.001. (ANOVA, WT versus k/o). Values for vehicle are not significantly different from no treatment.
To examine the effect of cannabinoids on the expression of genes involved in T-cell activation, an RT2 Profiler™ PCR Array for T-cell and B-cell activation was used. C57BL/6 responder splenocytes were pretreated for 3 h with 32 μM O-1966 or ethanol vehicle before the addition of mitomycin C inactivated C3HeB/FeJ splenocytes. After 18 hours, the cultures were harvested and purified by flow cytometry. The CD3+ T-cell population was collected and RNA extracted and analyzed. Figure 2 shows the differences in levels of gene expression between O-1966 and vehicle treated cells. Panel A shows a scatterplot of gene expression changes in T-cells of all the genes tested in the array. Genes that showed ≥ 4-fold changes in O-1966 treated cells, indicated by lying outside the dotted lines, were subsequently retested using individual qPCR reactions. These data presented in Panel B show that O-1966 treated T-cells from the MLR had a 4.8-fold and a 4.3-fold reduction in the expression of CD40 ligand and CyclinD3, respectively. In addition, there was a 4.3-fold increase in Suppressor of Cytokine Signaling 5 (SOCS5), a 3.9-fold increase of Src-like-adaptor 2 (SLA2), and a 4.8-fold increase of IL-10 mRNA expression.
Figure 2.
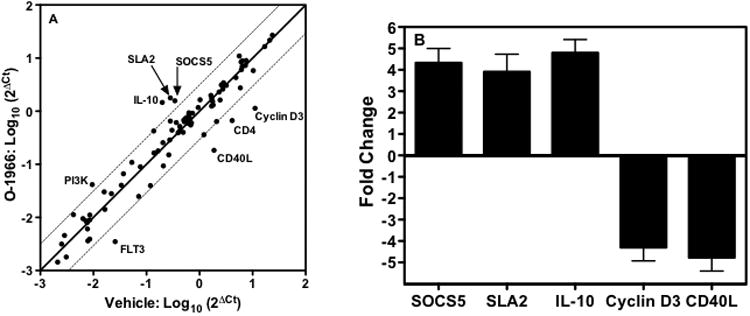
O-1966 treatment alters gene expression of T-cells in the MLR. C57BL/6 responder splenocytes were pretreated for 3 h with 32 μM O-1966 or 0.4 % ethanol vehicle. The cultures were incubated for 18 h and cells were harvested and sorted by flow cytometry for CD3+ T-cells. mRNA was extracted from this population and reverse transcribed. A Qiagen RT2 qPCR T-cell activation array was used to generate a gene expression profile. Panel A: Scatter plot showing gene expression in T-cells from vehicle treated cultures (x-axis) versus O-1966 treated cultures (y-axis). Points along the central line indicates unchanged expression and dotted lines designate 4-fold change cutoff of genes up-regulated in O-1966 treated cultures (left) and down-regulated in O-1966 treated cultures (right). Panel B: Genes showing ≥ 4-fold changes in the array were confirmed by 2 individual qPCR assays for each gene. Data are the mean ± S.E.M. of 2 qPCR reactions (array and individual reactions) from 2 separate experiments. p < 0.01 (Two-sample t-test, O-1966 versus vehicle)
O-1966 suppresses both CD4+ and CD8+ cells
Previously, we reported that treatment of CD3+ T-cells with O-1966 fully suppressed the MLR (Robinson et al. 2013). To determine whether O-1966 suppresses CD4+ cells, CD8+ cells, or both, splenocytes from wild-type mice were sorted by flow cytometry and CD4+ and CD8+ cells were selected. The populations were individually treated with O-1966 or ethanol vehicle, washed three times to remove the cannabinoid from the medium, and added back to the remainder of the untreated spleen cells, which were either CD4 or CD8 depleted, to restore the normal spleen population for the MLR. Figure 3 shows complete inhibition of the MLR was observed only in unsorted cultures and cultures containing cannabinoid treated CD4+ and CD8+ cells. In cultures that received cannabinoid treated CD4+ cells, the maximum suppression was 65% of the unsorted maximum and treatment of CD8+ cells was 50% of the maximum unsorted suppression, indicating O-1966 suppressed both CD4+ and CD8+ cells. Further, O-1966 treatment did not alter the CD4:CD8 ratio during the course of the MLR (data not shown).
Figure 3. O-1966 inhibits both CD4+ and CD8+ T-cells.
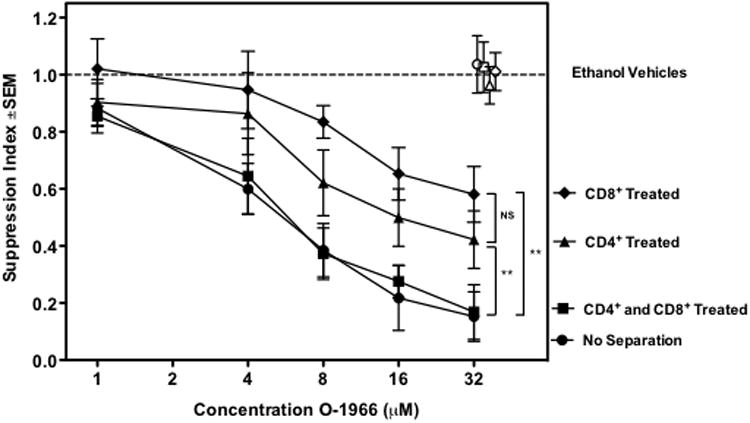
C57BL/6 splenocytes were sorted by flow cytometry into CD4+, CD8+, or CD4-CD8- fractions. CD4+ fractions were treated with O-1966 (▲) or ethanol vehicle (△), CD8+ fractions were treated with O-1966 (◆) or vehicle (◇), both CD4+ and CD8+ were treated with O-1966 (■) or vehicle (■), or unseparated populations were treated with O-1966 (●) or vehicle (○), for 3 h. Treated CD4+ or CD8+ populations were combined with untreated CD8+ or CD4+, respectively and CD4-CD8- cell subsets to reconstitute the normal splenocyte population for carrying out the MLR. Data are the mean of 2 separate experiments, with quadruplicate wells for each treatment. **p < 0.001. (ANOVA, CD4+ treated versus CD8+ treated, no separation versus CD4+ or CD8+ treated)
O-1966 treatment decreases CD4 expression in vitro
The expression of CD4 on the cell surface of CD3+Foxp3- cells was measured. MLR cultures were started using splenocytes from wild-type or CB2R k/o mice. The cells were pretreated for 3 h with O-1966 or ethanol vehicle and harvested 48 h into the assay, stained, and analyzed by flow cytometry. Figure 4A is a representative comparative histogram of the fluorescent intensity of CD4 in cultures treated with 32 μM O-1966 or ethanol vehicle control. While the percentage of CD4+ cells in the CD3+ population did not change, O-1966 treatment caused a negative shift of fluorescence intensity of these cells. Figure 4B shows that treatment with 8, 16, and 32 μM of O-1966 resulted in a dose dependent decrease of the mean fluorescence intensity of CD4 expression on the cell surface. Further, when splenocytes from CB2R k/o mice were used, there was no change in CD4 fluorescence intensity when treated with similar doses of O-1966, demonstrating that the decreased expression in wild-type mice is CB2 mediated.
Figure 4.
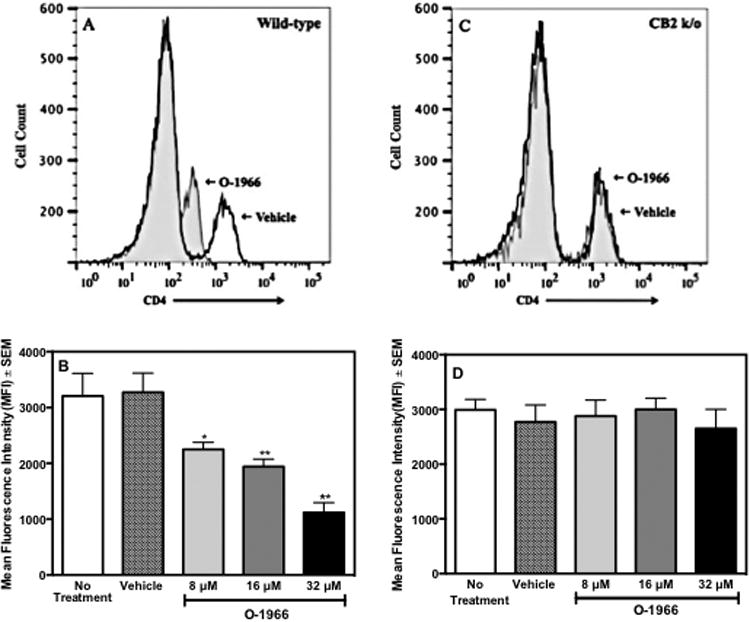
O-1966 treatment decreases CD4 expression in vitro. Wild-type (panels A and B) or CB2R k/o (panels C and D) C57BL/6 responder splenocytes were pretreated for 3 h with O-1966 or ethanol vehicle. MLR cultures were harvested at 48 hr and analyzed for CD4 expression on CD3+CD4+ populations by flow cytometry. An equal number of CD3+CD4+ cells were analyzed for each treatment group. Representative histograms of CD3+ cells from cultures treated with 32 μM O-1966 (gray filled) compared to vehicle treated cells (white filled) with responder cells from wild-type (Panel A) and CB2R k/o (Panel C). Mean Fluorescence Intensity (MFI) of CD4 in CD3+CD4+populations from MLR cultures that received no treatment (
 ), ethanol vehicle (
), ethanol vehicle (
 ), 8 μM O-1966 (
), 8 μM O-1966 (
 ), 16 μM O-1966 (
), 16 μM O-1966 (
 ), or 32 μM O-1966 (
), or 32 μM O-1966 (
 ). Data are mean ± S.E.M. of 3 separate experiments. *p < 0.01, **p < 0.001 (ANOVA, O-1966 versus vehicle). Values for vehicle are not significantly different from no treatment.
). Data are mean ± S.E.M. of 3 separate experiments. *p < 0.01, **p < 0.001 (ANOVA, O-1966 versus vehicle). Values for vehicle are not significantly different from no treatment.
O-1966 increases IL-10 release in the MLR
The release of IL-10 in the MLR was examined to support the observed increase of IL-10 mRNA expression detected in the array. Culture supernatants were collected 24 h after the start of the MLR. Figure 5 shows that O-1966, in doses ranging from 8 to 32 μM, significantly increased IL-10 release in a dose-related manner, indicating that O-1966 promotes an increase in this anti-inflammatory cytokine. Furthermore, when splenocytes from CB2R k/o mice were used, O-1966 treatment did not increase IL-10 release, indicating that this effect is CB2 receptor dependent.
Figure 5.
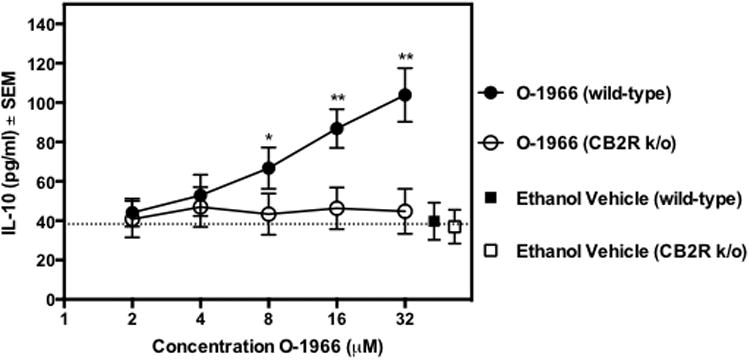
O-1966 increases IL-10 release. To determine the effect of O-1966 on the release of IL-10, CB2R k/o responder splenocytes (open symbols) or cells from wild-type littermates (closed symbols) were pretreated for 3 h with O-1966 (WT:●, k/o: ○) or ethanol vehicle (WT: ■, k/o: □). The cultures were incubated for 24 h; supernatants were collected; and concentrations of IL-10 were assessed by ELISA. Concentrations of ethanol vehicle correspond to the concentration needed to dissolve the highest concentration of cannabinoid. Data are the mean ± S.E.M. of 3 separate experiments with triplicate wells for each treatment. *p < 0.01, **p < 0.001. (ANOVA, WT versus k/o). Values for vehicle are not significantly different from no treatment.
O-1966 increases the percentage of Tregs in the MLR
Experiments were carried out to determine whether O-1966 treatment increased the presence of Tregs in the MLR cultures. To measure Tregs, MLR cultures were treated with 32 μM O-1966 or ethanol vehicle. Cells in the culture were harvested from wells at the start of the culture (T0), and 24 and 48 h into the assay. The cells were then stained for CD4, CD25 and Foxp3 and analyzed by flow cytometry. Figure 6 shows that cells harvested at 48h had a doubling in the percentage of CD25+Foxp3+ Tregs in the live CD4+ population, from 6.1% in untreated or vehicle treated cells to 12.7% in O-1966 treated cells.
Figure 6.
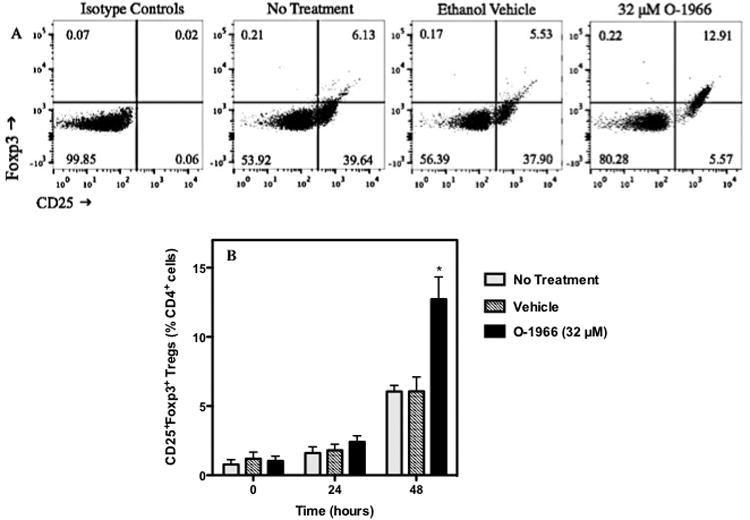
O-1966 increases the percentage of Tregs in the MLR. To determine if O-1966 induces Tregs in the MLR, cultures were analyzed by flow cytometry for live (LIVE/DEAD® dead cell stain negative) CD4+CD25+Foxp3+ Tregs. Panel A shows representative scatterplots of MLR cultures harvested at 48 hours and stained with isotype controls and left untreated or pretreated with 32 μM O-1966 or ethanol vehicle, stained with antibodies for CD25 and Foxp3. The cells were gated on live CD4+ cells and are expressed as a percentage of total live CD4+ cells. Panel B shows the average number of CD25+Foxp3+ Tregs as a percentage of total live CD4+ cells from 3 experiments from cultures left untreated (
 ) or pretreated with 32 μM O-1966 (
) or pretreated with 32 μM O-1966 (
 ) or ethanol vehicle (
) or ethanol vehicle (
 ), and harvested at time zero, or after 24 or 48 hours in culture. Data are the mean ± S.E.M. of 3 separate experiments. *p < 0.01. (ANOVA, O-1966 versus vehicle).
), and harvested at time zero, or after 24 or 48 hours in culture. Data are the mean ± S.E.M. of 3 separate experiments. *p < 0.01. (ANOVA, O-1966 versus vehicle).
Role of IL-10 in suppression of proliferation and increase in Tregs from O-1966 treatment
To assess the importance of increased IL-10 levels in the suppression of the MLR, cultures were treated with 2.5 or 5 μg/ml of anti-IL-10 antibody at T0 and 24 h into the assay to deplete the released IL-10. Figure 7A shows that the addition of 5 μg/ml anti-IL-10 antibody gave approximately 50% reversal of suppression induced by the 32 μM dose of O-1966. As IL-10 is reported to up-regulate Treg cells (Groux et al. 1997), the effect of IL-10 depletion on the increase of the percentage of Tregs in the MLR by O-1966 was also assessed. MLR cultures were treated with 2.5 or 5 μg/ml anti-IL-10 at T0 and 24 h, and cells harvested 48 h into the assay were stained and analyzed by flow cytometry for Tregs. Figure 7D shows that the addition of both 2.5 and 5 μg/ml anti-IL-10 antibody completely blocked the increase of CD4+Foxp3+ Tregs in the CD3+CD4+ population in cultures treated with 8 to 32 μM O-1966. The addition of 5 μg/ml of an isotype control antibody did not affect the suppression of proliferation or the increase of the percentage of Tregs at any dose of O-1966 tested. Together, these results indicate that the increased levels of IL-10 seen in O-1966 treated cultures is an important contributing mechanism for the suppression of proliferation and is essential for the increase of Tregs in the MLR. The cell subset that was the source of IL-10 was probed using flow cytometry. After surface staining for CD3, CD45A or CD11b to detect T-cells, B-cells and macrophages, intracellular staining for this cytokine was carried out. As shown in Figure 8, intracellular IL-10 was detected in T-cells and B-cells harvested at 48 hours from MLR cultures. There were too few macrophages in the harvested cells to determine if macrophages were a significant source of IL-10. Treatment with 32 μM of O-1966 increased the percentage of T-cells expressing IL-10 above that of vehicle treated cells, and doubled the percentage of B-cells expressing this cytokine.
Figure 7.
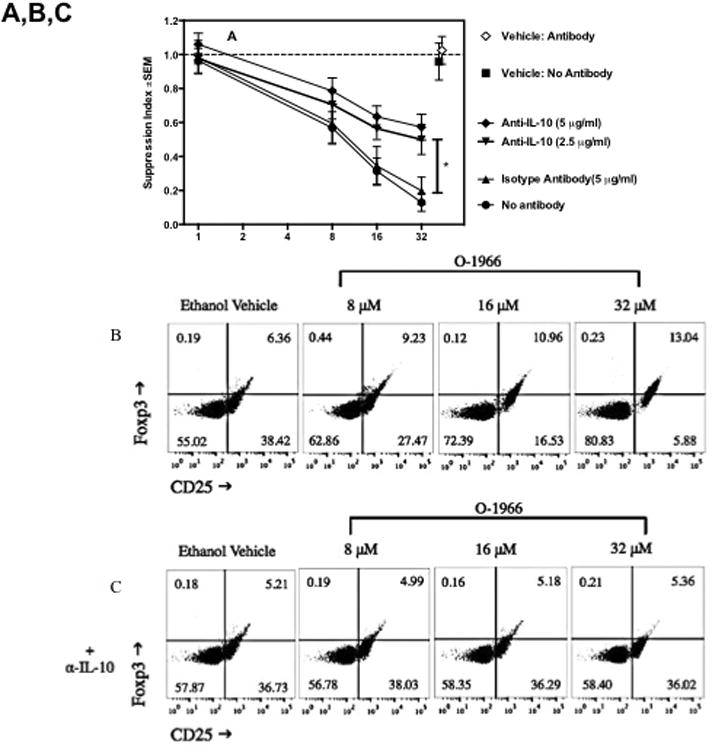
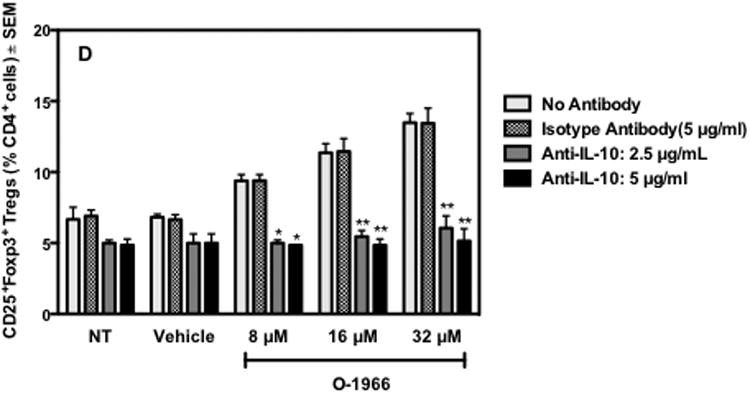
Anti-IL-10 antibody partially reverses suppression of proliferation and blocks Treg induction by O-1966. Responder splenocytes were pretreated with O-1966 for 3 hr and with a neutralizing anti-IL-10 antibody 1 hr before and 24 hr after the start of assay. Panel A- MLR response: Cultures were treated with O-1966 (●) or ethanol vehicle (■), O-1966 and 2.5 μg/ml anti-IL-10 antibody (▼), O-1966 and 5 μg/ml anti-IL-10 antibody (◆), O-1966 and 5 μg/ml isotype control antibody (▲), or O-1966 and antibody vehicle control (◇) and progressed to complete MLR to measure proliferation. Panels B and C: Representative scatterplots of MLR cultures pretreated with ethanol vehicle or 8, 16, or 32 μM O-1966. Panel C was treated with 5 μg/ml anti-IL-10 antibody. Cells were stained with antibodies for CD25 and Foxp3 and are gated on live CD4+ cells. Quadrants are expressed as a percentage of total live CD4+ cells, and were set using isotype control antibodies. Panel D: Average number of CD25+Foxp3+ Tregs as a percentage of total live CD4+ cells from 3 experiments from cultures pretreated as indicated along with no antibody (
 ), 5 μg/ml isotype control (
), 5 μg/ml isotype control (
 ), 2.5 μg/ml anti-IL-10 antibody (
), 2.5 μg/ml anti-IL-10 antibody (
 ), or 5 μg/ml anti-IL-10 antibody (
), or 5 μg/ml anti-IL-10 antibody (
 ), harvested 48 hr into culture and analyzed by flow cytometry for percentage of Tregs. MLR data are mean ± S.E.M. and flow cytometry data are mean percentage ± S.E.M. CD4+Foxp3+ Tregs of total live CD4+ cells (LIVE/DEAD® dead cell stain negative) in the culture. Both panels are the average of 3 separate experiments. *p < 0.01, **p < 0.001 (Two-way ANOVA, anti-IL- 10 versus isotype antibody).
), harvested 48 hr into culture and analyzed by flow cytometry for percentage of Tregs. MLR data are mean ± S.E.M. and flow cytometry data are mean percentage ± S.E.M. CD4+Foxp3+ Tregs of total live CD4+ cells (LIVE/DEAD® dead cell stain negative) in the culture. Both panels are the average of 3 separate experiments. *p < 0.01, **p < 0.001 (Two-way ANOVA, anti-IL- 10 versus isotype antibody).
Figure 8.
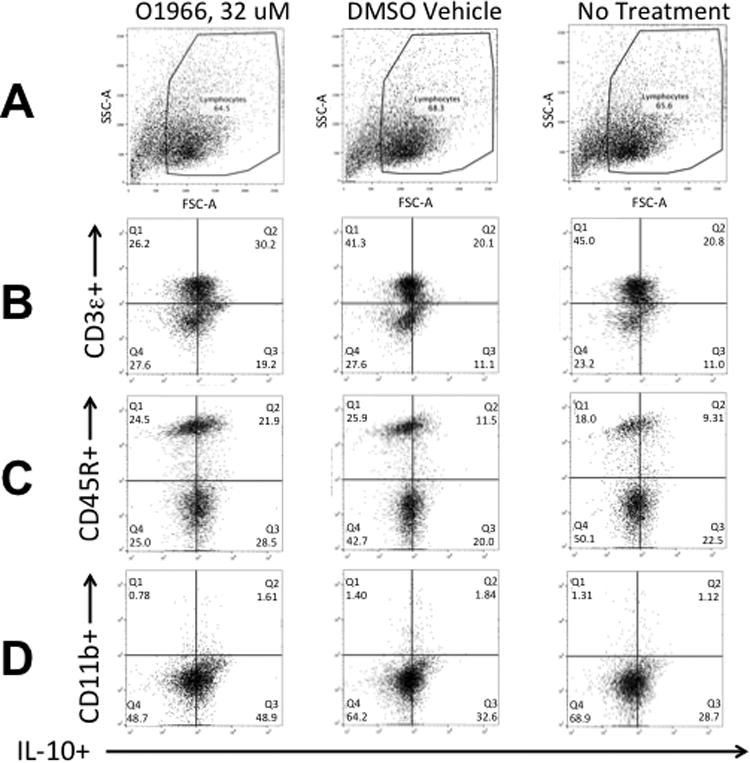
Detection of cell subsets expressing intracellular IL-10 in MLR cultures treated with O1966. Cells were first stained with eFluor 780 Fixable Viability Dye (eBioscience), then with antibodies to outer surface markers, and then stained intracellularly with APC-labeled anti-mouse IL-10. Row A: Plot of forward scatter (FSC-A) vs. side scatter (SSC-A), showing gating for lymphocytes of O-1966 treated cells, DMSO treated cells, and no treatment cells. Row B: Plot of eFluor 450 CD3e+ (T-cells) vs. IL-10+ cells. Row C: Plot of PE-Cy7 CD45R+(B220+) (B-cells) vs. IL-10+ cells. Row D: Plot of BV605 CD11b+ (macrophages) vs. IL-10+ cells. The quandrants indicate the percentage of live cells expressing both IL-10 and the indicated surface marker shown on the Y-axis. Quadrants were set using unstained cells.
Discussion
Recipients of allogeneic transplants must maintain lifelong immunosuppressive therapies to prevent rejection. Immunosuppressive agents most often target T-cells (Heeger 2003). Once alloreactive CD4+ T-cells are primed, they can differentiate into a pro-inflammatory type-1 phenotype, up-regulating CD40L and CD28 and releasing IL-2 and IFN-γ to activate cytotoxic CD8+ T-cells, which subsequently injure the graft (Lee et al. 1994) or directly mediate graft destruction (Grazia et al. 2010;Pietra et al. 2000). While most CD8+ T-cells require CD4+ T-cells for activation, some CD8+ T-cells can be primed independently by dendritic cells in a process called licensing (Albert et al. 1998;Buller et al. 1987;Matzinger and Bevan 1977). Even with continuous anti-rejection therapy, eventually many grafts are ultimately rejected. Therefore, new therapeutic approaches are necessary and could potentially be given alone or in combination with current standard treatments, in an effort to lower the required doses of tacrolimus and cyclosporine.
The studies presented in this paper provide pre-clinical evidence that a CB2-selective agonist is a potential treatment to prevent graft rejection, as it is able to decrease certain parameters of T-cell activation while increasing two potent anti-inflammatory parameters, IL-10 and Treg cells. Previously we have shown that CB2 expression increased 7-fold in the MLR and CB2-selective agonists inhibit the MLR with decreased IL-2 release (Robinson et al. 2013). Current studies explored additional mechanisms by which the CB2-selective agonist O-1966 suppresses T-cells. It was found that this CB2 agonist decreased levels of the active nuclear forms of NF-κB and NFAT in T-cells of wild-type mice, but not of CB2R k/o mice. In addition, O-1966 treatment significantly decreased mRNA expression for CD40L, a co-stimulatory molecule (van Kooten and Banchereau 2000), and Cyclin D3, a positive regulator for the transition from G1 to S phase during cell division (Ando et al. 1993). This compound also caused a dose-related decrease of CD4 expression on wild-type T-cells in the MLR, but not on T-cells lacking CB2. The high doses of CB2-selective agonists needed in vitro have been previously addressed (Robinson et al. 2013). It has been established that serum in cell cultures interferes with cannabinoid activity, so that there may be a poor correspondence between in vitro and in vivo doses (Klein et al. 1985;Nahas et al. 1977). Several groups have used CB2 selective agonists in vivo to reduce immune mediated effects in mouse models of spinal cord injury (Adhikary et al. 2011;Baty et al. 2008), stroke (Murikinati et al. 2010;Zhang et al. 2009b;Zhang et al. 2009c), inflammatory bowel disease (Cencioni et al. 2010), colitis (Storr et al. 2009), and sepsis (Tschöp et al. 2009). These agents have been found to have efficacy at doses in the range of 1 to 20 mg/kg. We have also found that O-1966 has efficacy in retarding graft rejection at 5 mg/kg (unpublished observations). Thus, the MLR would seem to predict in vivo efficacy, in spite of the rather high doses of cannabinoid required in vitro.
A number of similar observations have been made using THC, but not in the MLR (Börner et al. 2009;Lu et al. 2009;Ngaotepprutaram et al. 2012;Zhu et al. 2000). However, in previous investigations it was not determined whether the Δ9-THC was exerting its effects via the CB1 or the CB2 receptor. Δ9-THC binds to both CB1 and CB2 receptors, and T-cells and antigen-presenting cells express both receptors. In the present manuscript we extend these results and show that signaling through the CB2 receptor by a CB2-selective agonist is sufficient to achieve the same results. The CB2 agonists have a clear advantage over Δ9-THC in that they should not produce major psychoactive effects due to the low expression of the CB2 receptor on neurons (Galiegue et al. 1995). O-1966, administered intravenously in doses up to 30 mg/kg, did not produce any effects in behavioral analyses used to assess the psychoactive effects of cannabinoids (Zhang et al. 2007). Recently, however, there is some evidence that these compounds may have some subtler neuronal functions (Onaivi et al. 2008a;Onaivi et al. 2008b;Xi et al. 2011).
There is ample evidence in the literature to suggest preventing T-cell activation would provide sufficient protection against graft rejection (Heeger 2003), including the mechanisms induced by O-1966 reported here. Blocking the transcriptional activity of NFAT or NF-κB abrogates allograft rejection (Finn et al. 2001;Ueno et al. 2011). The inhibition of calcineurin by tacrolimus and cyclosporine, the standard anti-rejection drugs, blocks the translocation of the cytosolic component of NFAT to the nucleus (Ho et al. 1996). NFAT has been shown to be involved in the regulation of expression of CD40L on T-cells (van Kooten and Banchereau 2000). CD40L is expressed predominantly on activated CD4+ cells and induces an activating response when it binds to its receptor, CD40, which is expressed on a variety of cell types (Peng et al. 2001). Particularly important for transplant rejection, CD40-CD40L interactions have been shown to mediate the delivery of T-cell help by T helper CD4+ T-cells expressing CD40L to the CD40+ dendritic cells, which then activate CD8+ T-cells into killers (Ridge et al. 1998). CD40L expression is increased 4-fold in cases of acute rejection, and antibodies against CD40L have been found to be protective in mouse and monkey models of transplantation, including renal, pancreas, and skin allografts (Daoussis et al. 2004). Unfortunately, studies testing the effects of anti-CD40L blockade in humans were halted because of the development of thromboembolic phenomena (Kawai et al. 2000). Therefore, treatments that decrease the expression of CD40L may obtain the same immunosuppression without the adverse effects of the anti-CD40L antibodies. The 4-fold decrease in CD40L expression on T-cells in the MLR reported here may provide significant protection against graft rejection.
Additionally, O-1966 treatment reduced expression levels of CD4 on the cell surface. Through its interaction with class II major histocompatibility complex (MHC) on antigen presenting cells (APC), CD4 affects the activation and function of both T-cells and APC through the stabilization of TCR and APC interactions and subsequent MHC class II signaling (Al-Daccak et al. 2004;Miceli and Parnes 1991). Moreover, CD4 directly participates in T-cell signal transduction (Miceli and Parnes 1991) through the recruitment of the leukocyte-specific protein tyrosine kinase (Lck) (Li et al. 2004;Straus and Weiss 1992). Recently, treatment with Δ9-THC or the CB2-selective agonist, JWH-015, was shown to block the dephosphorylation of an inhibitory region of Lck that prevents autophosphorylation and subsequent initiation of TCR signaling in primary human T-cells and Jurkat T-cells activated with anti-CD3/CD28 antibodies (Börner et al. 2009). Whether CB2 agonists block T-cell activation in the MLR by reducing CD4 surface expression, thereby decreasing CD4 and MHC class II interactions or by directly dampening TCR signaling will be investigated further.
In addition to blocking T-cell activation, O-1966 also induced a potent suppressive response in the MLR, through enhanced IL-10 release, which has been shown to inhibit the MLR (Bejarano et al. 1992), and also by increasing the percentage of Tregs. It has previously been shown that nonselective CB1/CB2 cannabinoids increased IL-10 and Tregs levels (Arevalo-Martin et al. 2012;Hegde et al. 2008;Klein et al. 2000;Lu et al. 2009;McKallip et al. 2005;Smith et al. 2000). The current results extend the published observations with nonselective cannabinoids by using CB2-selective compounds. The increased number of Tregs in O-1966 treated MLR cultures was completely blocked by the addition of neutralizing anti-IL-10 antibodies. This is consistent with a study by Groux et al. that showed IL-10 was able to induce Tregs, which then overproduced IL-10 and suppressed the proliferation of CD4+ T-cells in response to antigen (Groux et al. 1997). The observation linking the action of a CB2 agonist with the interplay of IL-10 and Tregs is novel. It is worth noting that we have previously shown that IL-2 is depressed in MLR cultures treated with CB2 agonists (Robinson et al. 2013). Others have shown a similar CB2-induced decrease in IL-2 in an assay measuring activation of antigen-specific T-cells (Maresz et al. 2007). There is a conundrum in that IL-2 is reportedly needed for induction of Tregs (Davidson et al. 2007;Tone et al. 2008). While there is no ready explanation for this seeming contradiction, it may be that the decrease in IL-2 is gradual over the first 24 hr of the MLR assay, so that there is initially sufficient cytokine to allow Treg induction.
The results presented here also demonstrate that both T-cells and B-cells harvested from the MLR contain significant amounts of intracellular IL-10. We were unable to determine if the T-cell population that was IL-10 positive was the Treg subset, as there was interference between the intracellular stains for Foxp3 and IL-10. The unexpected observation of IL-10 secreting B-cells suggests that the CB2 agonist may have the capacity to induce B10 cells with immunosuppressive capacity (Candando et al. 2014;Rahim et al. 2005;Rosser et al. 2014). This finding is of interest because it has been reported that B-cells are needed for emergence of Treg cells (Sun et al. 2008;Tadmor et al. 2011). Further investigation will be needed to purify and test the functional and phenotypic characteristics of this cell population generated in the MLR.
The induction of IL-10 and Tregs by a CB2-selective agonist make this class of compounds particularly promising, because the generation of Tregs could increase the likelihood of graft survival while decreasing the need for long-term immunosuppressive therapies. IL-10 has been shown to inhibit antigen presentation, antigen-specific T-cell proliferation, and decrease Th1 cytokine production (Fiorentino et al. 1991;Mitra et al. 1995). Blocking the activity of IL-10 in vivo in transplantation models diminished the survival of grafts (Kingsley et al. 2002;McMurchy et al. 2011) and was essential for the induction and maintenance of Tregs (Wood et al. 2012). Indeed, following transplantation in both humans and animals, the presence of Tregs in the spleen, draining lymph notes, and at the site of the allograft, closely correlated with graft acceptance (Wood et al. 2012).
Together, the present data show that CB2 activation can suppress T-cells in the MLR by blocking T-cell function while favoring the induction of Tregs. We have also shown that in vivo administration of O-1966 at 5mg/kg every other day for 14 days retards skin graft rejection in mice, depresses the response of spleen cells from treated mice placed ex vivo in an MLR assay, and induces Tregs in spleens (manuscript in preparation). Overall, the results presented in this paper support CB2-selective agonists as a new class of compounds to prevent graft rejection and graft-versus-host disease in patients, and potentially as a new class of immunosuppressive and anti-inflammatory drugs for a variety of other immune mediated conditions.
Acknowledgments
We would like to thank Dr. Stefania Gallucci, Department of Microbiology and Immunology, Temple University School of Medicine for her helpful advice. This work was supported by The Pennsylvania Department of Health as part of the Tobacco Settlement Funds to Temple University, and NIDA grants DA13429, DA06650, and T32-DA07237.
Footnotes
Authorship: R.H.R designed and performed the experiments, interpreted data, and wrote the manuscript. J.J.M. contributed to the experimental design and data interpretation, and helped with the manuscript. X.F. helped with the cell sorting studies using the flow cytometer. D.Y. performed statistical analyses of data. M.W.A. provided advice on the proposed studies. T.K.E. designed the experiments, interpreted the data, and provided guidance in writing the manuscript.
Conflict of Interest Disclosure: The authors declare no conflict of interest.
References
- Adhikary S, Li H, Heller J, Skarica M, Zhang M, Ganea D, Tuma RF. Modulation of inflammatory responses by a cannabinoid-2-selective agonist after spinal cord injury. J Neurotrauma. 2011;28:2417–2427. doi: 10.1089/neu.2011.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Al-Daccak R, Mooney N, Charron D. MHC class II signaling in antigen-presenting cells. Curr Opinion Immunol. 2004;16:108–113. doi: 10.1016/j.coi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Ando K, Ajchenbaum-Cymbalista F, Griffin JD. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci U S A. 1993;90:9571–9575. doi: 10.1073/pnas.90.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous O. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN / SRTR 2011 Annual Data Report 2012 [Google Scholar]
- Arevalo-Martin A, Molina-Holgado E, Guaza C. A CB1/CB2 receptor agonist, WIN 55,212-2, exerts its therapeutic effect in a viral autoimmune model of multiple sclerosis by restoring self-tolerance to myelin. Neuropharmacology. 2012;63:385–393. doi: 10.1016/j.neuropharm.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res. 2011;51:26–38. doi: 10.1007/s12026-011-8210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty DE, Zhang M, Li H, Erb CJ, Adler MW, Ganea D, Loftus CM, Jallo JI, Tuma RF. Cannabinoid CB2 receptor activation attenuates motor and autonomic function deficits in a mouse model of spinal cord injury. Clin Neurosurg. 2008;55:172–177. [PubMed] [Google Scholar]
- Bejarano MT, de Waal Malefyt R, Abrams JS, Bigler M, Bacchetta R, de Vries JE, Roncarolo MG. Interleukin 10 inhibits allogeneic proliferative and cytotoxic T cell responses generated in primary mixed lymphocyte cultures. Int Immunol. 1992;4:1389–1397. doi: 10.1093/intimm/4.12.1389. [DOI] [PubMed] [Google Scholar]
- Börner C, Smida M, Hollt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009;284:35450–35460. doi: 10.1074/jbc.M109.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Buller RML, Holmes KL, Hügin A, Frederickson TN, Morse HC., III Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencioni MT, Chiurchiu V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE [Electronic Resource] 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: Evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharm. 2007;7:360–371. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Friedman H, Klein TW. Cannabinoid receptor proteins are increased in jurkat, human T-cell line after mitogen activation. J Pharmacol Exp Ther. 1996;276:776–783. [PubMed] [Google Scholar]
- Daoussis D, Andonopoulos AP, Liossis SN. Targeting CD40L: A promising therapeutic approach. Clinical & Diagnostic Laboratory Immunology. 2004;11:635–641. doi: 10.1128/CDLI.11.4.635-641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TS, Dipaolo RJ, Anderson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. Journal of Immunology. 2001;167:5994–6001. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. Journal of Immunology. 1991;146:3444–3451. [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Grazia TJ, Plenter RJ, Weber SM, Lepper HM, Victorino F, Zamora MR, Pietra BA, Gill RG. Acute cardiac allograft rejection by directly cytotoxic CD4 T cells: Parallel requirements for fas and perforin. Transplantation. 2010;89:33–39. doi: 10.1097/TP.0b013e3181be6bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Heeger PS. T-cell allorecognition and transplant rejection: A summary and update. American Journal of Transplantation. 2003;3:525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clinical Immunology & Immunopathology. 1996;80:S40–5. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Jose M. The CARI guidelines. calcineurin inhibitors in renal transplantation: Adverse effects. Nephrology. 2007;12:S66–S74. doi: 10.1111/j.1440-1797.2007.00731.x. [DOI] [PubMed] [Google Scholar]
- Kawai T, D, Colvin RB, Sachs DH, Cosmi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. Journal of Immunology. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Widen R, Friedman H. The effect of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol on T-lymphocyte and B-lymphocyte mitogen responses. J Immunopharmac. 1985;7:451–466. doi: 10.3109/08923978509026487. [DOI] [PubMed] [Google Scholar]
- Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H., Jr Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994;179:865–872. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- Li X, Kaminsky NE, Fischer LJ. Examination of the immunosuppressive effect of Δ9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes. Int Immunopharm. 2001;1:699–712. doi: 10.1016/s1567-5769(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Lu H, Kaplan BL, Ngaotepprutaram T, Kaminski NE. Suppression of T cell costimulator ICOS by Delta9-tetrahydrocannabinol. J Leukoc Biol. 2009;85:322–329. doi: 10.1189/jlb.0608390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nature Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P, Bevan MJ. Induction of H-2-restricted cytotoxic T cells: In vivo induction has the appearance of being unrestricted. Cell Immunol. 1977;33:92–100. doi: 10.1016/0008-8749(77)90137-x. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS. Δ9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. Journal of Immunology. 2005;174:3281–3289. doi: 10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- McMurchy AN, Bushell A, Levings MK, Wood KJ. Moving to tolerance: Clinical application of T regulatory cells. Semin Immunol. 2011;23:304–313. doi: 10.1016/j.smim.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli MC, Parnes JR. The roles of CD4 and CD8 in T cell activation. Semin Immunol. 1991;3:133–141. [PubMed] [Google Scholar]
- Mitra RS, Judge TA, Nestle FO, Turka LA, Nickoloff BJ. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. Journal of Immunology. 1995;154:2668–2677. [PubMed] [Google Scholar]
- Munro S, Thomas KK, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murikinati S, Jütter E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. Faseb j. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- Nagarkatti M, Rieder SA, Hegde VL, Kanada S, Nagarkatti P. Do cannabinoids have a therapeutic role in transplantation? Trends Pharmacol Sci. 2010;31:345–350. doi: 10.1016/j.tips.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas GG, Morishima A, Desoize B. Effects of cannabinoids on macromolecular synthesis and replication of cultured lymphocytes. Fed Proc. 1977;36:1748–1752. [PubMed] [Google Scholar]
- Ngaotepprutaram T, Kaplan BL, Crawford RB, Kaminski NE. Differential modulation by Δ9-tetrahydrocannabinol (Δ9-THC) of CD40 ligand (CD40L) expression in activated mouse splenic CD4+ T cells. J Neuroimm Pharmacol. 2012;7:969–980. doi: 10.1007/s11481-012-9390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Geller EB, Eppihimer MJ, Eisenstein TK, Adler MW, Tuma RF. Win 55212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis mode. Mult Scler. 2004;10:158–164. doi: 10.1191/1352458504ms1009oa. [DOI] [PubMed] [Google Scholar]
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci. 2008a;1139:426–433. doi: 10.1196/annals.1432.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Liu QR, Chirwa SS, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci. 2008b;1139:434–449. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106:1003–1010. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Jr, Adler MW, Eisenstein TK. Splenic macrophages and B-cells mediate immunosuppression following abrupt withdrawal from morphine. J Leukoc Biol. 2005;78:1185–1191. doi: 10.1189/jlb.0304123. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Robinson RH, Meisser JJ, Breslow-Deckman JM, Gaughan J, Adler MW, Eisenstein TK. Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. J Neuroimmuno Pharmacol. 2013;8:1239–1250. doi: 10.1007/s11481-013-9485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser EC, Blair PA, Mauri C. Cellular targets of regulatory B cell-mediated suppression. Mol Immunol. 2014;62:296–304. doi: 10.1016/j.molimm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Servettaz A, Kavian N, Nicco C, Deveaux V, Chereau C, Wang A, Zimmer A, Lotersztajn S, Weill B, Batteux F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am J Pathol. 2010;177:187–196. doi: 10.2353/ajpath.2010.090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr MA, Keenan CM, Emmerdinger D, Zhang H, Yuce B, Sibaev A, Massa F, Buckley NE, Lutz B, Goke B, Brand S, Patel KD, Sharkey KA. Targeting endocannabinoid degradation protects against experimental colitis in mice: Involvement of CB1 and CB2 receptors. J Mol Med. 2008;86:925–936. doi: 10.1007/s00109-008-0359-6. [DOI] [PubMed] [Google Scholar]
- Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Sun JB, Flach CF, Czerkinsky C, Holmgren J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: Powerful induction by antigen coupled to cholera toxin B subunit. J Immunol. 2008;181:8278–8287. doi: 10.4049/jimmunol.181.12.8278. [DOI] [PubMed] [Google Scholar]
- Tadmor T, Zhang Y, Cho HM, Podack ER, Rosenblatt JD. The absence of B lymphocytes reduces the number and function of T-regulatory cells and inhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother. 2011;60:609–319. doi: 10.1007/s00262-011-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Tschöp J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschöp MH, Caldwell CC. The cannabinoid receptor 2 is critical for the host response to sepsis. Journal of Immunology. 2009;183:499–505. doi: 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Yamada A, Ito T, Yeung MY, Gorbatov R, Shimizu T, Abdi R, Sayegh MH, Auchincloss H, Jr, Najafian N. Role of nuclear factor of activated T cell (NFAT) transcription factors in skin and vascularized cardiac allograft rejection. Transplantation. 2011;92:e26–7. doi: 10.1097/TP.0b013e318228061c. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. Journal of Pharmacology & Experimental Therapeutics. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nature Reviews Immunology. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: A regulatory system in states of inflammation. Brit J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cheng CL, Chen M, Manivannan A, Cabay L, Pertwee RG, Coutts A, Forrester JV. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J Leukoc Biol. 2007;82:532–541. doi: 10.1189/jlb.0307159. [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB2 receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009a;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009b;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, Tuma RF. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimm Pharmacol. 2009c;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, Dubinett SM. Δ9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. 2000;165:373–380. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]


