Abstract
We have designed and produced a prototypic malaria vaccine based on a highly versatile self-assembling polypeptide nanoparticle (SAPN) platform that can repetitively display antigenic epitopes. We used this platform to display a tandem repeat of the B cell immunodominant repeat epitope (DPPPPNPN)2D of the malaria parasite Plasmodium berghei circumsporozoite protein (CSP). Administered in saline, without the need for a heterologous adjuvant, the SAPN construct P4c-Mal conferred a long lived protective immune response to mice with a broad range of genetically distinct immune backgrounds including the H-2b, H-2d and H-2k alleles. Immunized mice produced a CD4+ T cell dependent, high titer, long lasting, high avidity antibody response against the B cell epitope. Mice were protected against an initial challenge of parasites given up to 6 months after the last immunization or for up to 15 months against a second challenge after an initial challenge of parasites had successfully been cleared. Furthermore, we demonstrate that the SAPN platform not only functions to deliver an ordered repetitive array of B cell peptide epitopes but operates as a classical immunological carrier to provide cognate help to the P4c-Mal specific B cells.
Introduction
Malaria, an infectious disease that is manifest in hundreds of millions of people each year, has no effective vaccine on the market. Many potential vaccines have been investigated, using various vaccine platforms including recombinant proteins, nucleic acid, multiple antigenic peptides, virus like particles (VLP) and attenuated micro-organisms, both bacterial and viral (1, 2). Recombinant protein vaccines and DNA vaccines, unlike live vector vaccines, often need the co-administration of an immuno-stimulator or adjuvant to sufficiently activate the immune effector mechanisms. In human trials the nucleic acid and live vector vaccines have not been efficacious on their own nor when given in a prime-boost regimen (3–6). Given the sparsity of human-use approved adjuvants the broad development of protein based vaccines for malaria has not significantly advanced (7). Only the Plasmodium falciparum vaccine RTS,S has proven effective when combined with one of a series of adjuvants (AS01B, AS02A, AS02D) developed by the maker of RTS,S, GlaxoSmithKline Biologicals (GSK) (8). Clinical and field trials of other malaria recombinant proteins co-administered with the GSK adjuvants have not demonstrated the same protective efficacy (1). While these results may be more a choice of antigen than adjuvant it has clearly been shown that choice of adjuvant does affect the level of the immune response (9).
Recently, a few nanoparticle based vaccines have shown to be effective in the induction of immune responses in animal models without the need for an adjuvant (10–15). But most of these particles are based on polymers that encapsulate antigen or create a solid support to which protein antigens are chemically coupled. The actual physical array of epitopes on the antigen presenting platform appears to influence the immune response and this cannot be well controlled in solid support platforms. Well ordered repetitive displays on VLPs (16) are amongst the strongest immunogens that are known (17, 18). A case for ordered antigen display was made more than 20 years ago, when trying to produce high-titer/high-affinity monospecific antibodies against the different capsid proteins of bacteriophage T4 (19–23). More recently, Liu and Chen (24) have shown that antibody affinity constants are as much as 2 logs higher when antigens are displayed in optimal density arrays. It is possible that antigens presented in an optimum configuration could induce a powerful immune response in the absence of extraneous adjuvant. For a recent review see Jennings, et al. (25) .
Raman et al., have developed mechanically and chemically stable nanoparticles using the intrinsic property of peptides and proteins to self-assemble to specific oligomers which then leads to the formation of particles of well defined size and shape (26–28). We use these self-assembling polypeptide nanoparticles (SAPN) as the basis for delivery of antigenic peptides of Plasmodium berghei as a model system for the development of a new vaccine platform.
The structural foundation of the SAPN is the molecular design of a monomeric linear polypeptide (LP) of about 100 amino acids (Fig. 1 A). Each LP contains both a pentameric and a trimeric coiled-coil oligomerization domain, joined by a two-glycine residue flexible linker. The pentameric coiled-coiled is derived from COMP (29) while the trimeric domain is a de-novo designed coiled-coil domain (30). Upon refolding from denaturing conditions the peptides co-assemble into SAPN (Fig. 1 B). The assembly is driven by the multiple coiled-coils formed by the two oligomerization domains. Ideally such SAPN will have icosahedral symmetry and thus resemble VLPs in their architecture (26). Since both the N- and C-terminal ends of the peptide are exposed on the surface, the SAPN are nicely suited for the presentation of B cell epitopes in a repetitive antigen display.
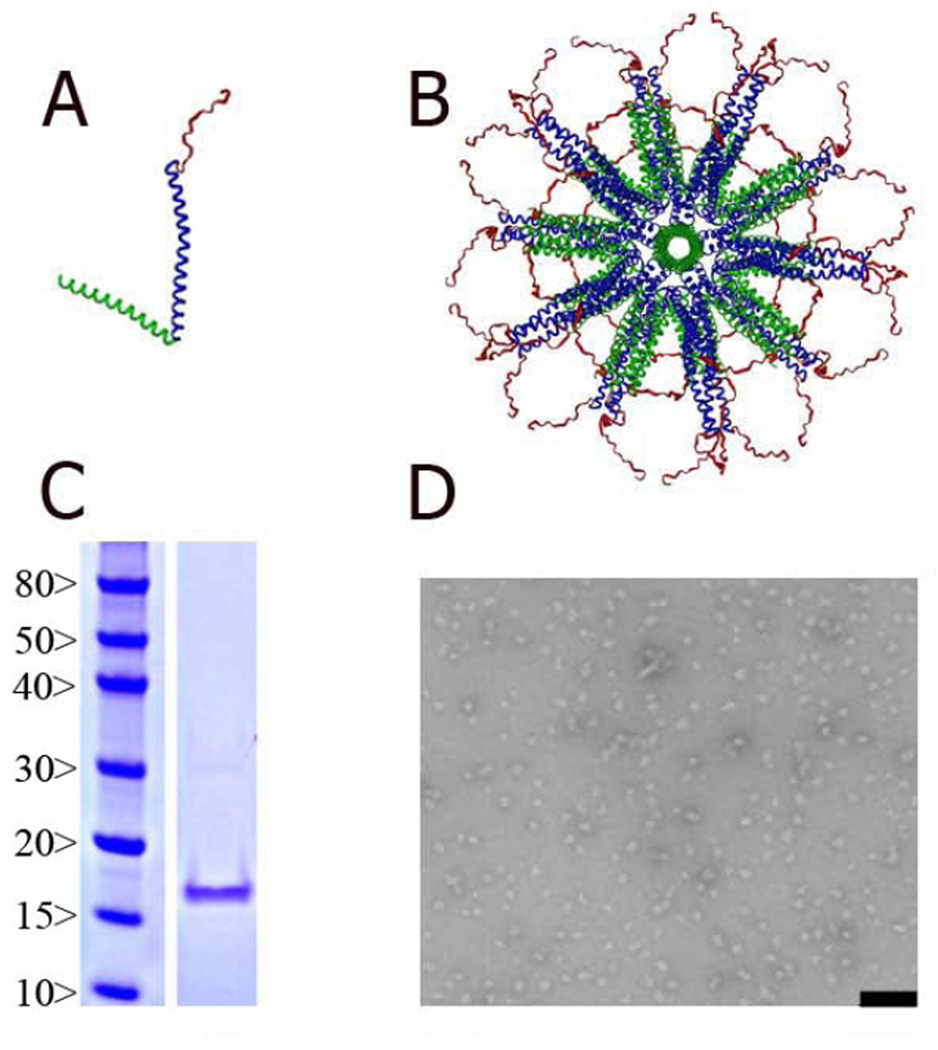
Figure 1. Assembly of P4c-Mal SAPN.
(A) Monomeric building block of the P4c-Mal construct composed of the five-fold coiled-coil domain from COMP (green), the de-novo designed trimeric coiled-coil domain (blue) and the B cell epitope (red) in extended conformation. (B) Diagram of an assembled SAPN with icosahedral symmetry; the view is down the five-fold symmetry axis of the icosahedron. The B cell epitope is presented at the surface of the SAPN. The figure was prepared using the program DINO (http://www.dino3d.org). (C) Coomassie Blue stained SDS-PAGE gel analysis of P4c-Mal linear polypeptide. On left are relative molecular weight markers (kDa). (D). Transmission EM of P4c-Mal SAPN particle preparation. Bar is 200 nm.
To test the vaccine efficacy of the platform to display peptides of malaria parasites we selected the tandem repeat (DPPPPNPN)2D of the B-cell immunodominant repeat epitope of the malaria parasite Plasmodium berghei circumsporozoite protein (PbCSP) (31) to be displayed on the SAPN surface. It has previously been shown that this tandem repeat when displayed as a Multiple Antigenic Peptide (MAP) and administered in Complete Freund’s Adjuvant (CFA) can confer protection in mice against a lethal challenge of live P. berghei sporozoites (32). Here we show that malaria epitope displaying SAPN, P4c-Mal, administered in phosphate buffered saline (PBS) can confer a long lived protective immune response to mice without the need of a heterologous adjuvant and that the antibodies that are produced in response to the SAPN are of higher avidity than antibodies produced against a near full-length recombinant PbCSP (R-PbCSP) delivered in the adjuvant Montanide ISA-720. Mice that received P4c-Mal were protected against an initial challenge of parasites even 6 months after the last immunization and for up to 15 months against a second challenge after an initial challenge of parasites had successfully been cleared. Furthermore, we show that immunization with P4c-Mal SAPN generate CD4+ T cell dependent antibodies which account for this protection.
Materials and Methods
Strains of mice
Six-to-eight-week old female C57BL/6 (H-2b) and Balb/c (H-2d) mice were used for most protection experiments. In addition, CD8 knockout strain B6/129S2-Cd8atm1Mak/J, the MHC Class II knockout strain B6.129S2-H2dlAb1-Ea/J, the CD4 knockout strain B6.129S2-Cd4tm1Mak/J, or the athymic nude strain B6.Cg-Foxn1nu/J of mice were used to dissect the immune response to the SAPN. Two strains of TLR4 deficient (C57BL/10ScNJ and C3H/He3) mice were used to test for the presence of bound endotoxin. All mice were obtained from Jackson Laboratory (Bar Harbor, ME).
Gene cloning
The sequence encoding the SAPN peptide was ligated into the BamHI/EcoRI restriction sites of mpPEP-T expression vector which corresponds to pPEP-T lacking the laminin oligomerization domain (33). Oligonucleotides encoding the P. berghei CSP B-cell epitope (DPPPPNPN)2D (PbCSP) were then cloned into the XmaI/EcoRI restriction sites of the SAPN expression construct to yield the final amino acid sequence
MGHHHHHHGDWKWDGGLVPRGSDEMLRELQETNAALQDVRELLRQQVRQITFLRCLLMGGRLLCRLEELERRLEELERRLEELERAINTVDLELAALRRRLEELARGGSGDPPPPNPNDPPPPNPND
A construct, P4c-RD, was made that contained a random peptide sequence, IPSTAFTDIAWVRLPNHY at the C-terminal end in place of the PbCSP epitope.
Protein purification, refolding and analysis of the nanoparticle polypeptide
Recombinant protein was expressed, purified and refolded essentially as previously described (26). Briefly, the LP was expressed as a recombinant protein in E. coli and purified by metal affinity chromatography followed by two polishing ion-exchange chromatography steps. The first, Q-sepharose, captured the endotoxin, and the second, SP-sepharose, concentrated the protein. Throughout the purification protocol the LP was kept in a denatured state in 8 M urea then the LP was slowly folded by stepwise dialysis to remove the urea. The shape and size of the P4c-Mal nanoparticles were studied by using Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS) (27). TEM analysis was preformed as previously described (29) and photographed on a Zeiss EM910 transmission electron microscope (Carl Zeiss, Oberkochen, Germany). The hydrodynamic diameter of the SAPN was measured using a Zetasizer Nano S (Malvern Instruments Ltd, UK) dynamic light scattering instrument in PBS at 25°C and pH 7.5.
SAPN containing different ratios of C-terminal peptides
To determine the percent of PbCSP peptide in a SAPN that was needed to still be protective we prepared a series of SAPN containing different ratios of LP containing either the PbCSP repeat peptide epitope or the random peptide sequence RD. After each LP-PbCSP or LP-RD was expressed and purified they were mixed in the ratio of (LP-PbCSP : LP-RD) 0:100, 10:90: 25:75, 50:50, 75:25, 100:0. Urea was then removed by dialysis and final SAPN analyzed as described (27).
Endotoxin analysis of the final product
Endotoxin levels in the final product were determined using the Pyrochrome Limulus Amebocyte Lysate (LAL) Test (Associates of Cape Cod, Inc., East Falmouth, MA, USA) following the manufacturer instructions and as reported previously (34).
Immunization of mice
All animal study protocols were reviewed and approved by the Walter Reed Army Institute of Research (WRAIR) IACUC and conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals. Mice were randomly divided into groups of 5 or 10 and immunized ip three times at 14-day intervals. Where indicated a positive control group were immunized with irradiated P. berghei sporozoites (ira-spz) according to (35).
Passive transfer of serum and splenocytes
Ten Balb/c or C57BL/6 mice were immunized three times at 14 days intervals with 10µg of P4c-Mal per mouse per injection (11) in the presence or absence of Montanide ISA-720. One week after the third immunization animals were sacrificed and bled by cardiac puncture for processing of serum. Spleens were surgically removed for harvesting of splenocytes. Serum or splenocytes from the ten animals were pooled. Groups of ten naïve Balb/c or C57BL/6 mice were transfused with 100 µL of pooled serum (non-diluted), or 1×107 cells or both. The infectivity control group received neither cells nor serum. Animals were challenged with live sporozoites 16 h after the transfer.
Challenge of mice with live P. berghei sporozoites
P. berghei sporozoites (ANKA strain), maintained by cyclical transmission in mice and Anopheles stephensi, were dissected from mosquitoes 21–23 days after their infectious blood meal and used within 6 h. Fourteen days after the final immunization or at other specific times on long term memory experiments mice were challenged with a lethal dose of live P. berghei sporozoites by intravenous inoculation. C57BL/6, MHC KO and nude mice were injected with 1000 sporozoites while Balb/c mice were injected with 4000 sporozoites per mouse.
Determination of infection following parasite challenge
Parasitemia was determined by examining Giemsa-stained thin smears prepared with blood from each mouse from days 6–15 after challenge. Infected animals were euthanized and an animal was considered protected if no parasites were detected by 20 days post challenge.
Cellular response (ELISpot, ICS)
Splenocytes from P4c-Mal immunized or naïve animals were resuspended at a concentration of 4×106/mL in complete media. One hundred microliters of cell suspension was distributed into 96-well ELISpot plates for IL-2 release determination and stimulated with P4c-Mal protein (5 µg/well) or Con-A (5 µg/mL) or left in media alone in the presence of 4µg/mL of anti-CD28 and anti-CD49 antibodies, with or without anti-CD4+ blocking antibody. The number of cells secreting IL-2 was determined.
Antibody titer in response to vaccination
Antibody responses to the PbCSP peptide (DPPPPNPN)2D were measured by the enzyme-linked immunosorbent assay (ELISA) as previously described (36). Briefly, 96-well microplates (Dynax, Chantilly, VA) were coated with 100 ng of the synthetic PbCSP peptide (GenScript Corporation, Piscataway, NJ) per well, overnight at 4°C. The antigen was blocked for 1 h at 37°C with phosphate-buffered saline (PBS, pH 7.4) containing 0.05% Tween20 and 1% casein (Sigma, St Louis, MO). Plates were washed three times with PBS-T (PBS, 0.05% Tween20) and incubated for 2 h at RT with individual mouse sera in triplicate wells per serum sample. Plates were washed again and incubated for 1 h at RT with 1:5000 diluted (PBS) secondary anti-mouse immunoglobin(total IgG) or the different immunoglobin subclasses (IgG1, IgG2a, IgG2b and IgG2c) antibodies labeled with horseradish peroxidase (Southern- Biotechnology Associates, Birmingham, AL). Plates were washed and developed by adding 2, 2’-azino-di(3-ethylbenthiazoline sulfonate)-peroxidase (ABTS) substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Color reaction was measured in a Dynatech MR5000 Microplate reader by determining optical density at 405 nm (OD(405nm)). The results were calculated as mean OD(405nm) readings of duplicate assays ± standard deviations.
Antibody avidity index (AI) determination
An initial concentration of sodium isothiocynate (NaSCN) that eluted 50% of the total bound IgG was determined by the method of Pullen et al. (37). Then two side-by-side four-fold serial dilutions for a second ELISA were done (in triplicate). After three washes with PBS-T and one wash with PBS, 100 µL of the concentration of NaSCN (1.5 M dissolved PBS) determined in the initial avidity determination was added to one set of the serial dilutions on the plate and plain PBS was added to the other dilution series. The same elution concentration of NaSCN determined for total IgG was used for elution of IgG subclasses. After 15 min incubation, all wells were washed and developed as described above. The AI was calculated based on the ratio of the areas derived from under the curves obtained by the plot of optical density (OD(405nm)) and log of the sera dilution in the ELISA experiment with and without NaSCN treatment as described (38).
Statistics
Student’s t-tests were performed using Excel Software. The association of antibody titer and protection was determined using Logistic Regression Analysis in the program SAS.
Results
Preparation and characterization of P4c-Mal SAPN
The LP was expressed, purified and folded to form nanoparticles. TEM showed the particles to be about 25 nm in diameter (Fig.1 D) and DLS analysis indicated a uniform distribution of unclumped, single nanoparticles (data not shown). The endotoxin levels were below the level of detection of the LAL chromogenic assay. While the calculated MW of the LP was 14.7 kDa the protein ran at a relative MW of about 17 kDa on SDS-PAGE (Fig. 1C).
The P4c-Mal SAPN induce high antibody titer leading to protection
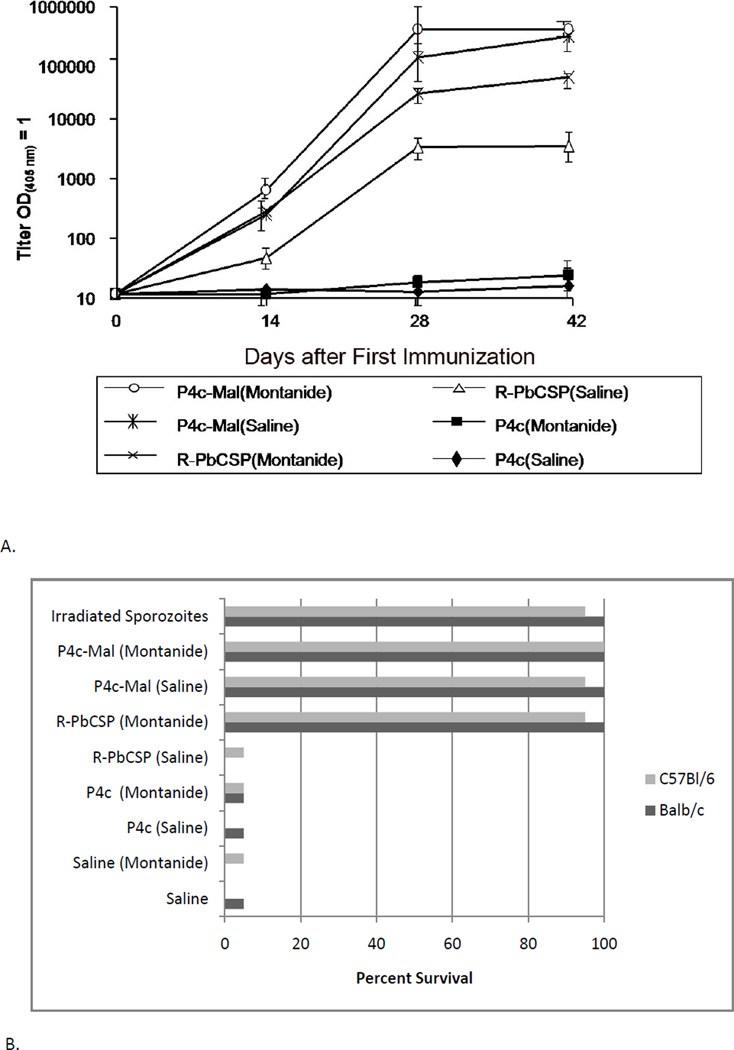
Groups (n=10) of C57BL/6 and Balb/c mice were immunized with 10 µg P4c-Mal or P4c (a SAPN without the PbCSP peptide epitope) in either PBS or Montanide ISA-720. Other groups of mice were immunized with 10 µg near full length recombinant PbCSP protein (39) in PBS (R-PbCSP) or in Montanide ISA-720 (R-PbCSP/M). Negative controls were age and sex-matched C57BL/6 and Balb/c mice which had received either saline only or saline and Montanide ISA-720. One day before the first immunization, and 14 days post each immunization mice were bled for determination of antibody titers. As shown in Fig. 2 A, antibody titers reached maximum levels after two immunizations when the P4c-Mal was mixed with Montanide ISA-720 while titers of the non-adjuvanted and adjuvanted P4c-Mal immunized mice achieved parity after the third immunization.
Figure 2. A. Titers of anti-PbCSP peptide antibody.
Balb/c mice were immunized at 0, 14 and 28 days with P4c in saline, P4c in Montanide ISA 720, R-PbCSP in saline, R-PbCSP in Montanide ISA 720, P4c-Mal in saline or P4c-Mal in Montanide ISA 720. Titers were determined on sera taken on days 0, 14, 28 or 42 as the dilution required to achieve and OD(405nm) =1. Values are from a pool of sera from a representative cohort of 10 mice per group. Similar titers were obtained with C57BL/6 mice. (B). Survival of mice whose titers are depicted in Figure 2A 15 days following challenge with live sporozoites. Values are the combined results of two separate experiments (two cohorts of 10 mice) per group. Additional mice received irradiated sporozoites, saline or Montanide ISA 720 in saline as described. No mouse that developed parasitemia survived beyond day 15 and no mouse developed parasitemia after day 15.
Fourteen days after the last immunization animals were challenged intravenously (iv) with live sporozoites. More than 95% of mice immunized with P4c-Mal, both with and without Montanide ISA-720, and also recombinant PbCSP in Montanide ISA-720 showed complete protection against challenge with viable sporozoites (Fig 2 B). This level of protection is equivalent to what is only achieved with the whole irradiated sporozoite immunization regime. In contrast, only as few as 5% of animals administered saline, saline and Montanide ISA-720 or R-PbCSP in saline survived until 11 days post challenge. These results show that that immunization with P4c-Mal had a significant ability to induce a protective immune response, in the presence as well as in the absence of adjuvant. The ability to protect against challenge was directly correlated with antibody titer (P<0.001).
To determine if the antibodies alone were responsible for protection, serum and spleen cells were collected from the protected animals and transferred into strain matched naïve animals. C57BL/6 or Balb/c mice were divided into 4 groups of 5 animals each, and each mouse received 100 µL of serum from immunized animals by intra-peritoneal (ip) injection, or 50 ×106 total splenocytes by intravenous (iv) injection from immunized donors, or a mixture of both splenocytes and serum. A group of animals for each strain was injected with PBS and served as infectivity controls. Animals from both strains that received serum or splenocytes plus serum showed 100% resistance to lethal challenge while all animals that received cells alone or those injected with PBS became parasitemic by day 10 (Table II).
Table II.
Protection is transferred by serum
| Group | Transfer | Number of Balb/c mice Developing Parasitemia by Day 10 |
Number of C57BL/6 mice Developing Parasitemia by Day 10 |
|---|---|---|---|
| 1 | 100 µl undiluted serum | 0/5 | 0/5 |
| 2 | 100 µl undiluted serum + 1×107 splenocytes |
0/5 | 0/5 |
| 3 | 1×107 splenocytes | 5/5 | 5/5 |
| 4 | none | 5/5 | 5/5 |
SAPN need to contain at least 50% of LP carrying a specific epitope
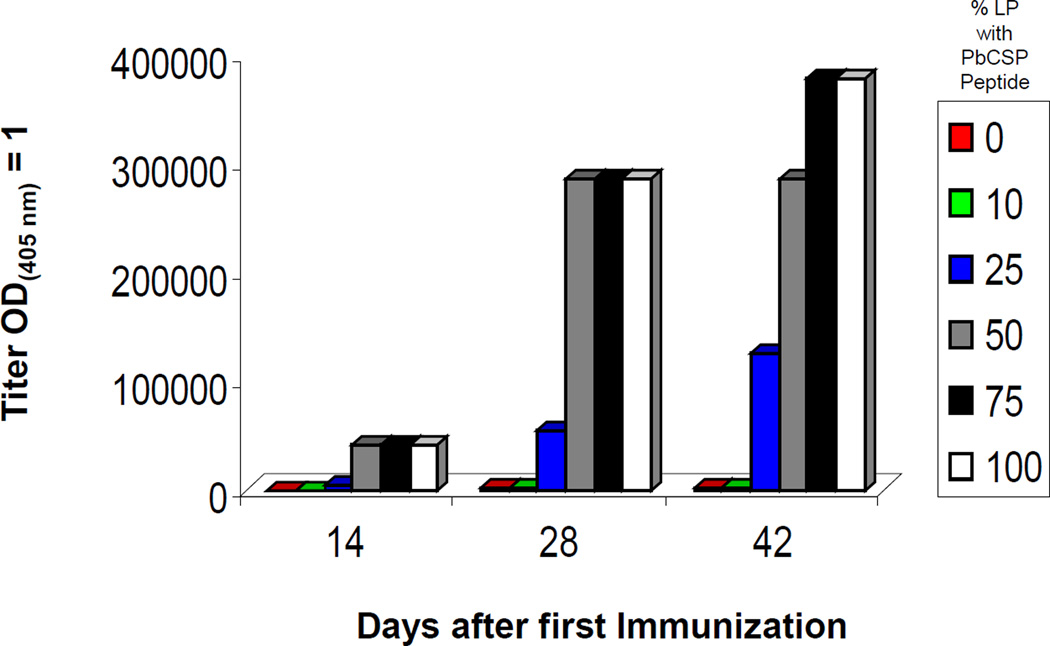
The SAPN are made by self-assembly of LPs that contain a B cell epitope on their C-terminal ends. By mixing different LP expressing different peptide epitopes it is, theoretically, possible to make a SAPN that displays several different peptide epitopes and therefore perhaps to create a multiple antigenic SAPN. We wanted to determine the percentage of LP in a SAPN carrying a unique epitope that would be required to achieve an immune response. By mixing LP with the PbCSP peptide and LP with a random (RD) peptide in different ratios before self-assembly allowed us to form SAPN with different percentages of PbCSP on their surface. Only SAPN that contained 25% or more of the PbCSP peptide induced high antibody titers against PbCSP peptide. SAPN with 50% or more of the PbCSP peptide developed high titers after three immunizations, but those with 75% or more induced the highest titers (Fig. 3).
Figure 3.
Development of PbCSP peptide specific antibody in mice immunized with SAPN constructs containing different ratios of LP containing PbCSP peptide and a random peptide. Mice were immunized with SAPN containing 0, 10, 25, 50, 75 or 100% of LP on days 0, 14, 28. Titers were determined on sera obtained on days 14, 28 and 42 as the dilution required to achieve an OD(405nm) =1.
Protective immune response is long lasting
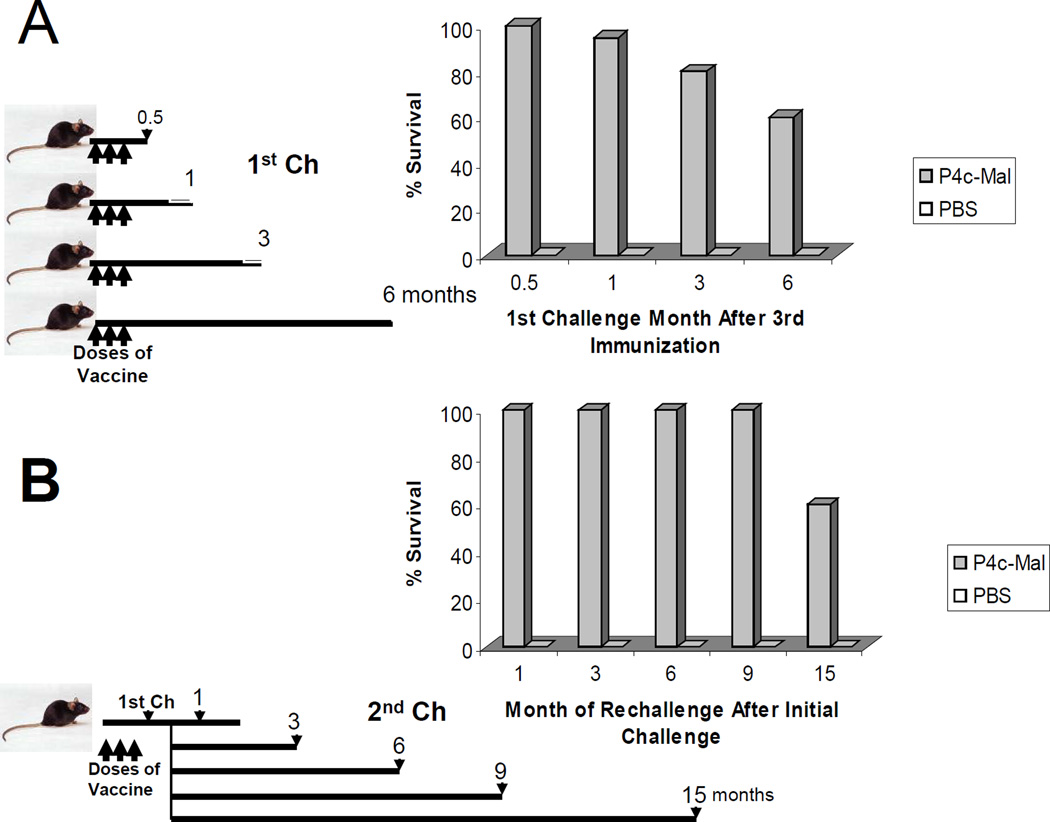
The ability to induce immune memory is an important component of any vaccine. Therefore we wanted to determine the extent of the longevity of the protective immune response after immunization. We asked the question in two ways: first, how long after the final immunization would an effective immune response exist and, second, analogous to a field situation where subsequent exposure to infected mosquitoes occurs, how long after an initial response to an effective challenge would the mouse remain protected? In the first experiment (Fig. 4 A) groups of 10 mice were immunized and then challenged either 2 weeks or 1, 3 or 6 months later. The ability to resist infection slowly dropped over time but a majority of mice were still able to resist infection from lethal challenge up to 6 months after the third immunization with P4c-Mal in PBS.
Figure 4. P4c-Mal induces long term immunity that is boostable by challenge with live sporozoites.
(A) Groups (n=5) mice received three doses of P4c-Mal or saline at 0, 2 and 4 wks. A group was challenged with live sporozoites either 14 days, or 1, 3 or 6 months later. (B) Groups (n=5) mice received three doses of P4c-Mal or saline at 0, 2 and 4 wks. All mice that received P4c-Mal were challenged with live sporozoites two weeks post 3rd dose of vaccine. All mice survived challenge. A group (n=5) of vaccinated and challenged mice or mice receiving saline only were challenged a second time either 1, 3, 6, 9 or 15 months later with live sporozoites.
In the second set of experiments, five groups (n=5) of mice received 3 doses of vaccine. All mice were challenged two weeks after the third P4c-Mal immunization with a lethal dose of sporozoites. All animals survived and individual groups were then challenged either1, 3, 6, 9 or 15 months later. As shown in Fig. 4 B all mice survived a second challenge up to 9 months later and after 15 months 60% of mice were still protected. At the time of challenge in all experiments five control mice (immunized with PBS and not initially challenged) were given sporozoites and in all instances they became parasitemic.
Antibody production is CD4+ T cell dependent
No P. berghei specific or universal T helper epitope was intentionally incorporated into the SAPN design. This raised the question as to whether the observed antibody response was T cell dependent or independent. To answer this question two different strains of mice deficient in functional CD4+ T cells, MHC II KO or nude, or their wild type control strain, C57BL/6, were immunized with P4c-Mal. Two weeks post third immunization the MHC II KO and nude mouse strains showed very little IgG production compared to the wild type (data not shown). When the mice were challenged with live sporozoites all MHC II KO and all nude mice became infected. All wild type control mice immunized with P4c-Mal produced PbCSP peptide specific IgG and were protected against live sporozoite challenge (Table I). Thus, the absence of functional CD4+ T cells impairs the ability of mice to generate protective antibodies when immunized with P4c-Mal.
Table I.
T-cell dependency of protective immune
| Mice | Immunized with |
% survived challenge* |
|---|---|---|
| C57BL/6 | PBS | 0 |
| C57BL/6 | P4c-Mal | 100 |
| Nude | PBS | 0 |
| Nude | P4c-Mal | 0 |
| MHC-II KO | PBS | 0 |
| MHC-II KO | P4c-Mal | 0 |
Survival on day 10 post challenge with 1000 sporozoites.
Testing for potentially bound endotoxin using TLR4−/− mice
It is possible that endotoxin could be tightly bound in the crevices between the adjacent coiled-coils of the SAPN and because bound endotoxin is not detected by the LAL assay we needed to rule out that the adjuvant-like capacity of the SAPN was not due to endogenously bound bacterial lipopolysaccharide (LPS). Because LPS is a TLR4 agonist we reasoned that if the immunostimulatory activity observed with SAPN immunizations was due to contaminated with bound endotoxin we would not be able to protect TLR4 null mice with a P4c-Mal preparation. Therefore, we immunized two different TLR4 null, endotoxin resistant mouse strains C57BL/6/10ScNJ (TLR4−/−) and C3H/He3 (defective LPS response allele Tlr4Lps-d) as well as their wild type equivalent C57BL/6 and C3H mice, respectively, with P4c-Mal. After immunization with P4c-Mal in PBS and sporozoite challenge three out of five TLR4−/− and four out of five Tlr4Lps-d deficient mice and five out of five of each of their wild type counterparts were protected. The fact that we were notable to protect 100% of all the TLR4 null mice is not surprising. It has been shown that there is an increased susceptibly to pathogens when TLR4 signaling is impaired (40). However the ability to protect 70% of TLR4 null mice strongly indicates that LPS is not involved in the stimulation of the immune system in response to SAPN immunization.
Antigen specific CD4+ cells are generated in response to SAPN immunization
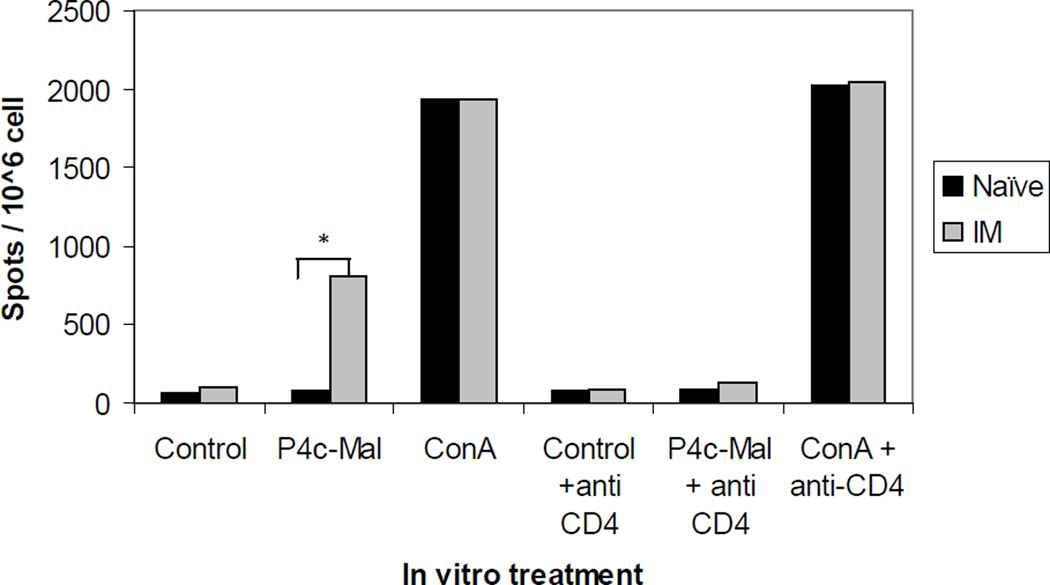
Having determined that CD4+ T cells are needed to initiate the humoral immune response raised the question of how these cognate CD4+ helper cells are generated. The SAPN is a complex protein structure and we suspected that a CD4+ stimulatory epitope was likely to be part of the LP that makes up the SAPN. To test this hypothesis a group of C57BL/6 mice (n=5) were immunized with P4c-Mal and two weeks post third immunization mice and control groups were euthanized, spleens were removed, and splenocytes were pooled and cultured in ELISpot plates. During culture splenocytes were incubated with media only, media containing P4c-Mal (10 µg/mL) or Con A (5 µg/mL), in the presence or absence of 2 µg/mL of the CD4+ T cell specific anti-CD28 and anti-CD49 antibodies. The incubation of splenocytes from immunized mice with P4c-Mal resulted in the induction of IL-2 production while incubation of P4c-Mal with splenocytes from naïve mice did not (Fig. 5). The IL-2 production was abrogated by inclusion of blocking anti-CD4+ antibodies in the media, thus indicating that the IL-2 producing cells were CD4+ T cells. Inclusion of Con A in the culture caused IL-2 production in splenocytes from naïve and immunized mice but was not affected by anti-CD4+ antibodies showing that the ablation of the CD4+ T cell response was not an artifact of the antibodies used. Thus the SAPN platform functions not only to deliver an ordered repetitive array of B cell peptide epitopes but operates as a classical immunological carrier to provide cognate help to the P4c-Mal specific B cells.
Figure 5. P4c-Mal induces the production of IL-2 in CD4+ T cells.
Splenocytes from P4c-Mal immunized or naïve C57BL/6 mice were cultured with P4c-Mal nanoparticles, Con A or media with or without anti-CD4+ T cell blocking antibodies. After 18 h the numbers of cells secreting IL-2 were determined. Values are representative of one of four separate experiments.*: P>0.001.
IgG subclass antibody responses
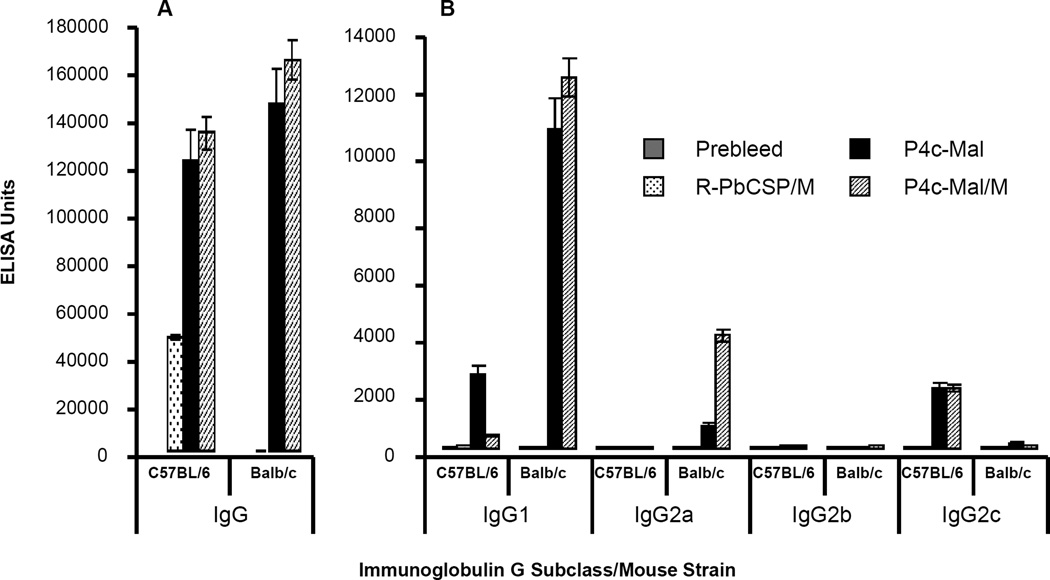
Anti-PbCSP peptide antibody levels were determined in serum from C57BL/6 and Balb/c mice immunized with P4c-Mal. The geometric mean values of the total IgG anti-PbCSP antibodies or IgG subclass antibodies in both strains are illustrated in Fig. 6. We measured IgG2a in Balb/c mice and IgG2c in C57BL/6 mice. C57BL/6 mice lack the Igh-1a allele that codes for the IgG2a but instead express IgG2c from the Igh-1b allele (41, 42). Sera were pooled from ten mice in each group two week’s post 3rd immunization. Immunization with P4c-Mal and P4c-Mal/M resulted predominantly in the induction of specific IgG1 and IgG2a/c antibodies. C57BL/6 mice immunized with R-PbCSP/M resulted in the induction of only IgG1 antibodies (Figs 6 B, 7 A).
Figure 6. P4c-Mal induces a mixed TH1/TH2 IgG subclass profile.
C57BL/6 or Balb/C mice were immunized with P4c-Mal in saline or P4c-Mal in Montanide ISA 720. A group of C57BL/6 mice also received R-PbCSP in Montanide ISA 720. (A) Total IgG and (B) IgG subclass titers were determined on pooled sera obtained two weeks after the last immunization. Error bars depict the SD of the geometric mean values.
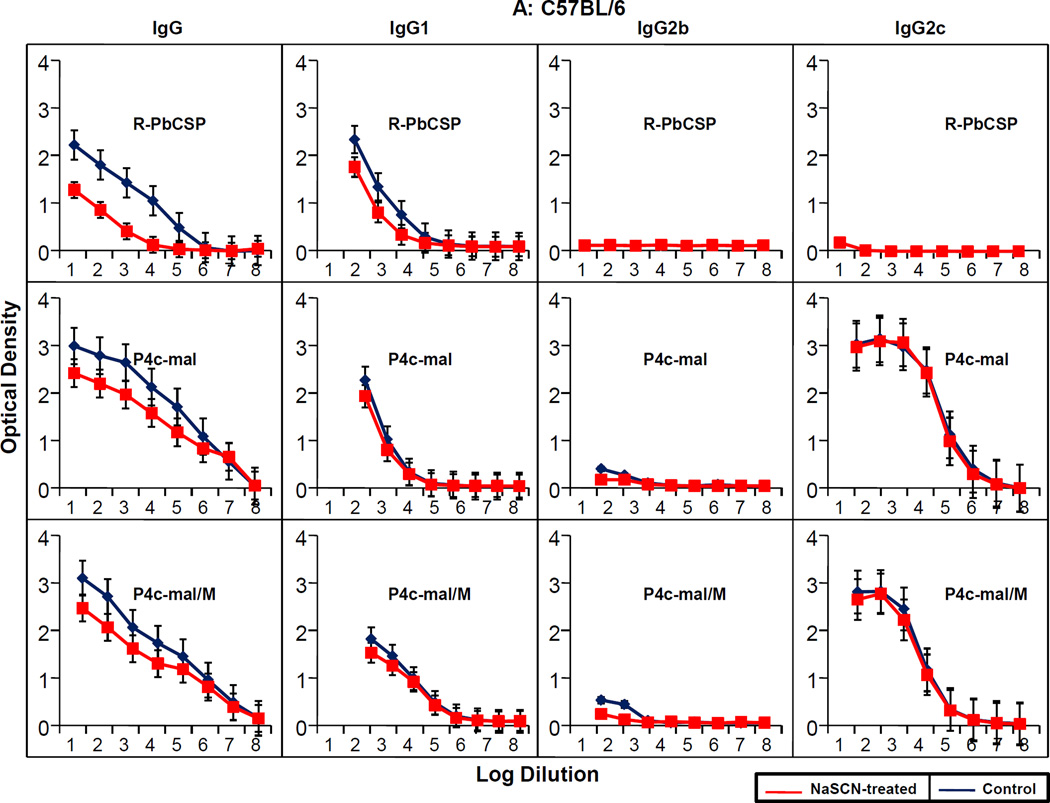
Figure 7. Avidity measurements of anti-PbCSP peptide total IgG and IgG subclasses.
(A) C57BL/6 or (B) Balb/C mice were immunized with R-PbCSP, P4c-Mal or P4c-Mal/M. The amount of total IgG or IgG subclass antibody that bound to PbCSP peptide was determined after incubation with PBS (•) or NaSCN (▪). Antibody determined in sera obtained two weeks after the last immunization.
In C57BL/6 mice P4c-Mal induced a significantly larger amount of IgG1 antibody than P4c-Mal/M (Fig 6B) (P<0.001). While over four times the amount of IgG1 was produced in Balb/c mice than in C57BL/6 (P<0.0002) no significant difference was observed in the amount of IgG1 subclass antibodies when Balb/c were immunized with either P4c-Mal or P4c-Mal/M (P=0.2). The formulation of P4c-Mal with Montanide ISA-720 significantly affected IgG2a responses in Balb/c mice (P<0.001). IgG2c antibody levels produced in C57BL/6 mice immunized with either P4c-Mal or P4c-Mal/M are statistically the same (P= 0.4).
Determination of avidity index (AI) of antibodies made against PbCSP repeat epitope
The chaotropic ELISA elution assay was used for determination of the AI of the antibodies induced by SAPN presented or recombinant protein presented epitopes. In Fig. 7 the OD(405nm) values of the ELISA from each group of mice were plotted against the respective log of the sera dilutions. Rather than using a single specific point on the curve to determine the AI the area under the curve was used (38). Table III shows the calculated AI for sera from R-PbCSP/M, P4c-Mal and P4c-Mal/M antigens. Immunization with R-PbCSP/M induced only IgG1 subclass antibody. The AI value (0.4) for total IgG for R-PbCSP/M was significantly lower (P=0.005) than for any SAPN-PbCSP peptide induced IgG regardless of whether the epitope was delivered in adjuvant (P> 0.5) or saline (P> 0.5). AI values for the total IgG induced by P4c-Mal or P4c-Mal/M were statistically similar. For IgG1, there was no significant difference among the AI values for R-PbCSP/M (P>0.1) and the P4c-Mal (P>0.5) or P4c-Mal/M (P>0.5). For IgG2a/c and IgG, there was no significant difference between the AI values antibodies induced by either of the SAPN-PbCSP peptides. Remarkably, the AI for IgG2c was close to 1 indicating that this particular IgG subclass had an extremely high avidity for the CSP peptide epitope.
Table III.
Avidity Index measurements of PbCSP peptide specific Ab produced in C57BL/6 or Balb/C mice.
| C57BL/6 Mice | |||||
|---|---|---|---|---|---|
| Immunogen | IgG | IgG1 | IgG2a | IgG2b | IgG2c |
| R-PbCSP | 0.40 (P=0.005) | 0.62 (P=0.136) | * | * | * |
| P4c-Mal | 0.79 (P=0.614) | 0.84 (P=0.674) | * | 0.67 (P=0.763) | 0.97 (P=0.549) |
| P4c-Mal/M | 0.78 (P=0.614) | 0.88 (P=0.674) | * | 0.60 (P=0.763) | 0.92 (P=0.549) |
| Balb/c Mice | |||||
| IgG | IgG1 | IgG2a | IgG2b | IgG2c | |
| P4c-Mal | 0.06 (P=0.733) | 0.82 (P=0.470) | 0.53 (P=0.846) | 0.54 (P=0.279) | * |
| P4c-Mal/M | 0.52 (P=0.733) | 0.71 (P=0.470) | 0.51 (P=0.846) | 0.90 (P=0.279) | * |
insufficient antibody made to make calculations.
Discussion
In spite of the recent limited successes of RTS,S in field trials (8, 43) the development of a vaccine for malaria has been a disappointing one. Neither live attenuated viral vectors nor DNA based vaccines, alone or in combination, have worked effectively (3–5, 44–47). In addition, the effectiveness of protein based vaccines, even RTS,S, has been limited by their selection of a co-administered adjuvant (48). We present here a new platform technology based on self-assembling polypeptides that form nanoparticles (27) to induce a protective immune response against whole organism challenge in vivo. We have found that the SAPN functions as a vehicle for presentation of the selected antigenic epitopes and acts as a powerful immuno-stimulatory entity.
To test the feasibility of this new platform to induce an immune response against an otherwise lethal infectious agent we chose to construct a vaccine that presents a known protective B cell epitope as a test antigen. We reasoned that the platform would have to be substantially better in the induction of an immune response and in demonstrating protective efficacy than many of the other existing protein vaccine technologies before carrying it forward into development. The P. berghei CSP repeat sequence, (DPPPPNPN)2D, has been shown, when chemically conjugated to tetanus toxoid (32) or administered in FCA (49), to induce antibodies that prevent P. berghei sporozoites from establishing a blood stage infection in mice. In the former the antibody titer was directly proportional to the dose of immunogen; however the dose was limited because of the toxicity of the tetanus toxoid carrier. In the latter, a chemically synthesized multiple antigenic peptide (MAP), suffered because the synthesis of the vaccine could not be easily controlled and reproduced. A similar approach of immunization with the same B cell and Th epitopes was later tested with a hepatitis B core antigen VLP (50). This VLP, displaying the PbCSP repeat sequence on its immunodominant loop in combination with the Th epitopes T1 and T*, ultimately failed as it too was not immunogenic enough on its own and repeated administration with a strong adjuvant caused serious side effects (51).
Immunization of mice with the P4c-Mal SAPN in saline provided complete protection to a lethal challenged of sporozoites one month after the third dose of vaccine. Subsequent challenge of the same mice showed a sustained immune response capable of protective efficacy against an otherwise lethal challenge of live sporozoites even 15 months later. This level of protection has only been demonstrated previously with the whole cell irradiated sporozoite model of immunization (35). We have shown that antibodies are the effector of protection as serum was required to transfer protection whereas cells alone could not and that the response was T cell dependent as MHC II knockout animals were incapable of mounting a protective response.
The T cells were demonstrated to be SAPN specific providing cognate help to the P4c-Mal peptide specific B cells. It is well established that in order to achieve a T cell dependent B cell response B cells need to interact with the cognate T cells which provide help for the antibody production. At the same time, only activated cognate T cells can provide help, and T cell activation only occurs upon simultaneous stimulation of the T cell receptor and the ligand for co-stimulatory molecules. Conventionally, adjuvant induces expression of high levels of co-stimulatory molecules on the dendritic cells presenting antigen to T cells thus enabling them to provide signal 1 (antigen) and signal 2 (co-stimulatory molecule). Since with this platform we did not need to use adjuvant this raised the question as what was initializing dendritic cell maturation to provide signal 2. Studies in our laboratory (manuscript in preparation) have shown that it is an intrinsic property of the SAPN that is responsible for the induction of the over expression of the co-stimulatory molecules.
Other reports using nanoparticles to present antigen have used inert substrates such as gold particles coated with azobenzene disulfide dye (52) or polymers of proteinaceous materials as the substrate. Polypeptides containing T or B cell epitopes bound to polymers of glutamic acid or poly-L-lysine coated polystyrene (10, 53–55) have been used to enhance DNA vaccine efficacy (12). Most of the immunostimulatory effects of those nanoparticles could be attributed to their interaction with DC in the skin or the lymph nodes. Recent studies have presented convincing evidence that small size (20–30 nm) particles are preferentially transported to the lymph nodes where the particles are presented to immature DC (14, 15, 56, 57). Once internalized in the DC the nanoparticles are processed and antigenic epitopes are released to activate MHC class I and II pathways leading to induction of both CD4+ and CD8+ T cell activation.
The high levels of IgG1, IgG2a/c as well as a considerable IgG2b subclass of immunoglobulin reflect a mixed Th1/Th2 response. In mice, IgG1 responses are usually associated with Th2 responses, whereas high levels of IgG2a, sometimes associated with IgG2b and IgG3, are thought to reflect Th1 responses. Several parameters influence the IgG subclass responses to proteins, including the dose of antigen used, use of adjuvants or routes of immunization as well as the intrinsic immunogenicity of the protein itself (58–64). We did not see major differences in IgG subclasses with different routes of immunization (data not shown) and adjuvants added to SAPN did not influence IgG subclass distribution. Therefore the major effector of the IgG subclass appears to be associated with an intrinsic immunogenic property of the SAPN.
A surprising finding in these studies was the high avidity and subclass specificity of the antibodies produced. Of note is the extremely high avidity of the IgG2c subclass antibodies. Immunization with R-PbCSP in the adjuvant Montanide ISA-720 did not induce any detectable IgG2c antibodies. Clearly the effectiveness of a vaccine that works predominately by induction of neutralizing antibodies, as is the mode of action of the immune response induced in these studies, is dependent not only on the amount of antibody induced but also the avidity of those antibodies. The complex cellular events that are needed to generate high affinity antibody secreting cells are not clearly understood. B cells in the germinal centers (GC) undergo somatic hypermutation of V(D)J immunoglobulin genes and competition for antigen retained on the GC follicular DC results in the selection of B cells secreting the highest affinity antibodies (65). Many things influence the combined affinity (avidity) of antibodies to antigenic determinates including the valence of the antibody and the valence of the antigen. The physical antigen properties or adjuvant assets that influence the maturation process, which may be essential for the protective efficacy have not been characterized following immunization with T-dependent protein antigens but is clearly important in vaccine development (66). The coiled-coil nature of the SAPN may have an influence on the presentation of the antigen and maturation of the antibody affinity because linear recombinant protein with the same epitope given in a strong adjuvant (R-PbCSP/M) or a modified P4c-Mal LP that would not form SAPN (data not shown) did not induce high titer or high avidity antibodies and failed to provide a protective immune response.
The nanoparticles used in this study appear to display B cell epitopes in a very immunostimulatory array. The particles are small, about 25 nm in size, and contain strong hydrophobic oligomerization domains which dictate their self-assembly. Recently we have shown that they are efficiently taken up by and activate mouse macrophages and bone-marrow derived DC (manuscript in preparation). The platform has recently been used to effectively present an otherwise poor antigenic determinant to make monoclonal antibodies against a conserved actin domain (28) as well as to induce in vitro neutralizing antibodies against a SARS virus epitope (26). We have now extended the use of this platform to induce an in vivo immune response that is protective against challenge with a lethal parasite. When administered in saline alone the SAPN are capable of inducing maturation of a long term memory response to a B cell epitope. The induced antibodies protect against an otherwise lethal challenge of sporozoites. It should be noted that when the leading malaria vaccine candidate, RTS,S, itself a VLP vaccine formulation, was administered to mice in saline no significant anti-CSP repeat or HbSAg antibody was detected (67). This clearly shows that nanoparticles or VLP, a priori, are not the key to effective immune responses. We are now determining if the SAPN platform can be used to make a vaccine against P. falciparum and P. vivax malaria, whether it can induce a CD8+ mediated immune response and whether it is effective in higher order mammals such as rabbits and non-human primates.
Acknowledgements
This work was performed while SAK held a National Research Council Research Associate award at WRAIR. Funds were received (DEL) from the Military Infectious Disease Research Program. The authors' views are private and are not to be construed as official policy of the Department of Defense or the U.S. Army. R-PbCSP was a gift from E. Angov, WRAIR, Silver Spring, MD.
Abbreviations
- SAPN
Self-Assembling polypeptide nanoparticle
- VLP
virus like particle
- LP
linear polypeptide
- PbCSP
Plasmodium berghei circumsporozoite protein
- R-PbCSP
recombinant-PbCSP
Footnotes
CM, DT and SR were supported by the grants “8433.1” and “Peptide NP” from the Kommission für Technologie und Innovation (Switzerland) and the Swiss Nanoscience Institute.
References
- 1.Ballou WR, Arevalo-Herrera M, Carucci D, Richie TL, Corradin G, Diggs C, Druilhe P, Giersing BK, Saul A, Heppner DG, Kester KE, Lanar DE, Lyon J, Hill AV, Pan W, Cohen JD. Update on the clinical development of candidate malaria vaccines. The American journal of tropical medicine and hygiene. 2004;71:239–247. [PubMed] [Google Scholar]
- 2.Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: are we getting closer? Current opinion in molecular therapeutics. 2007;9:12–24. [PubMed] [Google Scholar]
- 3.Bejon P, Mwacharo J, Kai O, Mwangi T, Milligan P, Todryk S, Keating S, Lang T, Lowe B, Gikonyo C, Molyneux C, Fegan G, Gilbert SC, Peshu N, Marsh K, Hill AV. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS clinical trials. 2006;1:e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, Andrews L, Bejon P, Poulton I, Butcher G, Watkins K, Sinden RE, Leach A, Moris P, Tornieporth N, Schneider J, Dubovsky F, Tierney E, Williams J, Heppner DG, Jr, Gilbert SC, Cohen J, Hill AV. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine. 2006;24:2850–2859. doi: 10.1016/j.vaccine.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Moorthy VS, Imoukhuede EB, Milligan P, Bojang K, Keating S, Kaye P, Pinder M, Gilbert SC, Walraven G, Greenwood BM, Hill AS. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS medicine. 2004;1:e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, Berthoud T, Andrews L, Andersen RF, Moore A, Gilbert SC, Poulton I, Dubovsky F, Tierney E, Correa S, Huntcooke A, Butcher G, Williams J, Sinden RE, Hill AV. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infection and immunity. 2006;74:2706–2716. doi: 10.1128/IAI.74.5.2706-2716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotkin SA. Vaccines: past, present and future. Nature medicine. 2005;11:S5–S11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 9.Rafi-Janajreh A, Tongren JE, Kensil C, Hackett C, Candal F, Lal A, Udhayakumar V. Influence of adjuvants in inducing immune responses to different epitopes included in a multiepitope, multivalent, multistage Plasmodium falciparum candidate vaccine (FALVAC-1) in outbred mice. Experimental parasitology. 2002;101:3–12. doi: 10.1016/s0014-4894(02)00029-2. [DOI] [PubMed] [Google Scholar]
- 10.Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials. 2007;28:3427–3436. doi: 10.1016/j.biomaterials.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Kalkanidis M, Pietersz GA, Xiang SD, Mottram PL, Crimeen-Irwin B, Ardipradja K, Plebanski M. Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods (San Diego, Calif. 2006;40:20–29. doi: 10.1016/j.ymeth.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Minigo G, Scholzen A, Tang CK, Hanley JC, Kalkanidis M, Pietersz GA, Apostolopoulos V, Plebanski M. Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25:1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 13.Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Molecular pharmaceutics. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 14.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends in immunology. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Noad R, Roy P. Virus-like particles as immunogens. Trends in microbiology. 2003;11:438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science (New York, N.Y. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann MF, Zinkernagel RM. The influence of virus structure on antibody responses and virus serotype formation. Immunology today. 1996;17:553–558. doi: 10.1016/s0167-5699(96)10066-9. [DOI] [PubMed] [Google Scholar]
- 19.Aebi U, ten Heggeler B, Onorato L, Kistler J, Showe MK. New method for localizing proteins in periodic structures: Fab fragment labeling combined with image processing of electron micrographs. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:5514–5518. doi: 10.1073/pnas.74.12.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buhle EL, Jr, Aebi U. Specific labeling of protein domains with antibody fragments. Journal of ultrastructure research. 1984;89:165–178. doi: 10.1016/s0022-5320(84)80012-x. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood J, Willis AE, Perham RN. Multiple display of foreign peptides on a filamentous bacteriophage. Peptides from Plasmodium falciparum circumsporozoite protein as antigens. Journal of molecular biology. 1991;220:821–827. doi: 10.1016/0022-2836(91)90354-9. [DOI] [PubMed] [Google Scholar]
- 22.Kistler J, Aebi U, Onorato L, ten Heggeler B, Showe MK. Structural changes during the transformation of bacteriophage T4 polyheads: characterization of the initial and final states by freeze-drying and shadowing Fab-fragment-labelled preparations. Journal of molecular biology. 1978;126:571–589. doi: 10.1016/0022-2836(78)90059-1. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Chen YH. High epitope density in a single protein molecule significantly enhances antigenicity as well as immunogenicity: a novel strategy for modern vaccine development and a preliminary investigation about B cell discrimination of monomeric proteins. European journal of immunology. 2005;35:505–514. doi: 10.1002/eji.200425749. [DOI] [PubMed] [Google Scholar]
- 25.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biological chemistry. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 26.Pimentel TA, Yan Z, Jeffers SA, Holmes KV, Hodges RS, Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chemical biology & drug design. 2009;73:53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raman S, Machaidze G, Lustig A, Aebi U, Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomedicine. 2006;2:95–102. doi: 10.1016/j.nano.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder U, Graff A, Buchmeier S, Rigler P, Silvan U, Tropel D, Jockusch BM, Aebi U, Burkhard P, Schoenenberger CA. Peptide nanoparticles serve as a powerful platform for the immunogenic display of poorly antigenic actin determinants. Journal of molecular biology. 2009;386:1368–1381. doi: 10.1016/j.jmb.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. Science. Vol. 274. New York, N.Y: 1996. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? pp. 761–765. [DOI] [PubMed] [Google Scholar]
- 30.Burkhard P, Meier M, Lustig A. Design of a minimal protein oligomerization domain by a structural approach. Protein Sci. 2000;9:2294–2301. doi: 10.1110/ps.9.12.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eichinger DJ, Arnot DE, Tam JP, Nussenzweig V, Enea V. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Molecular and cellular biology. 1986;6:3965–3972. doi: 10.1128/mcb.6.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zavala F, Tam JP, Barr PJ, Romero PJ, Ley V, Nussenzweig RS, Nussenzweig V. Synthetic peptide vaccine confers protection against murine malaria. The Journal of experimental medicine. 1987;166:1591–1596. doi: 10.1084/jem.166.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandenberger R, Kammerer RA, Engel J, Chiquet M. Native chick laminin-4 containing the beta 2 chain (s-laminin) promotes motor axon growth. The Journal of cell biology. 1996;135:1583–1592. doi: 10.1083/jcb.135.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta S, Lalitha PV, Ware LA, Barbosa A, Moch JK, Vassell MA, Fileta BB, Kitov S, Kolodny N, Heppner DG, Haynes JD, Lanar DE. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infection and immunity. 2002;70:3101–3110. doi: 10.1128/IAI.70.6.3101-3110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 36.Brando C, Ware LA, Freyberger H, Kathcart A, Barbosa A, Cayphas S, Demoitie MA, Mettens P, Heppner DG, Lanar DE. Murine immune responses to liver-stage antigen 1 protein FMP011, a malaria vaccine candidate, delivered with adjuvant AS01B or AS02A. Infection and immunity. 2007;75:838–845. doi: 10.1128/IAI.01075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. Journal of immunological methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 38.Perciani CT, Peixoto PS, Dias WO, Kubrusly FS, Tanizaki MM. Improved method to calculate the antibody avidity index. Journal of clinical laboratory analysis. 2007;21:201–206. doi: 10.1002/jcla.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheiblhofer S, Chen D, Weiss R, Khan F, Mostbock S, Fegeding K, Leitner WW, Thalhamer J, Lyon JA. Removal of the circumsporozoite protein (CSP) glycosylphosphatidylinositol signal sequence from a CSP DNA vaccine enhances induction of CSP-specific Th2 type immune responses and improvesprotection against malaria infection. European journal of immunology. 2001;31:692–698. doi: 10.1002/1521-4141(200103)31:3<692::aid-immu692>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 40.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nature reviews. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 41.Jouvin-Marche E, Morgado MG, Leguern C, Voegtle D, Bonhomme F, Cazenave PA. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29:92–97. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 42.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. Journal of immunological methods. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 43.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, Omari S, Urassa A, Mshinda H, Jumanne A, Salim N, Shomari M, Aebi T, Schellenberg DM, Carter T, Villafana T, Demoitie MA, Dubois MC, Leach A, Lievens M, Vekemans J, Cohen J, Ballou WR, Tanner M. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. The New England journal of medicine. 2008;359:2533–2544. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 44.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, Andersen RF, Bejon P, Goonetilleke N, Poulton I, Webster DP, Butcher G, Watkins K, Sinden RE, Levine GL, Richie TL, Schneider J, Kaslow D, Gilbert SC, Carucci DJ, Hill AV. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infection and immunity. 2006;74:5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert SC, Plebanski M, Harris SJ, Allsopp CE, Thomas R, Layton GT, Hill AV. A protein particle vaccine containing multiple malaria epitopes. Nature biotechnology. 1997;15:1280–1284. doi: 10.1038/nbt1197-1280. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Locke E, Bruder J, Clarke D, Doolan DL, Havenga MJ, Hill AV, Liljestrom P, Monath TP, Naim HY, Ockenhouse C, Tang DC, Van Kampen KR, Viret JF, Zavala F, Dubovsky F. Viral vectors for malaria vaccine development. Vaccine. 2007;25:2567–2574. doi: 10.1016/j.vaccine.2006.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moorthy VS, Imoukhuede EB, Keating S, Pinder M, Webster D, Skinner MA, Gilbert SC, Walraven G, Hill AV. Phase 1 evaluation of 3 highly immunogenic prime-boost regimens, including a 12-month reboosting vaccination, for malaria vaccination in Gambian men. The Journal of infectious diseases. 2004;189:2213–2219. doi: 10.1086/421118. [DOI] [PubMed] [Google Scholar]
- 48.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. The New England journal of medicine. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 49.Romero PJ, Tam JP, Schlesinger D, Clavijo P, Gibson H, Barr PJ, Nussenzweig RS, Nussenzweig V, Zavala F. Multiple T helper cell epitopes of the circumsporozoite protein of Plasmodium berghei. European journal of immunology. 1988;18:1951–1957. doi: 10.1002/eji.1830181213. [DOI] [PubMed] [Google Scholar]
- 50.Birkett A, Lyons K, Schmidt A, Boyd D, Oliveira GA, Siddique A, Nussenzweig R, Calvo-Calle JM, Nardin E. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infection and immunity. 2002;70:6860–6870. doi: 10.1128/IAI.70.12.6860-6870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveira GA, Wetzel K, Calvo-Calle JM, Nussenzweig R, Schmidt A, Birkett A, Dubovsky F, Tierney E, Gleiter CH, Boehmer G, Luty AJ, Ramharter M, Thornton GB, Kremsner PG, Nardin EH. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infection and immunity. 2005;73:3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishii N, Fitrilawati F, Manna A, Akiyama H, Tamada Y, Tamada K. Gold nanoparticles used as a carrier enhance production of anti-hapten IgG in rabbit: a study with azobenzene-dye as a hapten presented on the entire surface of gold nanoparticles. Bioscience, biotechnology, and biochemistry. 2008;72:124–131. doi: 10.1271/bbb.70499. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo K, Yoshikawa T, Oda A, Akagi T, Akashi M, Mukai Y, Yoshioka Y, Okada N, Nakagawa S. Efficient generation of antigen-specific cellular immunity by vaccination with poly(gamma-glutamic acid) nanoparticles entrapping endoplasmic reticulum-targeted peptides. Biochemical and biophysical research communications. 2007;362:1069–1072. doi: 10.1016/j.bbrc.2007.08.112. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto S, Yoshii H, Akagi T, Akashi M, Ishikawa T, Okuno Y, Takahashi M, Yamanishi K, Mori Y. Influenza hemagglutinin vaccine with poly(gamma-glutamic acid) nanoparticles enhances the protection against influenza virus infection through both humoral and cell-mediated immunity. Vaccine. 2007;25:8270–8278. doi: 10.1016/j.vaccine.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto S, Yoshii H, Ishikawa T, Akagi T, Akashi M, Takahashi M, Yamanishi K, Mori Y. Single dose of inactivated Japanese encephalitis vaccine with poly(gamma-glutamic acid) nanoparticles provides effective protection from Japanese encephalitis virus. Vaccine. 2008;26:589–594. doi: 10.1016/j.vaccine.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 56.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. European journal of immunology. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 57.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature biotechnology. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 58.Ahlborg N, Ling IT, Holder AA, Riley EM. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 (MSP1(19)) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP1(19) Infection and immunity. 2000;68:2102–2109. doi: 10.1128/iai.68.4.2102-2109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Oliveira GA, Clavijo P, Nussenzweig RS, Nardin EH. Immunogenicity of an alum-adsorbed synthetic multiple-antigen peptide based on B- and T-cell epitopes of the Plasmodium falciparum CS protein: possible vaccine application. Vaccine. 1994;12:1012–1017. doi: 10.1016/0264-410x(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 60.Guy B, Krell T, Sanchez V, Kennel A, Manin C, Sodoyer R. Do Th1 or Th2 sequence motifs exist in proteins? Identification of amphipatic immunomodulatory domains in Helicobacter pylori catalase. Immunology letters. 2005;96:261–275. doi: 10.1016/j.imlet.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Hisaeda H, Yasutomo K, Himeno K. Malaria: immune evasion by parasites. The international journal of biochemistry & cell biology. 2005;37:700–706. doi: 10.1016/j.biocel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Lee EA, Palmer DR, Flanagan KL, Reece WH, Odhiambo K, Marsh K, Pinder M, Gravenor MB, Keitel WA, Kester KE, Diggs C, Kaslow D, Apostolopoulos V, Ballou WR, Hill AV, Krzych U, Plebanski M. Induction of T helper type 1 and 2 responses to 19-kilodalton merozoite surface protein 1 in vaccinated healthy volunteers and adults naturally exposed to malaria. Infection and immunity. 2002;70:1417–1421. doi: 10.1128/IAI.70.3.1417-1421.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Livingston B, Crimi C, Newman M, Higashimoto Y, Appella E, Sidney J, Sette A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol. 2002;168:5499–5506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 65.McHeyzer-Williams LJ, Driver DJ, McHeyzer-Williams MG. Germinal center reaction. Current opinion in hematology. 2001;8:52–59. doi: 10.1097/00062752-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Siegrist CA, Pihlgren M, Tougne C, Efler SM, Morris ML, AlAdhami MJ, Cameron DW, Cooper CL, Heathcote J, Davis HL, Lambert PH. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23:615–622. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 67.Mettens P, Dubois PM, Demoitie MA, Bayat B, Donner MN, Bourguignon P, Stewart VA, Heppner DG, Jr, Garcon N, Cohen J. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS,S vaccine antigen. Vaccine. 2008;26:1072–1082. doi: 10.1016/j.vaccine.2007.12.018. [DOI] [PubMed] [Google Scholar]