Abstract
Objective
To assess the efficacy and safety of a 24-week course of abatacept in the treatment of active lupus nephritis. An additional exploratory objective was to assess the potential of abatacept to induce ‘clinical tolerance’, defined as sustained clinical quiescence of lupus nephritis after discontinuation of immunosuppressive therapy.
Methods
Patients (n=134) with active lupus nephritis were studied in a randomized, double-blind phase II add-on trial in which they received either abatacept or placebo in conjunction with the Euro-Lupus regimen of low-dose cyclophosphamide followed by azathioprine. The primary efficacy outcome was the frequency of complete response (CR) at week 24. Thereafter, patients who met either complete or partial response criteria continued blinded treatment through week 52. During this phase of the study, subjects in the abatacept treatment group who had achieved CR status at week 24 discontinued immunosuppressive therapy other than prednisone (10 mg/d).
Results
There were no statistically significant differences between groups with respect to the primary outcome or any of the secondary outcomes, including measures of safety. Thirty-three percent of subjects in the treatment group and 31% of subjects in the control group achieved CR status at week 24. Fifty percent of subjects in the treatment group who met CR criteria and therefore discontinued immunosuppressive therapy at week 24 maintained their CR status through week 52.
Conclusion
The addition of abatacept to a regimen of cyclophosphamide followed by azathioprine did not improve the outcome of lupus nephritis at either 24 or 52 weeks. No worrisome safety signals were encountered.
There are no consistently safe and effective treatments for lupus nephritis. Induction therapy for active nephritis typically consists of moderate-to-high dose glucocorticoids (GC) combined with an additional potent immunosuppressive drug, followed by maintenance therapy involving long-term sustained immune suppression [1]. Despite this aggressive approach to treatment, many patients continue with active nephritis and/or recurrent flares, and all patients are exposed to the risks of therapy, including the potential for fatal complications.
For several decades, the standard of care for active lupus nephritis consisted of monthly intravenous pulses of cyclophosphamide (CTX) for at least six months, with a target of achieving modest depression of circulating leukocyte counts between doses. This approach had emerged from a relatively small trial that compared high-dose GC alone with several alternative regimens consisting of GC in combination with other immunosuppressive agents [2]. Progression to renal failure occurred most often among patients who received GC alone. Although the trial did not distinguish convincingly among the various combination regimens, the community adopted pulse CTX as the preferred approach. In recent years, two other approaches have been compared to high-dose pulse CTX and appear to have equivalent efficacy. One approach is based on the Euro-Lupus Nephritis Trial (ELNT). It utilizes a shorter and less intense regimen of CTX followed by maintenance therapy with azathioprine (AZA) [3, 4]. The other approach utilizes mycophenolate mofetil (MMF) instead of pulse CTX [5–8]. There is reason to believe that these regimens may be safer than high-dose pulse CTX.
Against this background, there has been great hope that the advent of targeted biologic therapies would lead to breakthroughs in the treatment of lupus nephritis. Thus far, however, these hopes have not been realized [1, 9]. CTLA4Ig is among the biologic interventions that have generated great interest. The rationale for testing CTLA4Ig in lupus nephritis is very strong. CTLA4Ig blocks binding of antigen-presenting cells to CD28 on T cells, thereby inhibiting activation of primary T-dependent immune responses [10]. CTLA4Ig may also have direct inhibitory effects on the B cell lineage, as CD28 is expressed on plasma cells; whether CD28 engagement mediates positive or negative regulation remains an area of controversy [11–13]. In murine models for SLE, CTLA4Ig acts synergistically with CTX to arrest lupus nephritis [14, 15]. In humans, CTLA4Ig (abatacept) is effective in the treatment of rheumatoid arthritis [16, 17]. Moreover, a post-hoc analysis of a large trial of abatacept (ABA) in people with lupus nephritis strongly suggested clinical benefit [18]. Finally, a recent study of patients with focal segmental glomerulosclerosis showed that treatment with ABA induced disease remission, apparently by binding to CD80 on renal podocytes [19]. Taken together, these observations provide a strong foundation for postulating that ABA may be effective in people with lupus nephritis.
PATIENTS AND METHODS
Study design and treatment protocol
The ACCESS trial was a 1:1 randomized, double-blind, controlled phase II multicenter trial of ABA vs placebo on a background of treatment with GC plus CTX followed by AZA in patients with active lupus nephritis. The trial consisted of two phases. In the first phase, patients with active lupus nephritis were randomized to receive monthly infusions of either placebo or ABA. Subjects in both groups also received six biweekly pulses of CTX followed by oral AZA based on the ELNT regimen [3] as well as a tapering regimen of oral GC. The primary outcome measure was the proportion of subjects who achieved a complete response (CR) at week 24.
Treatment was initiated with monthly infusions of either placebo or ABA at doses that were adjusted for body weight according to the ABA dose that is recommended for rheumatoid arthritis (<60 kg, 500 mg; 60–100 kg, 750 mg; >100 kg, 1 gram). All patients received six intravenous pulses of 500 mg of CTX at two-week intervals followed by oral AZA at 2 mg/kg/d based on the ELNT regimen. This control regimen differed slightly from the original ELNT regimen with respect to the approach to GC treatment. Unlike the ELNT trial, the ACCESS trial did not employ an initial intravenous pulse of GC, but rather left that decision to the site investigator’s discretion. Oral GC treatment was begun at 60 mg/d for two weeks in all subjects, followed by a prescribed taper to 10 mg/d over the next 10 weeks.
The second phase of the trial (weeks 24–52) was exploratory and was intended to generate preliminary data regarding the potential of ABA to restore self-tolerance, defined as sustained quiescence of nephritis off immunosuppressive therapy. In this phase, patients who met CR criteria on ABA at week 24 then discontinued immunosuppression with ABA and AZA at week 28 and continued only on prednisone 10 mg/d. Patients who had achieved only a partial response on ABA continued therapy with monthly infusions of ABA and daily oral AZA. In the control group, patients who had achieved either a complete or partial response continued AZA. Patients who were non-responders at week 24 discontinued the trial at that point. Institutional review boards at all sites approved the study design, and all subjects provided written informed consent.
Study subjects
Eligible subjects were 16 years of age or older. They fulfilled the American College of Rheumatology (ACR) criteria for SLE, and they had a positive antinuclear antibody (ANA) and/or a positive anti-double-stranded DNA antibody test at study entry. All subjects had active lupus nephritis, defined by: (a) kidney biopsy documentation within the last 12 months of International Society of Nephrology/Renal Pathology Society (ISN/RPS) proliferative nephritis (class III or class IV, with or without features of class V); and (b) urine protein-to-creatinine ratio (UPCR) >1. Overall, 137 subjects were enrolled, of whom 134 met entry criteria and comprised the intent-to-treat population for the efficacy analysis. Study subjects came from 19 sites in the United States and two sites in Mexico. Enrollment began in November 2008 and concluded in June 2012.
Study endpoints and assessments
The primary efficacy endpoint was the proportion of subjects who achieved a complete renal response (CR) at week 24. CR was defined as all of the following: (i) UPCR <0.5 based on a 24-hour urine collection; (ii) serum creatinine ≤1.2 mg/dL or ≤125% of baseline; and (iii) adherence to the prednisone taper to 10 mg/d by week 12. Prospectively defined secondary efficacy endpoints included: (i) the proportion of subjects who achieved a partial response (PR) at week 24, defined by the same criteria as the CR definition except that the UPCR component of the PR definition required only a 50% improvement from baseline rather than a decline to <0.5; (ii) the proportion of subjects who met the UPCR and GC criteria for CR at week 24; (iii) the proportion of subjects who met the UPCR and GC criteria for PR at week 24; (iv) the proportion of subjects who met either CR or PR criteria at week 52; (v) the proportion of subjects who achieved CR status at week 24 and who maintained that response to week 52; (vi) time to CR or PR; and (vii) lupus disease activity as reflected by reduction of anti-dsDNA antibodies, resolution of hypocomplementemia (C3 or C4), patient global assessment, the 36-Item Short-Form Health Survey (SF36) score [20], and the British Isles Lupus Assessment Group (BILAG)-2004 score [21]. Secondary efficacy endpoints also included frequency of lupus flares, either renal or non-renal. For subjects who had achieved CR status at week 12 or any time thereafter, a renal flare was defined as recurrence of proteinuria >1 gm/24 hours. For all others, a renal flare was defined as either of the following: (i) serum creatinine at least 25% higher than baseline or above the upper limit of normal, plus proteinuria at least 75% of baseline; or (ii) doubling of UPCR compared with the lowest previous value. A non-renal flare was defined by the BILAG-2004 guidelines as any new ‘A’ finding in a nonrenal organ system. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Power/sample size
Using data from the ELNT [3, 4], the statistical analysis plan was based on the assumption that the proportion of CR outcomes at week 24 in the control group would be 20%. Our goal was to detect a 30% increase in the CR rate in the ABA group (50% compared to 20%). Subjects dropping out of the study prior to week 24 were handled as clinical response failures for the primary analysis. As such, after adjusting for an expected 10% dropout rate equally distributed to the two groups, this difference corresponds to an expectation of 45% CR in the ABA group and 18% CR in the control group. To detect this 27% difference at 90% power using a two-sided Fisher’s exact test at the 0.05 level of significance, a sample size of 67 subjects per group was required.
RESULTS
Study population
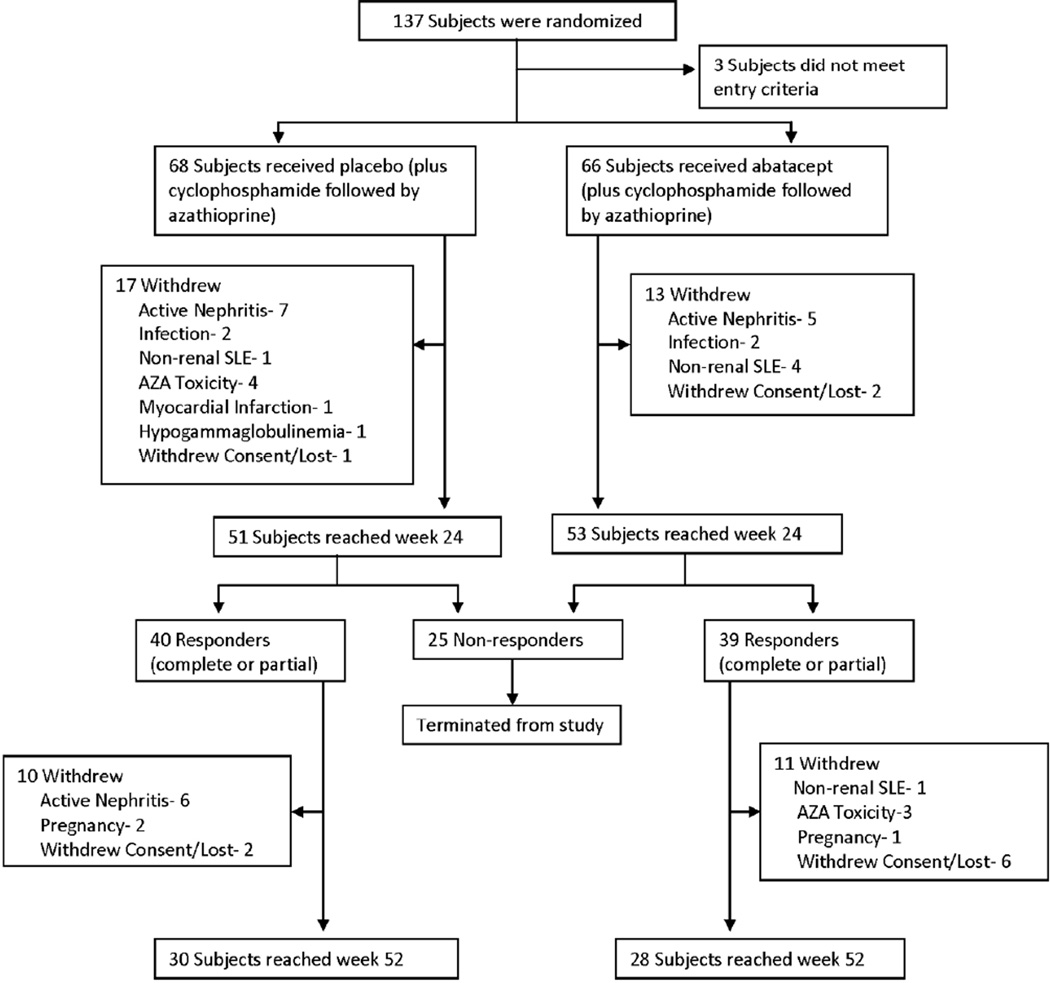
Sixty-eight subjects were randomized to the control group, and 66 subjects were randomized to the ABA treatment group (Figure 1). Baseline characteristics were well-matched between treatment groups (Table 1). Approximately 90% of subjects were women. The mean age at entry was 32 years. The study population was racially and ethnically diverse, including 39% African American subjects and 40% Hispanic or Mestizo subjects. Thirty-four percent of the subjects had ISN/RPS LN class III with or without features of class V, and 66% had class IV with or without features of class V. Forty-six percent of subjects in the control group and 41% of subjects in the treatment group entered the trial with UPCR>3. Seventy-one percent of subjects in each group had duration of nephritis <1 year. At the time of study entry, 60% of subjects were receiving an antimalarial drug and 73% of subjects were receiving either an angiotensin-converting-enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). The use of these agents was comparable between the two treatment groups.
Figure 1.
Treatment assignments and withdrawals in the intention-to-treat population.
Table 1.
Patient demographics and baseline characteristics*
| Variable | Control (n=68) |
Abatacept (n=66) |
|---|---|---|
| Age (years) – mean±SD | 32.7 ± 12.0 | 32.0 ± 10.1 |
| Females | 64 (94%) | 58 (88%) |
| Primary Race | ||
| White | 33 (49%) | 34 (51%) |
| Black | 25 (37%) | 27 (41%) |
| Asian | 3 (4%) | 3 (5%) |
| Mixed or Undeclared | 7 (10%) | 2 (3%) |
| Ethnicity | ||
| Hispanic/Mestizo | 28 (41%) | 25 (38%) |
| Weight (kg) | 75 ± 23 | 74 ± 18 |
| Blood pressure (mm Hg) | ||
| Systolic | 133 ± 17 | 130 ±17 |
| Diastolic | 83 ± 11 | 79 ± 12 |
| Time from onset of lupus nephritis | ||
| <1 year | 48 (71%) | 47 (71%) |
| ISN/RPS** classification | ||
| Class III | 11 | 10 |
| Class IV | 24 | 24 |
| Segmental | 8 | 10 |
| Global | 16 | 14 |
| Class III + V | 12 | 12 |
| Class IV + V | 20 | 20 |
| Renal function | ||
| Serum creatinine (mg/dl) | 1.3 ± 0.6 | 1.2 ± 0.7 |
| Estimated GFR (ml/min/1.73 m2) | 58 ± 28 | 65 ± 36 |
| Urine protein | ||
| 24-hour total (gm/d) | 4.5 ± 4.0 | 3.8 ± 3.1 |
| Protein:creatinine ratio (mg/mg) | 4.1 ± 3.4 | 3.6 ± 2.6 |
| UPCR >3 | 31 (46%) | 27 (41%) |
| Serum albumin (g/dl) | 2.7 ± 0.7 | 2.8 ±0.6 |
| Serology (at randomization) | ||
| ANA positive (≥1:80) | 68 (100%) | 66 (100%) |
| Anti-dsDNA positive | 50 (75%) | 49 (75%) |
| C3 complement low | 44 (70%) | 47 (78%) |
| C4 complement low | 37 (59%) | 39 (65%) |
| Patient global assessment | 45 ± 28 | 42 ±30 |
| SF-36 | ||
| Physical component score | 39 ± 10 | 39 ± 11 |
| Mental component score | 40 ± 13 | 40 ± 13 |
Except where noted, values are the number (%). Percentages are calculated out of the number of subjects with evaluable data.
ISN/RPS – International Society of Nephrology/Renal Pathology Society. Biopsies were read and classified by pathologists at the local sites.
Primary Outcome Measure
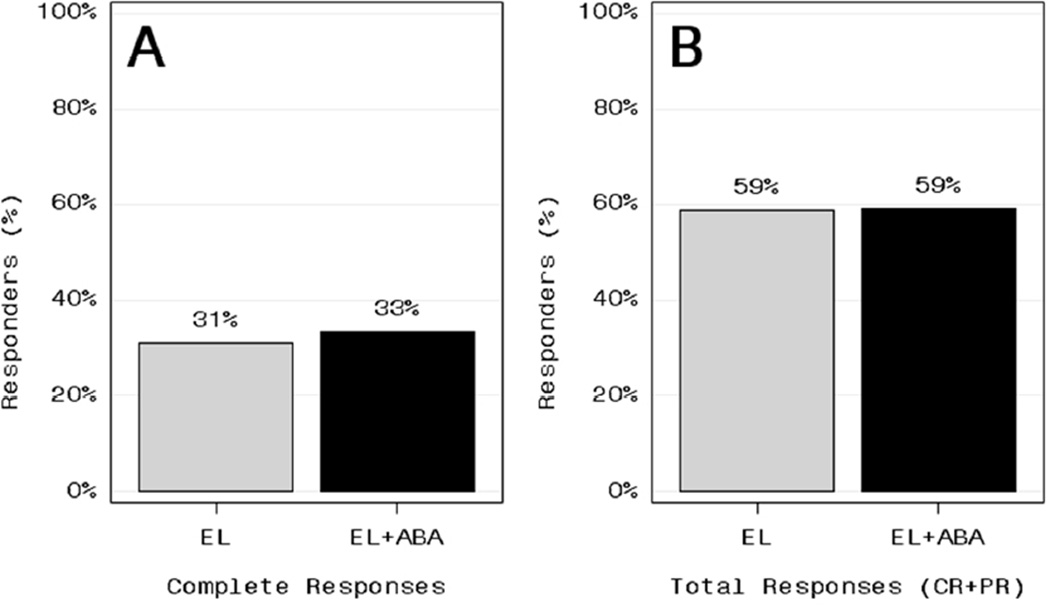
There was no significant difference in the CR rate at week 24 between the ABA and control groups (Figure 2a) (Fisher’s exact test). CRs occurred in 21/68 (31%) of control subjects and 22/66 (33%) of ABA-treated subjects.
Figure 2.
Complete response rate (A) and total response rate (complete responses plus partial responses) (B) at week 24 among control subjects treated with either the Euro-Lupus (EL) regimen or the EL regimen plus abatacpet (EL+ABA).
Secondary Efficacy Outcome Measures
The frequency of total responses (complete or partial) was identical in the two groups at 59% (Figure 2b). There also were no statistically significant differences in any of the other pre-specified secondary outcome measures (Table 2), including: (i) the proportion of subjects who met the proteinuria and prednisone requirements for CR or PR; (ii) the proportion of subjects who had a 75% reduction in anti-dsDNA antibodies; (iii) the proportion of subjects with negative anti-dsDNA; (iv) resolution of hypocomplementemia as measured by C3 or C4 levels; (v) time to CR or PR; (vi) patient global assessment; (vii) SF-36 physical and mental scores; (viii) BILAG-2004 score, or (ix) frequency of flares. The mean UPCR at week 24 was 1.1 ± 1.2 in the control group compared to 1.1 ± 1.3 in the ABA group (p=ns). The CR rate was lowest among Black subjects in the control group (16% vs 40% among non-Black control subjects), but no such difference was observed in the ABA group (33% CR in both Black and non-Black subjects) nor were any of the differences among racial and ethnic groups statistically significant. CR and PR rates were not significantly different when comparing subjects with recent onset of nephritis (<1 year) to subjects with a longer history of nephritis (≥1 year) (data not shown).
Table 2.
Secondary outcome measures at week 24*
| Outcome measure | Control | Abatacept |
|---|---|---|
| Urinary protein-to-creatinine ratio, mean ± SD | 1.1 ± 1.2 | 1.1 ± 1.3 |
| Disappearance of anti-dsDNA antibody | 4/36 (11) | 9/38 (24) |
| Correction of low C3 concentration | 11/30 (37) | 14/38 (37) |
| Correction of low C4 concentration | 10/25 (40) | 17/32 (53) |
| Total BILAG score, mean ± SD | 3.4 ± 1.8 | 3.8 ± 3.0 |
| Patient’s global assessment, mean ± SD | 28 ± 25 | 18 ± 22 |
| SF-36, mean ± SD | ||
| Physical component score | 45.3 ± 11 | 45.3 ± 11 |
| Mental component score | 46.5 ± 11 | 45.9 ± 12 |
| Subjects with lupus flares | ||
| Renal flare | 3/68 (4.4) | 3/66 (4.5) |
| Nonrenal flare | 6/68 (9) | 8/66 (12) |
| Complete response by race/ethnicity | ||
| Race | ||
| White | 14/33 (42) | 12/34 (35) |
| African American | 4/25 (16) | 9/27 (33) |
| Asian | 1/3 (33) | 1/3 (33) |
| Mixed or undeclared | 2/7 (29) | 0/2 (0) |
| Ethnicity | ||
| Hispanic/Mestizo | 10/28 (36) | 8/25 (32) |
| Not Hispanic/Mestizo | 11/40 (28) | 14/41 (34) |
| Total response by race/ethnicity† | ||
| Race | ||
| White | 21/33 (64) | 22/34 (65) |
| African American | 14/25 (56) | 15/27 (56) |
| Asian | 2/3 (67) | 1/3 (33) |
| Mixed race or undeclared | 3/7 (43) | 1/2 (50) |
| Ethnicity | ||
| Hispanic/Mestizo | 16/28 (57) | 15/25 (60) |
| Not Hispanic/Mestizo | 24/40 (60) | 24/41 (59) |
Except where indicated otherwise, values are the number of patients/number evaluated (%). Values for urinary protein-to-creatinine ratio were compared using a 2-sided t-test from an analysis of covariance (ANCOVA) model on log(urinary protein-to-creatinine ratio) that was adjusted for log(baseline values). Values for disappearance of anti—double-stranded DNA (anti-dsDNA) antibody and lupus flares were compared using a 2-sided Pearson’s chi-square test. Values for total British Isles Lupus Assessment Group (BILAG) score were compared using a 2-sided t-test from an analysis of variance model. Values for patient’s global assessment and Short Form 36 (SF-36) were compared using actual values between experimental and control groups and a 2-sided t-test from an ANCOVA model that adjusts for baseline values. None of the differences were statistically significant.
Total response included patients in whom a complete response or a partial response was achieved.
Safety
There were no clinically or statistically significant differences between groups at week 24 in total adverse events, lupus-related adverse events, serious adverse events, serious infectious adverse events, opportunistic infections, or withdrawals due to adverse events (Table 3). There was one death, due to sepsis, in the control group.
Table 3.
Proportion of subjects with AEs through week 24*
| Control (n = 68) |
Abatacept (n = 66) |
|
|---|---|---|
| Any AE | 56 (82) | 56 (85) |
| Infection-related AEs | 32 (47) | 31(47) |
| Grade 3 or higher AEs | 24 (35) | 21(32) |
| Infection-related grade 3 or higher AEs | 5 (7) | 8 (12) |
| Serious AEs | 20 (29) | 19 (28) |
| Deaths | 1 | 0 |
| AEs resulting in withdrawal from study | 17 (25) | 11(16) |
Values are the number (%). AE = adverse event.
Outcomes at Week 52
Forty subjects from the control group and thirty-nine subjects from the ABA group continued per protocol beyond the primary endpoint at week 24. Their outcomes are shown in Table 4. According to the protocol, the 22 subjects in the ABA group who met CR criteria at week 24 discontinued ABA and AZA at week 28 and continued on prednisone alone 10 mg/d thereafter. Eleven of these subjects (50%) maintained CR status through week 52, compared to 13/21 (62%) subjects in the control group who maintained CR status while continuing AZA through week 52 (p=ns). One subject in each group deteriorated from CR at week 24 to PR at week 52. Two subjects in the ABA group and four subjects in the control group either withdrew due to a renal flare or failed to meet either CR or PR criteria at week 52. One patient in the ABA group withdrew due to a non-renal flare, and several subjects in each group withdrew for reasons unrelated to lupus or study treatment. Overall, 13 subjects in the control group withdrew from the trial due to active nephritis, compared to 5 subjects in the ABA group (Fig. 1).
Table 4.
Outcome at week 52*
| Control | Abatacept | |
|---|---|---|
| Patients with a complete response at week 24 | ||
| No. of patients | 21 | 22 |
| Status at week 52 | ||
| Complete response, no. (%) | 13 (62) | 11 (50) |
| Partial response | 1 | 1 |
| Loss of renal response† | 4 | 2 |
| Withdrew (nonrenal SLE flare) | 0 | 1 |
| Withdrew (unrelated to SLE or abatacept) | 3 | 7 |
| Patients with a partial response at week 24 | ||
| No. of patients | 19 | 17 |
| Status at week 52 | ||
| Complete response, no. (%) | 9(47) | 7(41) |
| Partial response | 4 | 6 |
| Loss of renal response† | 5 | 1 |
| Withdrew (nonrenal SLE flare) | 0 | 0 |
| Withdrew (unrelated to SLE or abatacept) | 1 | 3 |
| Total response at week 52, no./no. evaluated (%)‡ | 27/40(68) | 25/39(64) |
Except where indicated otherwise, values are the number of patients. SLE = systemic lupus erythematosus.
Either withdrew due to renal flare or did not meet complete response or partial response criteria at week 52.
Total response included patients in whom a complete response was achieved and patients in whom a partial response was achieved.
All subjects who were classified as PR at week 24 continued their immunosuppressive treatment during weeks 24–52. Again, there were no significant differences between groups. The most common outcome in both groups was to improve from PR status at week 24 to CR status at week 52 (Table 4).
There were no statistically significant differences in any of the secondary outcome measures at week 52 (not shown). With regard to safety between weeks 24 and 52, there were ten SAEs (5 in each group), no discontinuations due to infection, and no deaths.
DISCUSSION
This trial did not demonstrate any benefit for ABA when added to a regimen consisting of low-dose pulse CTX followed by AZA in patients with lupus nephritis. This finding suggests that ABA may not be effective in lupus nephritis. However, there are alternative explanations that might account for the outcome. This trial explored only one dose regimen, which was based on the dose of ABA that is approved for the treatment of rheumatoid arthritis. It is possible, therefore, that a higher dose might be required for lupus nephritis. Background therapy may be important. In this trial, we chose to use CTX as the foundation for the background regimen, based on studies in murine lupus suggesting potential synergy between ABA and CTX [14, 15]. By using a low-dose approach to CTX therapy, we may have used too little to achieve a synergistic benefit. A post-hoc analysis of a prior trial of ABA in lupus nephritis suggested possible benefit on a background of MMF [18], so perhaps a combination with MMF would be more effective. Finally, this add-on trial demonstrated that ABA does not provide additional benefit when superimposed on background therapy initiated with high-dose GC followed by CTX and AZA. However, it does not establish whether ABA might demonstrate comparable efficacy in a head-to-head trial design. In that context, it is even possible that the background GC therapy or the multiple doses of CTX in this trial interfered with the mechanism of action of ABA. It may be noteworthy in that respect that the preclinical mouse studies that contributed to the foundation for this trial did not employ GC at all. Although the alternative explanations for the trial results are all plausible, it would be a daunting task to put them to the test.
A unique aspect of this trial was the opportunity it provided to acquire preliminary data on the impact of discontinuing immunosuppressive therapy in patients who achieve CR status within 24 weeks. This opportunity resulted from three factors. First, there is a biologic rationale for postulating that CTLA4Ig might induce tolerance among autoreactive T cells [10, 22]. Second, studies in mice indicate that the beneficial effect of CTLA4Ig on murine lupus nephritis persists even after treatment is discontinued [23]. Third, this trial was supported by the Immune Tolerance Network, which has a mission to evaluate therapies that have the potential to induce clinical tolerance, defined as quiescence of autoimmune disease in the absence of ongoing immunosuppressive therapy. This goal is particularly important in lupus, where it is unknown whether the risks of long-term immunosuppression exceed the risks of discontinuing therapy in patients who achieve a complete response. There is little information in the literature to address this issue. One trial demonstrated that continued immunosuppression with pulse methylprednisolone plus CTX was preferable to reliance on pulse methylprednisolone alone as maintenance therapy, but that result was based on an examination of the overall study population and did not focus on the minority of subjects who had achieved complete responses [24]. Similarly, retrospective analyses of longitudinal cohorts have demonstrated the benefit of maintenance therapy for the entire cohort, but have not provided data about the risk:benefit ratio specifically for subjects without evidence of active disease [25]. A recent report described successful withdrawal of immunosuppressive therapy from lupus nephritis patients in remission, but in that report the duration of treatment for lupus nephritis varied between 2.5 and 10 years prior to gradual tapering and eventual discontinuation of therapy (26). In the ACCESS trial, we discontinued immunosuppressive therapy in 22 subjects in the ABA treatment group who met CR criteria at week 24, because those were the subjects in whom the scientific rationale for discontinuing therapy was strongest. Eleven of those subjects maintained their CR status through week 52. The 50% success rate at maintaining CR in this group was similar to the 62% (13/21) success rate among subjects in the control group maintained on AZA who met CR criteria at week 24. These exploratory findings cannot be interpreted to imply that ABA contributed to the sustained quiescence, but they raise the possibility that, once a complete response is achieved, it may be possible to discontinue immunosuppression, monitor patients closely, and avoid the risks of ongoing immunosuppression. There was no difference between the groups in the number of subjects who lost their renal response or had a non-renal flare between weeks 24 and 52 (3/22 complete responders who discontinued immunosuppressive therapy compared to 4/21 complete responders who continued AZA maintenance therapy). The number of subjects in this exploratory analysis is small, but the results suggest that further study is warranted to determine whether maintenance therapy should be continued after establishment of CR.
The ACCESS trial provided an important opportunity to explore the effectiveness of the Euro-Lupus treatment strategy in a racially and ethnically diverse North American population. Previous studies of this regimen strongly suggested that a less aggressive approach to pulse CTX might be as effective as, and safer than, the more intense CTX regimen that has long been the standard of care [3, 4]. However, those studies involved primarily Northern European lupus patients, of whom most were White patients with newonset rather than refractory nephritis. Due to the nature of the study population, there has been a reluctance to generalize those results to other populations, especially Black and Hispanic populations who tend to have more severe and refractory disease [27–32]. By succeeding in recruiting a racially and ethnically diverse population of lupus patients that more closely resembles the overall demographics of lupus, we have been able to show that the response rates for the Euro-Lupus regimen in this population closely match, or slightly exceed, the response rates for high-dose CTX or MMF reported from other trials [6, 9], although the efficacy of this regimen in Blacks warrants further study.
There have now been two trials of ABA in patients with lupus nephritis. Neither trial achieved its primary outcome goal. The prior trial employed a control regimen of MMF rather than CTX/AZA [18]. Although that trial failed to meet its primary endpoint, a post-hoc analysis suggested that there may have been efficacy that wasn’t captured by the prospectively defined endpoint [18]. Therefore, a second large, multicenter international trial of ABA on a background of MMF is currently underway (NCT01714817). The results of that trial will provide additional data to determine whether ABA will have a role in the treatment of lupus nephritis.
Acknowledgments
This research was performed as a project of the Immune Tolerance Network (NIH contract N01-AI-15416; protocol number ITN034AI), an international research consortium headquartered at the University of California, San Francisco. The Immune Tolerance Network is funded by the National Institute of Allergy and Infectious Diseases. Abatacept was provided by Bristol-Myers Squibb (BMS).
The principal investigators of the ACCESS Trial, Betty Diamond and David Wofsy, have received consulting fees from BMS in the past, but did not receive any remuneration from BMS for the conduct or analysis of this trial. Their participation in this trial was supported in part by their institutions, the Feinstein Institute for Medical Research at Northshore-Long Island Jewish Health System and the Russell/Engleman Rheumatology Research Center at the University of California San Francisco.
REFERENCES
- 1.Hahn BH, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64(6):797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin HA, 3rd, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314(10):614–619. doi: 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- 3.Houssiau FA, et al. Immunosuppressive therapy in lupus nephritis: the Euro- Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–2131. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 4.Houssiau FA, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69(1):61–64. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 5.Ginzler EM, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 6.Appel GB, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dooley MA, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886–1895. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 8.Chan TM, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343(16):1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 9.Rovin BH, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 10.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 11.Bahlis NJ, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109(11):5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair JR, et al. CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment. J Immunol. 2011;187(3):1243–1253. doi: 10.4049/jimmunol.1100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Njau MN, et al. CD28-B7 interaction modulates short- and long-lived plasma cell function. J Immunol. 2012;189(6):2758–2767. doi: 10.4049/jimmunol.1102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daikh DI, Wofsy D. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J Immunol. 2001;166(5):2913–2916. doi: 10.4049/jimmunol.166.5.2913. [DOI] [PubMed] [Google Scholar]
- 15.Schiffer L, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171(1):489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 16.Genovese MC, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 17.Kremer JM, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 18.Wofsy D, Hillson JL, Diamond B. Abatacept for lupus nephritis: alternative definitions of complete response support conflicting conclusions. Arthritis Rheum. 2012;64(11):3660–3665. doi: 10.1002/art.34624. [DOI] [PubMed] [Google Scholar]
- 19.Yu CC, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369(25):2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHorney CA, et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Hay EM, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86(7):447–458. [PubMed] [Google Scholar]
- 22.Dai Z, Lakkis FG. The role of cytokines, CTLA-4 and costimulation in transplant tolerance and rejection. Curr Opin Immunol. 1999;11(5):504–508. doi: 10.1016/s0952-7915(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 23.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265(5176):1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 24.Illei GG, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001;135(4):248–257. doi: 10.7326/0003-4819-135-4-200108210-00009. [DOI] [PubMed] [Google Scholar]
- 25.Mok CC, et al. Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum. 2004;50(8):2559–2568. doi: 10.1002/art.20364. [DOI] [PubMed] [Google Scholar]
- 26.Moroni G, et al. What happens after complete withdrawal of therapy in patients with lupus nephritis. Clin Exp Rheumatol. 2013;31(Suppl. 78):S57–S81. [PubMed] [Google Scholar]
- 27.McCarty DJ, et al. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38(9):1260–1270. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez M, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57(4):576–584. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 29.Pons-Estel GJ, Alarcon GS. Lupus in Hispanics: a matter of serious concern. Cleve Clin J Med. 2012;79(12):824–834. doi: 10.3949/ccjm.79a.12048. [DOI] [PubMed] [Google Scholar]
- 30.Richman IB, et al. European genetic ancestry is associated with a decreased risk of lupus nephritis. Arthritis Rheum. 2012;64(10):3374–3382. doi: 10.1002/art.34567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez LA, et al. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus. 2013;22(12):1214–1224. doi: 10.1177/0961203313502571. [DOI] [PubMed] [Google Scholar]
- 32.Feldman CH, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–763. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]