Abstract
Objectives
Abnormal responses to social stimuli are seen in people vulnerable to suicidal behavior, indicating possible disruptions in the neural circuitry mediating the interpretation of socio-emotional cues. These disruptions have not been empirically related to psychological and cognitive pathways to suicide. In the present study of older suicide attempters, we examined neural responses to emotional faces and their relationship to impulsivity, one of the components of the suicidal diathesis.
Methods
Using functional magnetic resonance imaging, we recorded neuro-hemodynamic responses to angry faces in a carefully-characterized sample of 18 depressed elderly with history of suicide attempts, 13 depressed non-suicidal patients, and 18 healthy individuals, all aged 60+. Impulsivity was assessed with the Social Problem Solving Inventory Impulsivity/Carelessness Style subscale and Barratt Impulsiveness Scale. The Suicide Intent Scale planning subscale was used to describe the degree of planning associated with the most lethal attempt.
Results
Depression and history of attempted suicide were not associated with neural responses to angry faces, failing to replicate earlier studies. Higher impulsivity, however, predicted exaggerated responses to angry faces in fronto-opercular and dorsomedial prefrontal cortex (pcorr < .05). Poorly planned suicide attempts also predicted increased fronto-opercular responses. Results were robust to effects of medication exposure, comorbid anxiety and addiction, severity of depression, burden of physical illness, and possible brain injury from suicide attempts.
Conclusions
Impulsive traits and history of unplanned suicide attempts partly explain the heterogeneity in neural responses to angry faces in depressed elderly. Displays of social emotion command excessive cortical processing in impulsive suicide attempters.
Keywords: aging, suicide, impulsivity, social threat, neuroimaging, prefrontal cortex
Suicide among older adults remains common and evades efforts to predict and prevent it (1, 2). Although pre-existing psychiatric disorders increase one’s liability, suicidal behavior does not appear to be simply an extreme expression of depression (3, 4). The diversity of cognitive and psychosocial markers of suicide (e.g., cognitive deficits, interpersonal dysfunction, hopelessness, and impulsive-aggressive traits) attests to its highly heterogeneous nature and highlights the fact that it cannot be pinned down to a single neural substrate or mechanism. Instead, research examines different pathways to suicide, such as the stress-diathesis model. In this model, suicidal behavior arises from the interaction of depression, addiction or psychosis with various neurobiological and psychosocial vulnerability factors. The present study focuses on the coupling of two putative vulnerability factors: chronic interpersonal dysfunction (5) associated with a maladaptive approach to social problems in older suicide attempters (6–9), and impulsive aggressive traits (10). To uncover alterations in neural systems that underlie deficits in social functioning and trait impulsivity in elderly suicide attempters, we investigated neural processing of socio-emotional stimuli (angry faces).
Accumulating evidence indicates that, although depression may often be a necessary precondition for suicidal behavior, the risk architecture of suicidal behavior extends beyond depression. Depression is associated with frontostriatal alterations that parallel an altered encoding of rewards (11–14) and a failure of cognitive control (15, 16), as well as with alterations in the paralimbic cortex (default-mode network) that may parallel abnormal self-referential processing (17). Although these features are present and even exaggerated in suicidal individuals (6, 18–20), cognitive and neural correlates of suicidal behavior, beyond the features typical of depression, are closely associated with impulsive traits and poor decision-making. For example, depressed individuals express oversensitivity to punishment in reinforcement learning tasks, whereas suicide attempters pay excessive attention to superficial features of the task like reward immediacy, indicating a non-strategic, short-sighted approach to problem-solving and decision-making (21). These behaviors are mirrored by alterations in the ventromedial prefrontal cortex and in the basal ganglia, which are also related to impulsive traits (11, 21). Hence, in order to isolate neural alterations unique to suicidal behavior, the present study contrasts suicide attempters, depressed non-suicidal patients and healthy individuals, with a specific focus on impulsivity in social context.
More specifically, older suicide attempters often perceive life problems as threatening and unsolvable, and exhibit an impulsive and avoidant approach to social problems (7). Altered processing of emotional expressions may influence how these individuals approach social conflict (22–24), as suicide attempters also make more errors in identifying emotional states from facial expressions (9). Until now, only two studies have examined the functioning of neural systems underlying the processing of emotional expressions in attempted suicide and found evidence of alterations (25). Angry faces elicited greater activity in the right lateral orbitofrontal cortex in adult male suicide attempters than in depressed individuals, although neither group was different from the healthy comparison group (19). In suicidal adolescents, response to angry faces elicited greater activity in the prefrontal, primary sensory and temporal cortices (26). Additional evidence for association between impulsivity, abnormal perception of socio-emotional cues, and disrupted neural dynamics during processing of emotional faces is seen in clinical populations that express extreme levels of trait impulsivity and high rates of suicidal behavior: patients with borderline personality disorder (BPD) (27) (28) and intermittent explosive disorder (IED) (29, 30). Overall, these findings indicate that socially threatening stimuli may command excessive processing resources in suicidal individuals. No study, however, has investigated these alterations in suicidal elderly; nor has any study examined the association between impulsivity, which, as noted earlier, plays an important role in suicide diathesis, and functional abnormalities in suicide attempters.
The present study targets suicidal elderly because the suicide rate is high in this population. Suicidal behavior tends to be more lethal in this group with a much higher attempt to completion ratio than that of younger suicide attempters (31, 32). In addition, clinically and demographically older suicide attempters may be more likely to resemble cases of death by suicide than younger suicide attempters (33, 34). We investigate whether elderly suicide attempters process emotional faces differently than depressed non-suicidal and non-psychiatric comparison groups. Non-suicidal depressed elderly were included in the study to detect an association between altered processing and suicidal behavior beyond the effects of depression. We hypothesized that suicide attempters would have an exaggerated response in the orbitofrontal cortex in response to angry faces compared to non-suicidal depressed and healthy comparison groups. This finding would replicate those of Jollant et al. (19) and provide support for differential recruitment of cognitive resources in response to socially-threatening stimuli in suicide attempters. We also hypothesized that trait impulsivity, probed by a variety of clinical measures, would predict disrupted processing of emotional stimuli in suicide attempters. Finally, we investigated whether executive function and interpersonal dysfunction were related to processing of angry faces.
Methods
Participants
Forty-nine participants 60 years and older completed the study: 18 individuals with history of suicide attempts and major depression, 13 individuals with major depression but no history of suicide attempt, and 18 healthy individuals. Their demographic, clinical and cognitive characteristics are described in Table 1.
Table 1.
Demographic, Clinical, and Cognitive Characteristics
| Study Group
| ||||||
|---|---|---|---|---|---|---|
| Characteristic | Non-psychiatric Controls (n = 18) | Non-suicidal Depressed (n = 13) | Depressed Suicide Attempters (n = 18) | Statistic | p | Post-hoc |
| Male sex, No. (%) | 7 (40) | 4 (31) | 12 (67) | χ2(2, N = 49) = 4.65 | .10 | |
| Age in years (SD) | 70.05 (7.7) | 68.15 (6.1) | 67.44 (7.0) | F(2, 46)= 0.65 | .53 | |
| White, No. (%) | 16 (89) | 8 (62) | 14 (78) | χ2(4, N = 49) = 4.97 | .30 | |
| Educational level, y | 14.44 (2.0) | 15.08 (2.4) | 14.39 (3.3) | F(2, 46) = 0.30 | .75 | |
| Premorbid IQ (n = 41) | 107.06 (7.6) | 111.36 (10.5) | 103.07 (17.4) | F(2, 38) = 1.37* | .27 | |
| Dementia rating scale | 137.72 (2.6) | 136.92 (3.9) | 133.5 (6.9) | F(2, 46) = 3.66* | .03 | HC1 > SA2 |
| EXIT-25 (n = 48) | 6.89 (3.3) | 6.54 (3.2) | 7.76 (3.8) | F(2, 45) = 0.53 | .60 | |
| Physical illness burden | 6.67 (0.9) | 9.85 (3.2) | 9.7 (5.0) | F(2, 46) = 3.91* | .03 | HC < D3, SA |
| Hamilton Rating Scale for Depression-16 (without suicide item) | 2.56 (2.1) | 10.31(7.3) | 14.22 (7.6) | F(2, 46) = 17.09* | .01 | HC < D, SA |
| Beck Hopelessness Scale | 0.89 (0.7) | 9.38 (6.2) | 10.91 (7.4) | F(2, 46) = 18.14* | .01 | HC < D, SA |
| Barratt Impulsiveness Scale: Non-planning and Attention/Cognitive subscales (n = 39) | 10.83 (7.4) | 14.67 (3.5) | 16.87 (8.0) | F(2, 36) = 2.68* | .08 | |
| Social Problem-Solving Inventory: | ||||||
| Impulsive/Careless Style subscale | 2.83 (2.6) | 3.85 (2.4) | 4.94 (4.2) | F(2, 46) = 1.90 | .16 | |
| Inventory of Interpersonal Problems (IIP-15): | ||||||
| Interpersonal Sensitivity | 2.65 (2.4) | 6.69 (2.6) | 7.22 (4.1) | F(2, 46) = 10.5 | .01 | HC < D, SA |
| Interpersonal Ambivalence | 1.59 (3.4) | 3.92 (3.3) | 5.22 (3.2) | F(2, 46) = 4.82 | .01 | HC < SA |
| Interpersonal Aggression | 0.76 (1.3) | 4.08 (3.4) | 4.28 (2.2) | F(2, 46) = 11.66 | .01 | HC < D, SA |
| Suicide Intent Scale | NA | NA | 19.22 (5.2) | |||
| High lethality attempts, No. | NA | NA | 8 | |||
| Antidepressant exposure (n = 42) | NA | 2.20 (1.8) | 4.29 (3.0) | D < SA | ||
| Lifetime substance use disorders, No. | NA | 4 | 7 | χ2(2, N = 31) = 0.81 | .37 | |
| Lifetime anxiety disorders, No. | NA | 5 | 7 | χ2(2, N = 31) = 0.33 | .56 | |
Healthy Controls (HC);
Depressed suicide attempters (SA);
Non-suicidal depressed (D)
Post-hoc tests were performed using Tukey HSD.
Whenever variances were unequal between groups, the results of the ANOVA were verified with follow-up t-test comparisons for which equal variances were not assumed. The pattern of group differences was the same with both tests.
Major depression was diagnosed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (35, 36). We excluded individuals with a diagnosis of clinical dementia and/or a score of less than 24 on the Mini-Mental State Examination. Further details on the recruitment of participants and exclusion criteria can be found in our previous paper (11).
All participants provided written informed consent. The University of Pittsburgh institutional review approved the study.
Suicide attempters engaged in self-injurious act with an intent to die; 6 made their first suicide attempt before 50 years of age, 4 after 50 years of age and 8 after 60 years of age. The suicidal intent associated with suicide attempt was measured with Beck’s Suicide Intent Scale (SIS (37)) (M = 18.83, SD = 5.46). The SIS-planning subscale (38) was used to assess the degree of planning of suicide attempts (M = 6.27, SD = 2.61). A study psychiatrist (A.Y. D. or K. S.) verified a history of suicide attempts, based on the interview, medical records, information from family members and friends. We excluded participants with significant discrepancies between these sources.
Nonsuicidal depressed had no current or lifetime history of suicide attempts or suicidal ideation as established by clinical interview, review of medical records, SCID, and the Scale for Suicidal Ideation (lifetime). Participants were excluded from this group if they had a current passive death wish or a history of indirect self-destructive behaviors.
Nondepressed individuals were included as the benchmark group. They had no lifetime history of any psychiatric disorder as determined by SCID.
Clinical and cognitive assessments used to characterize the study groups are described in detail in the supplement materials.
Impulsivity, executive function, chronic interpersonal difficulties and attempt-related impulsivity
Impulsivity is a multifaceted construct (39). We selected two widely-used self-report measures of impulsivity with the intent to interpret shared features of neural activity. The Impulsive/Careless Style subscale of the Social Problem-Solving Inventory (SPSI-ICS) (7, 40) measures a narrow and hurried approach to social problem-solving situation. The Nonplanning and Attention/Cognitive subscales of the Barratt Impulsiveness Scale (BIS-nonplanning) (41) have been associated with suicide attempts in previous studies (42). High values on the BIS-nonplanning subscale correspond to acting without consideration of consequences and with a focus on immediate rather than long-term outcomes. The two self-report measures of impulsivity are moderately correlated with each other and with the measure of chronic interpersonal difficulties, measured by IIP-15 (43). Statistics for these and other inter-correlations reported in the supplemental materials.
The degree to which suicide attempts were planned (SIS-planning) captures preparation, premeditation, isolation, timing, and precautions against discovery. Our past research indicated that the planning of suicide attempts in the elderly is inversely related to the willingness to wait for larger rewards on a delay discounting task (44) and to paralimbic expected value signals (11). The SIS-planning subscale was only modestly and non-significantly correlated with the self-report measures of impulsivity.
Faces and shapes task
The faces and shapes fMRI task (Figure 1) has been used extensively to investigate the neural circuitry of processing facial emotions (45). On a single trial participants are simultaneously presented with two stimuli and a target stimulus. During the faces condition, they choose a match to a target stimulus from two facial expressions (angry or afraid). Twelve different images derived from a standard set of pictures of facial affect are used (46), six per block and three of each gender. During the control shapes condition, participants choose a match from two geometric shapes. Participants complete five blocks of matching shapes alternating with four blocks of matching faces. Each block lasts 30 sec (6 trials that last 5 seconds each).
Figure 1.
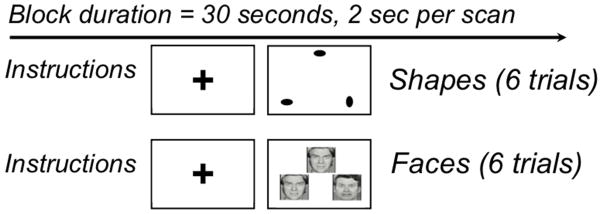
Faces and shapes task.
Data Acquisition
Imaging data were collected with a 3-T Siemens Trio Tim scanner located in the MR Research Center at the University of Pittsburgh. For functional image alignment we used T2*-weighted image depicting blood oxygenation level-dependent (BOLD) contrast; TR = 2000ms, TE =29ms, FOV=20cm, flip =75, 28 3mm slices). Stimulus presentation and response recording was controlled using the E-Prime software package (47).
Image Preprocessing
Functional images were preprocessed using tools from AFNI (48) and the FMRIB software library (FSL: (49). Functional volumes were aligned to the mean functional image using the FSL MCFLIRT program with sinc interpolation, and head motion parameters were estimated. Scans with scan-to-scan movement over 0.7 mm in any dimension were censored from analyses (< 2%). Next, slice-timing correction was performed using FSL slicetimer. Non-brain voxels were removed from functional images by masking voxels with extremely low intensities and by a brain-extraction algorithm implemented in FSL’s BET. Anatomical scans were registered to the MNI152 template (50) using both affine transformation (FSL FLIRT) and nonlinear deformation (FSL FNIRT). The alignment of functional images to each subject’s anatomical scan was computed using the white matter segmentation of each image and a boundary-based registration algorithm (51). Functional scans were then resampled into 3mm-isocubic voxels and warped into the MNI152 template’s space using the concatenation of the functional-structural and structural-MNI152 transforms. Images were spatially smoothed using a 5mm full-width at half-maximum kernel (FSL SUSAN). A .008 Hz temporal high-pass filter was then applied to remove slow-frequency-signal changes. Finally, images were normalized to a global-median intensity to allow for comparability of parameter estimates across subjects.
Statistical Analysis
For the purposes of the analyses the design was mixed, with condition (faces vs. shapes) as a block, and trials as events. Events were defined as the period between stimulus presentation and response (response times), convolved with condition (faces vs. shapes) (52) and with motor response (right vs. left). Each of resulting regressors was convolved with the canonical SPM5 double-gamma-hemodynamic-response function. Voxelwise BOLD signal was regressed on these estimates in the single-subject analyses using an AFNI’s 3dDeconvolve (48). Regressors included condition (faces vs. shapes) as the regressor of interest (aligned to stimulus onset and with a duration equal to the response time), as well as response time, motor responses (right vs. left), and the six motion parameters as nuisance regressors. Group differences in neural activation for faces versus shapes were estimated using AFNI’s 3dRegAna by regressing the beta weights against predictors that included depression, history of suicide attempts and age. Analyses of individual differences included the measure of interest (impulsivity, executive function, chronic interpersonal difficulties) in addition to depression and age in AFNI’s 3dRegAna.
To control type I error, we used a voxelwise threshold of p < .001. The cluster threshold for whole-brain analysis was set with Monte Carlo simulations based on the residual spatial smoothness of the derived maps using the AFNI’s 3dFWHM and 3dAlphaSim (48, 53) (17 voxels, yielding p < .05, corrected). Data from significant clusters in each map (task contrast, BIS, and SPSI-ICS) were extracted for further region of interest (ROI) analyses. In contrast to the whole-brain approach used with BIS and SPSI-ICS measures, we used an unbiased task-based ROI to test the association of the suicide attempt planning measure (SIS-planning) with the activity in the frontal operculum. Because the SIS-planning subscale is only applicable for the suicide attempters, the reduced sample size afforded insufficient power for whole-brain analysis.
Results
Main effect of task: faces versus shapes
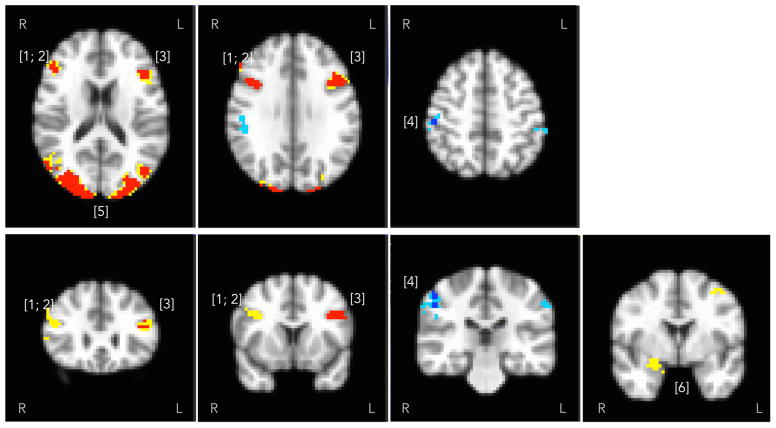
Figure 2 and Table 2 describe the network of regions activated by the task. At the conservative threshold of pvoxelwise < 0.001, pcorr < .05, viewing of angry faces positively modulated the BOLD signal in the visual areas (bilateral occipital cortex, Brodmann areas [BAs] 17 and 18) and bilateral frontal operculum (BA 44 and 45), and negatively modulated the signal in the right somatosensory cortex (BA 2). Figure 2 also shows positively-modulated activity in the amygdala that did not pass cluster-forming threshold (shown: 20 voxels at pvoxelwise < 0.005), but was observed within the MNI amygdala anatomical ROI (right: pvoxelwise = .001, left: pvoxelwise = .002, pcorr < .05 using a small volume correction for the amygdala).
Figure 2.
Task contrast (faces > shapes) positively modulated the blood oxygenation level-dependent signal in the prefrontal cortex ([1–3] right and left inferior frontal gyri; Brodmann areas [BAs] 44 and 45), occipital cortex ([5] BAs 17 and 18) and limbic regions ([6] right amygdala), shown in warm colors. The 20-voxel cluster in the right amygdala (psvc = .001) and the 15-voxel cluster in the left amygdala (psvc = .002, not shown) did not pass the whole-brain cluster threshold. Signal was negatively modulated in somatosensory areas, shown in cold colors ([4] - postcentral cortex; BA 2). Activity detected at Pcorr < .05, voxelwise threshold P.001 is shown in contrasting color over the activity detected at voxelwise P.005.
Table 2.
Functional MRI Activation for the Task Contrast (angry faces vs. shapes) in the Entire Sample.
| Region | MNI Coordinates | Peak t(47) | Cluster Size (mm3) |
|---|---|---|---|
| [1] Right Inferior Frontal Gyrus (BA 45) | 49, 33, 24 | 4.78 | 918 |
| [2] Right Inferior Frontal Gyrus (BA 44) | 41, 14, 36 | 6.07 | 1107 |
| [3] Left Inferior Frontal Gyrus (BA 45, 44) | −48, 19, 31 | 6.09 | 2484 |
| [4] Right Postcentral Gyrus / Primary somatosensory cortex (BA 2) | 53, −26, −30 | −5.25 | 1026 |
| [5] Occipital cortex (BA 17 and 18) | 2, −85, −6 | 10.70 | 86157 |
Comparison of responses to angry faces in suicide attempters vs. depressed vs. non-psychiatric comparison group
Our first hypothesis was not supported: responses to angry faces were not related to the history of attempted suicide or to major depression, even at the more liberal threshold of pvoxelwise = .005. Analyses using severity of depression measured by the HAM-D-16 (without suicide item) were similarly negative, controlling for age.
Responses to angry faces and impulsivity
Consistent with our second hypothesis, impulsivity was positively associated with prefrontal responses to angry faces, particularly in the left frontal operculum (Table 3 and Figure 3), controlling for age.
Table 3.
Indices of Impulsivity Co-varying for Age
| Social Problem Solving Inventory [Impulsivity/Carelessness Style subscale (45)]
| |||
|---|---|---|---|
| Region | MNI Coordinates | t(47) | Cluster Size (mm3) |
| [1] Dorsomedial prefrontal cortex, Left Superior Medial Gyrus | −7, 37, 42 | 4.56 | 675 |
| [2] Frontal operculum, Left Middle/Inferior Frontal Gyrus (BA 45) | − 43, 17, 39 | 4.93 | 3402 |
|
| |||
| Barratt Impulsiveness Scale (36)
| |||
| [3] Frontal operculum, Left Precentral Gyrus (BA 44) | −53, 8, 21 | 5.09 | 2754 |
Figure 3.
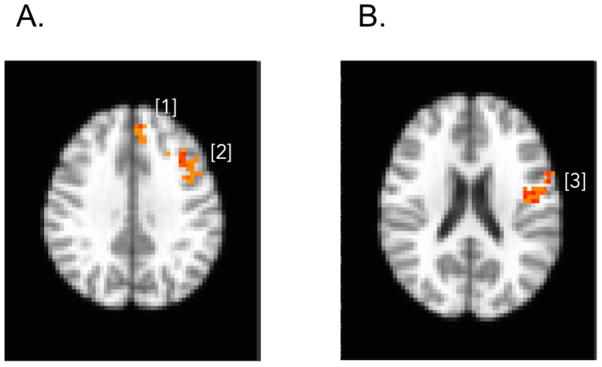
Brain activation map shows areas with greater BOLD response for high impulsivity compared with low in medial prefrontal cortex ([1] left superior medial gyrus) and the frontal operculum ([2] left middle/inferior frontal gyrus [BA 45]) for SPSI-ICS subscale, shown on Panel A, and the frontal operculum ([3] left precentral gyrus [BA 44]) for BIS, shown on Panel B.
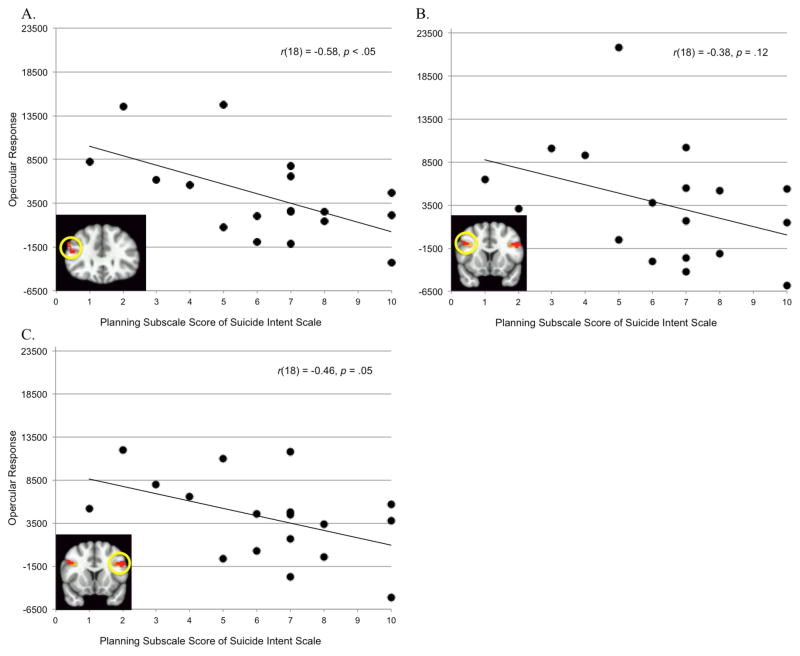
Furthermore, among suicide attempters, activity in the frontal operculum (independently-defined by the task contrast) was related to the attempt planning (SIS) (Figure 4). Exaggerated response was associated with poor attempt planning (in the right BA45: F[1,16] = 8.12, p < .05, ηp2 = .34; marginally-significant in the right BA44: F[1, 16] = 4.42, p < .06, ηp2 = .22; but did not reach significance in the left operculum: F[1,16] = 2.68, p < .13, ηp2 = .14). The effect sizes were reduced but effects were still present when we co-varied for age (BA 44: ηp2= .13, BA45: ηp2= .28, and left operculum: ηp2 = .08). Associations between the measures of impulsivity (self-reports and SIS-planning) and the frontal operculum are more fully illustrated in the supplemental materials.
Figure 4.
SIS-planning and activity in frontal operculum independently defined by task contrast (A: right BA 44; B: right BA 45; and C: left BA 44/45).
Exploratory analyses: responses to angry faces, executive function, and interpersonal dysfunction
Executive dysfunction, measured by the EXIT-25 (54), and chronic interpersonal difficulties, measured by the IIP-15, were not related to responses to angry faces.
Sensitivity analyses
The relationship between fronto-opercular responses to angry faces and impulsivity remained after controlling for lifetime substance use and anxiety disorders, severity of depression, burden of physical illness, cumulative antidepressant exposure in the current episode, and possible brain injury from suicide attempts (see supplemental material for the statistics).
Discussion
We found impulsivity-related individual differences in prefrontal responses to angry faces. However, we did not find evidence that processing of angry faces was altered in elderly suicide attempters as a group. Lending further credence to the association between impulsivity and prefrontal responses, individuals with more poorly-planned attempts also tended to have an exaggerated response in functionally similar prefrontal regions as identified by the task contrast. Our combined-sample analyses indicate a robust distributed response to angry faces consistent with the canonical pattern of activation (for a review see (55)). Specifically, we observed increased activation to angry faces versus shapes in the visual (occipital cortex), prefrontal (bilateral inferior frontal gyri), and limbic system regions (amygdala, parahippocampal gyrus, posterior cingulate), although the latter did not withstand the more conservative thresholding in the reported sample.
We detected no differences in neural responses to angry faces between older suicide attempters and the comparison groups, thus, failing to replicate the earlier findings of exaggerated lateral orbitofrontal (BA47) responses to angry faces in younger suicide attempters (25). Our findings of increased fronto-opercular responses (BA44,45) raise the possibility that impulsivity may have mediated the group differences observed by Jollant et al (25). Impulsivity measures were not reported for that sample, however, it is likely that the suicide attempters (adult males with mean age of 32) were more impulsive than the patient controls. The region mapped in our study is more dorsal, although it still lies in the granular lateral prefrontal “frontoinsular” cortex (56, 57), architectonically and functionally similar to the region identified by Jollant et al. Our more detailed model for single-subject analyses included reaction-time-convolved regressors for condition as well as stimulus presentation and motor response, while Jollant et al. included a block condition regressor only. The question remains whether the elderly in our study process emotional faces differently from younger adult males in Jollant et al.
We identified the frontal operculum as the primary locus of individual differences in self-reported trait impulsivity, social problem-solving, and planning of suicide attempts. This region implicates the cingulo-opercular network, thought to maintain stable control of goal-directed behavior and to allocate cognitive resources as information is processed downstream (58). Another influential account of cingulo-opercular function focuses on the stimuli’s salience (56) or informational value (59). In this context, our finding suggests that high impulsivity in the task context corresponds to greater salience of angry faces, resulting in excessive recruitment of cognitive resources (indexed by cortical processing).
Our results parallel those with the probabilistic reversal learning and delayed reward-discounting tasks (11, 44). Suicide attempts, particularly poorly planned ones, were related to blunted expected value signals in a paralimbic network centered around the ventromedial prefrontal cortex. This blunting was extreme in highly impulsive individuals and was paralleled by a tendency to neglect decision-relevant information on the probabilistic reversal and Cambridge Gamble tasks. Further, individuals with a history of unplanned suicide attempts showed an exaggerated preference for immediate versus delayed rewards and alterations in the gray matter of the basal ganglia (21, 44). While the regions implicated in each of these studies differ, neural alterations are invariably associated with facets of impulsivity. These alterations may underlie a failure to consider deterrents to suicide. In contrast, older adults who engage in premeditated suicidal behavior appear more intact in this regard, while displaying distinct cognitive and decision-making deficits (8, 60).
Limitations
Our failure to replicate earlier findings may be due to a relatively small sample size. In addition, the discrepancy in the findings across studies may be partly explained by the use of subtly different emotional faces tasks that may, therefore, target different neural mechanisms (61). At least one study offers evidence that the same psychopathology can reveal contrasting (and abnormal) patterns of functional activations depending on whether affected participants paid attention to emotional faces implicitly, by identifying the gender of faces, or explicitly, by matching facial expressions (62). Altogether, although emotional faces tasks may broadly probe the processing of the socio-emotional cues, there is a need to more carefully consider how task features may modulate the contribution of relevant neural mechanisms.
Conclusions
In summary, the present study did not find evidence of alterations during explicit processing of socio-emotional cues in older suicide attempters as a group. Instead, impulsivity and unplanned suicide attempts were associated with an exaggerated fronto-opercular response. This pattern may be indicative of disruptions at the fundamental level of socio-emotional processing, which may also contribute to the social problem-solving deficits experienced by the more impulsive suicide attempters. Taken together with prior research, these findings suggest that impulsive suicidal individuals may be neurobiologically distinct from other people with depression. In addition to depression treatment, these patients may benefit from pharmacologic and learning-based interventions targeting faulty decision processes subserved by cortico-limbic loops. A combination of trait impulsivity, behavioral indices of disadvantageous decision-making, and planning of suicide attempts may help define a subgroup of patients for future trials. Psychologically, impulsivity and impaired decision-making should be seen as a focus of treatment rather than a mere “character” flaw.
Supplementary Material
Acknowledgments
Support for this research comes from K23MH086620, K23MH070471, 5R01MH085651, P30MH90333, P60MD000207, UL1RR024153, and UL1TR000005 from the National Institute of Mental Health, John A. Hartford Foundation, American Foundation for Suicide Prevention, and the UPMC Endowment in Geriatric Psychiatry
Footnotes
Disclosures: Dr. Reynolds reports receiving pharmaceutical support for NIH-sponsored research studies from Bristol-Myers Squibb, Forest, Pfizer, and Lilly; receiving grants from the National Institute of Mental Health, National Institute on Aging, National Center for Minority Health Disparities, National Heart Lung and Blood Institute, Center for Medicare and Medicaid Services (CMS), Patient Centered Outcomes Research Institute (PCORI), the Commonwealth of Pennsylvania, the John A Hartford Foundation, National Palliative Care Research Center (NPCRC), Clinical and Translational Science Institute (CTSI), and the American Foundation for Suicide Prevention; and serving on the American Association for Geriatric Psychiatry editorial review board. He has received an honorarium as a speaker from MedScape/WEB MD. He is the co-inventor (Licensed Intellectual Property) of Psychometric analysis of the Pittsburgh Sleep Quality Index (PSQI) PRO10050447 (PI: Buysse).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC. Leading Causes of Death Reports. 2004 [Google Scholar]
- 2.Conwell Y, Van Orden K, Caine ED. Suicide in older adults. The Psychiatric clinics of North America. 2011;34:451–468. doi: 10.1016/j.psc.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 4.van Heeringen K, Mann JJ. The neurobiology of suicide. The Lancet Psychiatry. 2014;1:63–72. doi: 10.1016/S2215-0366(14)70220-2. [DOI] [PubMed] [Google Scholar]
- 5.Harrison KE, Dombrovski AY, Morse JQ, et al. Alone? Perceived social support and chronic interpersonal difficulties in suicidal elders. International Psychogeriatrics. 2010;22:445–454. doi: 10.1017/S1041610209991463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock LR, Williams JM. Problem solving and suicidal behavior. Suicide Life Threat Behav. 1998;28:375–387. [PubMed] [Google Scholar]
- 7.Gibbs LM, Dombrovski AY, Morse J, et al. When the solution is part of the problem: problem solving in elderly suicide attempters. International Journal of Geriatric Psychiatry. 2009;24:1396–1404. doi: 10.1002/gps.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szanto K, Clark L, Hallquist M, et al. The cost of social punishment and high-lethality suicide attempts. Psychology and Aging. 2014;29:84–94. doi: 10.1037/a0035339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szanto K, Dombrovski AY, Sahakian BJ, et al. Social Emotion Recognition, Social Functioning, and Attempted Suicide in Late-Life Depression. American Journal of Geriatric Psychiatry. 2012;20:257–265. doi: 10.1097/JGP.0b013e31820eea0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann JJ, Waternaux C, Haas GL, et al. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 11.Dombrovski AY, Szanto K, Clark L, et al. Reward Signals, Attempted Suicide, and Impulsivity in Late-Life Depression. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshel N, Roiser JP. Reward and Punishment Processing in Depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Waiter G, Ahearn T, et al. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 15.Korgaonkar MS, Fornito A, Williams LM, et al. Abnormal Structural Networks Characterize Major Depressive Disorder: A Connectome Analysis. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton JP, Furman DJ, Chang C, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gujral S, Dombrovski AY, Butters M, et al. Impaired executive function in contemplated and attempted suicide in late life. The American Journal of Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keilp JG, Gorlyn M, Russell M, et al. Neuropsychological function and suicidal behavior: attention control, memory and execut ive dysfunction in suicide attempt. Psychological Medicine. 2013;43:539–551. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachs-Ericsson N, Hames JL, Joiner TE, et al. Differences between suicide attempters and nonattempters in depressed older patients: Depression severity, white-matter lesions, and cognitive functioning. The American Journal of Geriatric Psychiatry. 2014;22:75–85. doi: 10.1016/j.jagp.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dombrovski AY, Siegle GJ, Szanto K, et al. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological Medicine. 2012;42:1203–1215. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh AA, Ambady N. The influence of the fear facial expression on prosocial responding. Cognition and Emotion. 2007;21:225–247. [Google Scholar]
- 23.Frank MG, Stennett J. The forced-choice paradigm and the perception of facial expressions of emotion. Journal of personality and social psychology. 2001;80:75. doi: 10.1037//0022-3514.80.1.75. [DOI] [PubMed] [Google Scholar]
- 24.Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25:909–919. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- 25.Jollant F, Lawrence N, Giampietro V, et al. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. American Journal of Psychiatry. 2008;165:740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 26.Pan L, Hassel S, Segreti A, et al. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychological medicine. 2013;43:2129–2142. doi: 10.1017/S0033291712002966. [DOI] [PubMed] [Google Scholar]
- 27.Domes G, Schulze L, Herpertz SC. Emotion recognition in borderline personality disorder-a review of the literature. Journal of personality disorders. 2009;23:6–19. doi: 10.1521/pedi.2009.23.1.6. [DOI] [PubMed] [Google Scholar]
- 28.Guitart-Masip M, Pascual JC, Carmona S, et al. Neural correlates of impaired emotional discrimination in borderline personality disorder: an fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1537–1545. doi: 10.1016/j.pnpbp.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Coccaro EF, McCloskey MS, Fitzgerald DA, et al. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proceedings of the National Academy of Sciences. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Leo D, Padoani W, Scocco P, et al. Attempted and completed suicide in older subjects: results from the WHO/EURO Multicentre Study of Suicidal Behaviour. Int J Geriatr Psychiatry. 2001;16:300–310. doi: 10.1002/gps.337. [DOI] [PubMed] [Google Scholar]
- 32.Dombrovski AY, Szanto K, Duberstein P, et al. Sex differences in correlates of suicide attempt lethality in late life. American Journal of Geriatric Psychiatry. 2008;16:905–913. doi: 10.1097/JGP.0b013e3181860034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frierson RL. Suicide attempts by the old and the very old. Archives of Internal Medicine. 1991;151:141. [PubMed] [Google Scholar]
- 34.Merrill J, Owens J. Age and attempted suicide. Acta Psychiatr Scand. 1990;82:385–388. doi: 10.1111/j.1600-0447.1990.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 35.Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. American Psychiatric Association; 1994. [Google Scholar]
- 36.First MSR, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) 1995. Version 2.0. [Google Scholar]
- 37.Beck AT, Shuyler D, Herman I. Development of suicidal intent scales. In: Beck AT, Resnik HLP, Lettieri DJ, Bowie MD, editors. The prediction of suicide. Charles Press; 1974. pp. 45–56. [Google Scholar]
- 38.Mieczkowski TA, Sweeney JA, Haas GL, et al. Factor composition of the Suicide Intent Scale. Suicide & Life-Threatening Behavior. 1993;23:37–45. [PubMed] [Google Scholar]
- 39.Sharma L, Markon KE, Clark LA. Toward a Theory of Distinct Types of “Impulsive” Behaviors: A Meta-Analysis of Self-Report and Behavioral Measures. 2013 doi: 10.1037/a0034418. [DOI] [PubMed] [Google Scholar]
- 40.D’Zurilla TJ, Nezu AM. Development and preliminary evaluation of the Social Problem-Solving Inventory. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2:156–163. [Google Scholar]
- 41.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Klonsky ED, May A. Rethinking impulsivity in suicide. Suicide and Life-Threatening Behavior. 2010;40:612–619. doi: 10.1521/suli.2010.40.6.612. [DOI] [PubMed] [Google Scholar]
- 43.Morse JQ, Pilkonis PA. Screening for personality disorders. J Personal Disord. 2007;21:179–198. doi: 10.1521/pedi.2007.21.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dombrovski AY, Szanto K, Siegle GJ, et al. Lethal Forethought: Delayed Reward Discounting Differentiates High- and Low-Lethality Suicide Attempts in Old Age. Biological Psychiatry. 2011;70:138–144. doi: 10.1016/j.biopsych.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 46.Ekman P, Friesen WV, Press CP. Pictures of facial affect. Consulting Psychologists Press; 1975. [Google Scholar]
- 47.Schneider W, Eschman A, Zuccolotto A. E-Prime reference guide. Psychology Software Tools Inc; Pittsburgh: 2002. [Google Scholar]
- 48.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 49.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 50.Fonov V, Evans A, McKinstry R, et al. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- 51.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christoff K, Prabhakaran V, Dorfman J, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 53.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 54.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. Journal of American Geriatrics Society. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 55.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 56.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuster JM. The prefrontal cortex. 4. Amsterdam ; Boston: Academic Press/Elsevier; 2008. [Google Scholar]
- 58.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jessup RK, O’Doherty JP. Distinguishing informational from value-related encoding of rewarding and punishing outcomes in the human brain. European Journal of Neuroscience. 2014 doi: 10.1111/ejn.12625. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGirr A, Dombrovski AY, Butters M, et al. Deterministic learning and attempted suicide among older depressed individuals: Cognitive assessment using the Wisconsin Card Sorting Task. Journal of Psychiatric Research. 2012;46:226–232. doi: 10.1016/j.jpsychires.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown SM, Manuck SB, Flory JD, et al. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- 62.Chen C-H, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.