Abstract
We previously demonstrated that intestinal myofibroblasts from immature tissue produce excessive IL-8 in response to Escherichia coli lipopolysaccharide (LPS) compared to cells from mature tissue. However, it is unknown whether other cytokines and TLR agonists contribute to this developmentally regulated response. The aim of this study was to further characterize differences in inflammatory signaling in human primary intestinal fibroblasts from fetal (FIF) and infant (IIF) tissue and examine their potential to activate the adaptive immune response in vitro. Cytokine profiles of LPS-stimulated FIF and IIF were assessed by cytokine profile array. IL-8, IL-6, and IL-10 production in response to TLR2, TLR2/6, TLR4, and TLR5 agonists was determined by quantitative ELISA. The potential of activated myofibroblasts to activate adaptive immunity was determined by measuring surface class II MHC expression using flow cytometry. LPS-stimulated FIF produced a distinct proinflammatory cytokine profile consisting of MCP-1, GRO-alpha, IL-6, and IL-8 expression. FIF produced significant IL-8 and IL-6 in response to TLR4 agonist. IIF produced significant levels of IL-8 and IL-6 in the presence of TLR5 and TLR2 agonists. IFN-γ-treated FIF expressed greater HLA-DR levels compared to unstimulated controls and IFN-γ- and LPS-treated IIF. Activated FIF produce a more diverse inflammatory cytokine profile and greater levels of IL-8 and IL-6 in response to TLR4 stimulation compared to IIF. FIF express class II MHC proteins associated with activation of the adaptive immune response. These data suggest that FIF may contribute to bacterial-associated gut inflammation in the immature intestine.
Introduction
The healthy intestinal mucosa is lined by a layer of epithelial cells that form tight junctions at their apical portions. Intestinal tight junctions function as a selective barrier between luminal contents and the underlying lamina propria. A compromised or immature intestinal barrier may result in bacteria, toxins, or other antigens accessing the submucosal compartment with subsequent activation of immune and immune-like mesenchymal cells. Activation of these cells may incite an exaggerated local and systemic immune response in the immature host, especially preterm very low birth weight (VLBW, birth weight<1,500 g) infants (Luoto and others 2013). The immature host therefore has a substantially higher risk of developing inflammatory-associated bowel diseases such as necrotizing enterocolitis (NEC), milk protein- or food-related allergy, and atopic disease as a result of a compromised intestinal barrier (Arvola and others 2006; Shiou and others 2011; Pietzcker and others 2012).
Subepithelial intestinal myofibroblasts (IMFs) are submucosal mesenchymal cells that lie adjacent to intestinal epithelial cells (IEC) at the base of intestinal crypts. They regulate several aspects of intestinal barrier function, including the innate and adaptive immune response. IMFs contribute to intestinal barrier repair by modulating glandular secretion (Beltinger and others 1999), epithelial cell differentiation (Joyce and others 1987; Fritsch and others 2002), proliferation and mobility (McKaig and others 1999), and secretion of extracellular matrix (Inatomi and others 2007).
The intestinal inflammatory response is also regulated by IMFs, which secrete cytokines, prostaglandins, and various growth factors. They contribute to tissue injury and healing in patients with inflammatory bowel disease (Powell and others 1999; Andoh and others 2007; De and others 2007; Di Sabatino and others 2007a, 2007b). In our previous study, we observed that human fetal intestinal fibroblasts produce excessive amounts of IL-8 in response to lipopolysaccharide (LPS) stimulation compared to cells from more mature tissue (Okogbule-Wonodi and others 2012). However, the role of other cytokines in this differential response is unknown.
Pathogen-associated molecular patterns engage toll-like receptors (TLRs) thereby initiating the innate immune response. Although we recently demonstrated that TLR4 partially mediates the hyper-responsiveness of human fetal intestinal fibroblasts to LPS stimulation leading to greater IL-8 production (Okogbule-Wonodi and others 2012), it is not known if other TLR agonists contribute to this developmental-dependent response.
We hypothesize that fetal IMFs may contribute to immune dysregulation in the premature gut. Our objective in this study was to further characterize the differences in the inflammatory responses between IMFs from immature and more mature tissue by comparing cytokine profiles in response to LPS stimulation and analyze the effects of TLR agonists on the acute-phase inflammatory response as well as the potential to initiate an adaptive immune cellular response.
Materials and Methods
Reagents
Ultrapurified Escherichia coli (E. coli) 0111:B4 LPS was purchased from List Biologicals Laboratory, Inc. (Campbell, CA). Purified Pam2CGDPKHPKSF, FSL-1 (TLR2/TLR6), and Pam2CSK4 (TLR2) agonists were obtained from InvivoGen (San Diego, CA). Flagellin (TLR5 agonist) was purified from Salmonella typhi and provided as a kind gift from Dr. Raphael Simon (Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, MD). The Proteome Profiler™ Human Cytokine Array Panel A, DuoSet human ELISA Kits, and recombinant human interferon gamma (IFN-γ) were purchased from R&D Systems (Minneapolis, MN). The ECL™ Prime Western Blotting Detection Reagent was purchased from GE Healthcare (Piscataway, NJ). Anti-human HLA-DR APC and mouse IgG2b K Isotype Control APC were obtained from eBioscience (San Diego, CA).
Intestinal fibroblast cell culture
The primary fetal intestinal myofibroblast (FIF) cell isolate, designated as CCD-112CoN by ATCC (Manassas, VA), was isolated from normal fetal intestine at the second trimester. They were received at population doubling level (PDL) 24, begin to senesce at PDL 76, and were split at a ratio of 1:2. The maximum PDL used for experiments was 28. A primary infant intestinal myofibroblast (IIF) cell isolate, designated by ATCC as CCD-18Co, was isolated from normal intestine of a 2.5-month-old infant and received at PDL 27. These cells begin to senesce at PDL 42 and the maximum PDL used was 32 splitting at a ratio of 1:4. Cells were cultured in DMEM (Corning Cell Gro. Manassas, VA), supplemented with 10% fetal bovine serum (Quality Biological, Inc., Gaithersberg, MD), Pen-Strep (10,000 U/mL penicillin, 100 μg/mL streptomycin; Calbiochem, La Jolla, CA), and 1% MEM Non-Essential Amino Acid Solution (Mediatech, Manassas, VA) and maintained at 37°C in 5% CO2. The medium was changed 3 times weekly. Cells were passaged 3-4 times after removal from liquid nitrogen and seeded at a density of 4 to 8×105/mL for cell culture assays.
Cytokine profile array
Supernatants from FIFs and IIFs stimulated with 0.1 μg/mL of LPS were evaluated for cytokine expression using the Proteome Profiler™ Human Cytokine Array Panel A (R&D Systems) based on the manufacturer's instructions. We chose to use cell culture supernatants stimulated with this concentration of LPS because it was the lowest dose that led to significant IL-8 production in our previous experiments (Okogbule-Wonodi and others 2012). Triplicate samples from each treatment group were pooled. Briefly, supernatants diluted 1:6 were mixed with the biotinylated detection antibody cocktail. The mixture was applied to the proteome membranes spotted with cytokine capture antibodies. The membranes were washed to remove unbound sample mixture and developed by chemiluminescence detection using Streptavidin-HRP and the ECL detection system. Quantification of each cytokine was accomplished by pixel density analysis.
TLR assay
Cells seeded in 96-well tissue culture plates were grown to ∼ 80% confluence and stimulated with increasing concentrations of TLR2 agonist Pam2CSK4 (1–100 ng/mL), TLR2/TLR6 agonist FSL-1 (1–100 ng/mL), and TLR5 agonist S. typhi flagellin (10–50 ng/mL). Cells cultured in media alone or stimulated with 1 μg/mL LPS served as negative and positive controls, respectively. Cell culture supernatants were collected after 24 h and analyzed for IL-8, IL-6, and IL-10. We used 1 μg/mL LPS for this and subsequent experiments. Similar to 0.1 μg/mL of LPS used in the cytokine profile array experiments, 1 μg/mL LPS stimulated significant levels of IL-8 production that was consistent across the different experimental assays used in this study.
Cytokine quantification
IL-8, IL-6, and IL-10 were quantified in cell culture supernatants using capture DuoSet® human ELISA Kits (R&D Systems) according to the manufacturer's instructions. The cytokine concentrations from each sample were calculated from a curve fitted to standards. Concentrations are expressed as pg/mL supernatant.
Flow cytometry
IMFs were stimulated with 100 U/mL of interferon gamma (IFN-γ) for 7 days and subsequently with 1 μg/mL LPS for 24 h. Cells cultured in media or with LPS alone served as negative and positive controls, respectively. At the end of the incubation period, culture supernatants were discarded and IMFs washed ×3 in PBS and harvested from plates using a cell dissociation buffer. Cells were stained with APC-conjugated anti-human HLA-DR or APC mouse IgG2b K isotype control according to the manufacturer's instructions and fixed in 2% paraformaldehyde. HLA-DR cell surface expression was measured with a BD LSR flow cytometer and data analyzed by FlowJo7 software (Tree Star). Median fluorescence intensity was calculated and compared to isotype and unstimulated controls.
Statistical analysis
Experimental conditions were performed in triplicate except where indicated and each experiment was repeated at least 2–3 times. Data are represented as mean±SD. Multiple comparisons were conducted using analysis of variance (ANOVA) with Tukey post hoc and Student t-tests. A P value<0.05 was considered significant. Statistical analysis was performed using SPSS 12.0 (IBM Corp., Armonk, NY).
Results
LPS-stimulated fetal intestinal fibroblasts produce a novel cytokine profile
Our previous observations demonstrated a differential response between fetal and infant cell IL-8 production (Okogbule-Wonodi and others 2012). To more accurately phenotype the soluble factors produced by LPS-activated fibroblasts, a membrane-bound cytokine capture blotting system was used to analyze over 30 cytokines. The conditioned media of LPS-stimulated and unstimulated FIF and IIF were compared (Fig. 1). Conditioned media of the LPS-stimulated FIF produced a unique cytokine profile when compared to media from the other cell populations. Significantly elevated cytokines secreted by activated FIF included the monocyte chemoattractant protein-1 (MCP-1, 0 vs. 10,303±725, P=0.002), growth-related oncogene-alpha (GRO-alpha, 0 vs. 10,771±666, P=0.002), and IL-6 (0 vs. 12,003±548, P=0.013). Elevated levels of IL-8 (0 vs. 16,463±752, P=0.001) were also confirmed. In addition to these 4 factors, the macrophage migration inhibitory factor (MIF) and Serpin E1 were present in the supernatants of all 4 groups. The cytokines, not shown in Fig. 1, were not detected in the supernatants.
FIG. 1.
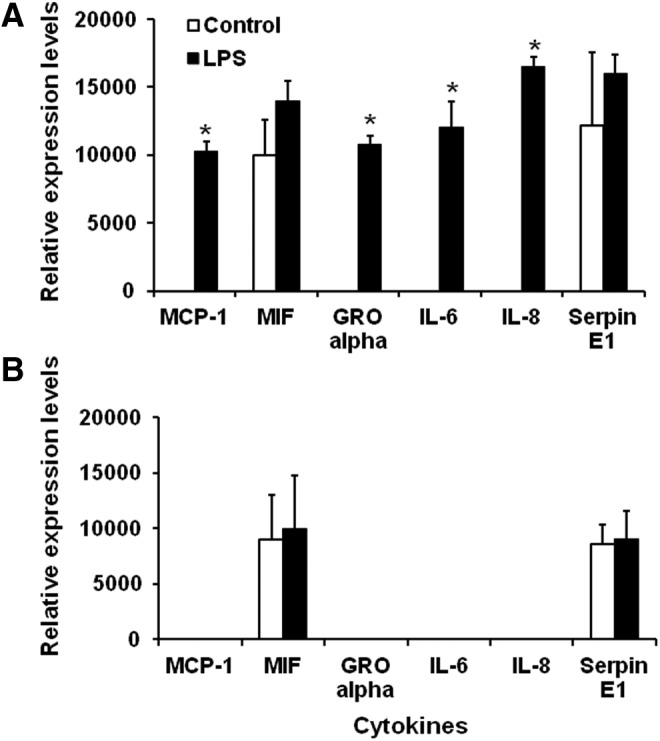
Cytokine profile of lipopolysaccharide (LPS)-stimulated fetal and infant intestinal myofibroblasts. Cytokine profile of LPS-stimulated fetal cells is consistent with acute inflammation. Cytokine profiler array measurements of (A) fetal intestinal myofibroblasts (FIF) and (B) infant intestinal fibroblasts (IIF) grown to confluence and stimulated with 0.1 μg/mL LPS for 24 h. Culture supernatants were incubated on membranes possessing capture antibodies for 30 different chemokines/cytokines. Data are expressed as relative pixel density. Results are reported as mean±SD and are representative of at least 3 experiments. *P<0.05, cytokines versus media control. LPS-stimulated FIF compared to controls expressed significant levels of monocyte chemoattractant protein-1 (MCP-1, 10,303±725 vs. 0, P=0.002); growth-related oncogene-alpha (GRO-alpha, 10,771±666 vs. 0, P=0.002); IL-6 (12,003±548 vs. 0, P=0.013); and IL-8 (16,463±752 vs. 0, P=0.001). Serpin E1 expression was comparable in stimulated and control FIF (15,966±1,455 vs. 12,193±5,334, P=0.4). LPS-stimulated and control IIF expressed similar levels of Macrophage Migration Inhibitory Factor (MIF, 10,007±4,784 vs. 9,018±3,998, P=0.33) and Serpin E1 (9,009±2,555 vs. 8,546±1,753, P=0.56). MCP-1, GRO-alpha, IL-6, and IL-8 were not expressed by IIF.
TLR2 agonists induce less IL-8 and IL-6 production by fetal intestinal fibroblasts compared to infant cells
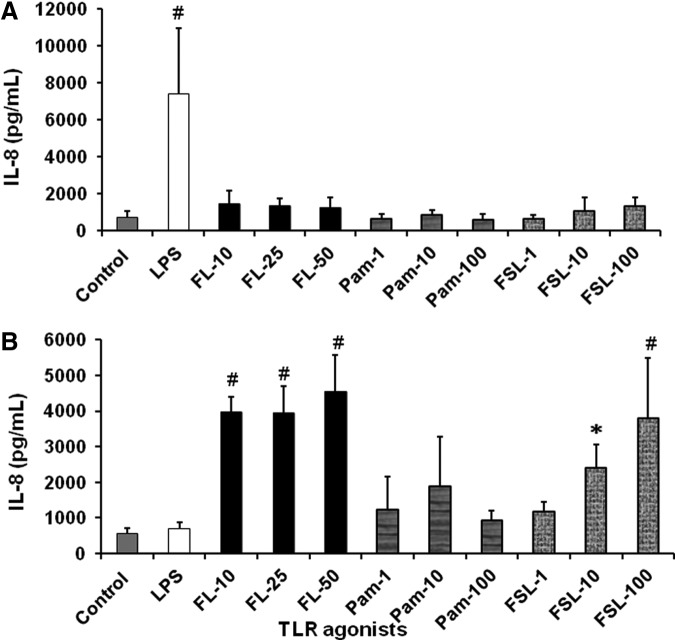
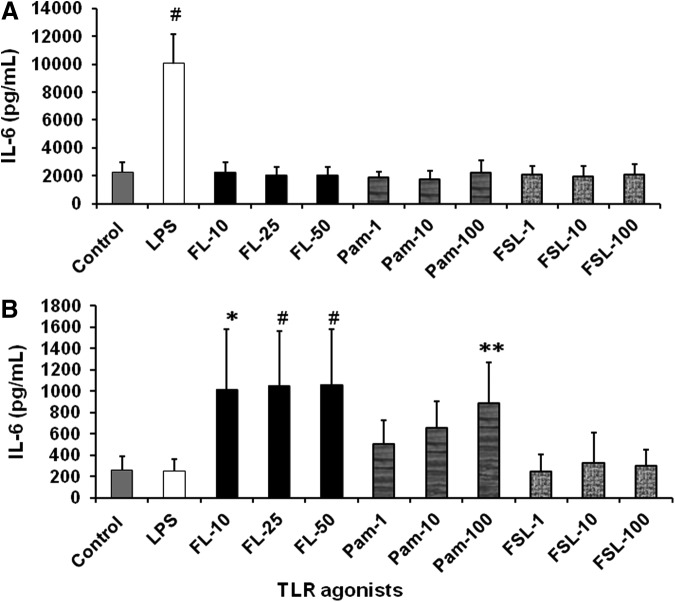
To identify the role of various TLRs in proinflammatory mediator production by FIF and IIF, we used agonists to stimulate TLR 2, TLR2/6 heterodimer, TLR4, and TLR5. Similar to our previous published findings (Okogbule-Wonodi and others 2012), FIF (Fig. 2A) stimulated with the TLR4 agonist, LPS led to a 10-fold increase in IL-8 secretion compared to unstimulated controls (7,397±3,554 pg/mL vs. 738±307 pg/mL, P<0.001). However, flagellin, Pam2CSK4, and FSL-1, which are TLR5, TLR2, and TLR2/TLR6 agonists, respectively, did not stimulate significant IL-8 production by FIF. IIF (Fig. 2B) appeared to respond to flagellin in a dose-dependent manner. There was a 7.1-fold (3,973±415 pg/mL, P<0.0001), 7.1-fold (3,937±770 pg/mL, P<0.0001), and 8.2-fold (4,556±1024 pg/mL, P<0.0001) increase in IL-8 production in response to 10, 25, and 50 ng/mL flagellin, respectively, versus unstimulated controls (556±153 pg/mL). IIF stimulated with 10 and 100 ng/mL FSL-1 produced 4.3-fold (2,406±656 pg/mL, P=0.001) and 2.2-fold (3,796±1,689 pg/mL) greater levels of IL-8, respectively, compared to unstimulated control cells (556±153 pg/mL). IL-6 production by LPS-stimulated FIF (Fig. 3A) was 4.5-fold greater compared to controls (10,040±2,109 pg/mL vs. 2,238±700 pg/mL, P<0.0001). Flagellin-induced IL-6 secretion by IIF (Fig. 3B) was dose dependent. Stimulation with 10, 25, and 50 ng/mL led to a 4-fold (1,011±566 pg/mL, P=0.001), 4.1-fold (1,049±508 pg/mL, P = <0.0001), and 4.1-fold (1,057±522 pg/mL, P = <0.0001) increase in IL-6 levels, respectively, compared to unstimulated IIF (255±130 pg/mL). IL-6 production by IIF was also induced 3.5-fold following stimulation with 100 ng/mL of Pam2CSK4 compared to controls (887±376 pg/mL vs. 255±136 pg/mL, P=0.014). IL-10 secretion by FIF and IIF was undetectable.
FIG. 2.
Toll-like receptor (TLR) agonists stimulated IL-8 production by FIF and IIF. FIF produce significant levels of IL-8 in response to TLR4 agonist, while IIF produce higher levels in response to TLR5 and TLR2/6 agonist. IL-8 ELISA quantification of (A) FIF and (B) IIF cell culture supernatants following stimulation with 1 μg/mL LPS, flagellin (FL, 10–50 ng/mL), Pam2CSK4 (Pam, 1–100 ng/mL), or FSL-1 (1–100 ng/mL) for 24 h. Data are expressed as mean±SD. N=3 experiments. #P<0.0001 and *P=0.001 TLR agonists versus media control.
FIG. 3.
TLR agonists stimulated IL-6 production by FIF and IIF. FIF produce significant levels of IL-6 in response to TLR4 agonist, while IIF produce higher levels in response to TLR5 and TLR2 agonists. IL-6 ELISA quantification of (A) FIF and (B) IIF cell culture supernatants following stimulation with 1 μg/mL LPS, flagellin (FL, 10–50 ng/mL), Pam2CSK4 (Pam, 1–100 ng/mL), or FSL-1 (1–100 ng/mL) for 24 h. Data are expressed as mean±SD. N=3 experiments. #P<0.0001, *P=0.001, and **P<0.014 TLR agonists versus media control.
IFN-γ-stimulated fetal intestinal fibroblasts have increased expression of MHC II
Adult intestinal and gastric fibroblasts express MHC class II molecules in response to proinflammatory stimuli (Saada and others 2006; Pinchuk and others 2013). Thus, we sought to determine if FIF express class II MHC following activation through HLA-DR staining using flow cytometry (Fig. 4). Fetal and infant cells were cultured in the presence of IFN-γ for 7 days prior stimulation with LPS. As seen in Fig. 4A and B, fetal cells cultured with IFN-γ expressed more HLA-DR compared to cells stimulated with LPS alone or media controls. HLA-DR expression on IFN-γ-treated cells cocultured with LPS-expressed HLA-DR at levels similar to cells treated with IFN-γ alone. Infant fibroblasts cultured with LPS alone expressed similar levels of HLA-DR when compared to unstimulated controls. Incubation of infant fibroblasts with IFN-γ appeared to increase expression of HLA-DR although this was not statistically significant and not all cells expressed the molecule. LPS did not enhance HLA-DR expression on IIF cells preincubated with IFN-γ.
FIG. 4.
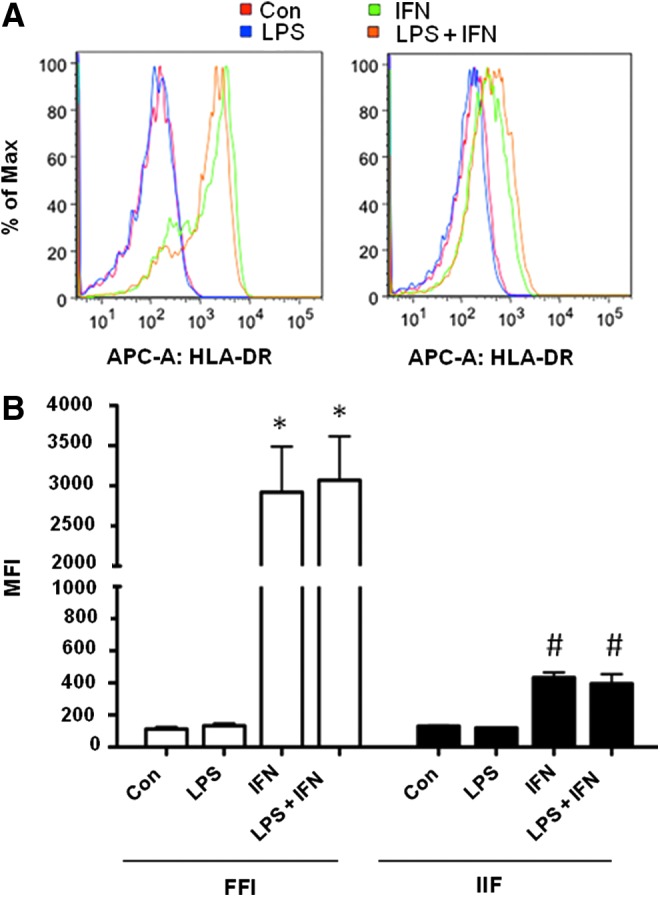
Class II MHC expression by FIF and IIF treated with interferon-gamma (IFN-γ) and LPS. FIF express increased Class II MHC in response to IFN-γ compared to controls and IIF. Flow cytometry measurement of HLA-DR expression on FIF and IIF (A and B) represented by mean fluorescence intensity (MFI). Cells pretreated with 100 U/mL IFN-γ for 7 days prior stimulation with 1 μg/mL LPS for 24 h were stained with APC-conjugated anti-human HLA-DR or isotype antibody. *P<0.0001 FIF IFN, FIF LPS+IFN versus FIF media control, #P<0.0001 FIF IFN versus IIF IFN and FIF LPS+IFN versus IIF LPS+IFN.
Discussion
In the present study, we provide additional evidence of the inherent differences between intestinal fibroblasts derived from fetal and infant tissue that make fetal tissue-derived cells more responsive to certain pathogen-associated molecular patterns. These results suggest that FIF may play a role in the innate and adaptive intestinal immune responses of the immature host and thus contribute to tissue injury in neonatal inflammatory-related bowel diseases.
To maintain intestinal homeostasis, a balance between local and systemic pro- and anti-inflammatory signaling and mediator production is required (Bahrami and others 2003). Physiological immaturity of the GI tract, the presence of bacterial pathogens, and altered levels of the commensal GI microbiota predispose VLBW infants to a dysregulated inflammatory response. We recently described heightened IL-8 production by FIF in response to LPS (Okogbule-Wonodi and others 2012). The present results extend our previous observations and provide a more comprehensive picture of the cytokine profile elicited from LPS-stimulated FIF, consistent with the promotion of acute inflammation. Interleukins, IL-8 and IL-6, are associated with acute systemic and local intestinal inflammation. IL-8 is a key mediator of acute inflammation and a neutrophil chemoattractant. It is secreted by and regulates the activity of several cell types, including fibroblasts (Dunican and others 2000; Cheng and others 2008; Castellani and others 2010). In a previous study by Otte et al., stimulation of infant intestinal fibroblasts used in this study and fibroblasts derived from normal human lung with TLR2 and 4 agonists resulted in upregulation of TLR2, 4 mRNA, and protein expression and increased IL-8 secretion (Otte and others 2003). Several investigators have studied the role of IL-8 in neonatal intestinal inflammatory diseases. Human fetal IEC stimulated with tumor necrosis alpha and IL-1 beta in vitro produce significantly greater levels of IL-8 compared to unstimulated cells (Claud and others 2003). Viscardi and others (1997) demonstrated an association between IL-8 mRNA expression in full-thickness and mucosal intestinal sections from neonates and acute inflammation. IL-6, produced to a greater degree by the stimulated fetal fibroblasts in our study, is associated with inflammatory-related bowel and systemic disease in the mature and immature host. Patients with inflammatory bowel disease have increased circulating levels of IL-6 and the soluble IL-6 receptor (Rose-John and others 2009). IL-6 is elevated in the serum of VLBW infants with NEC and spontaneous intestinal perforations (Chan and others 2012; Benkoe and others 2013). Increased IL-6 mRNA expression is evident in sigmoid colon tissue biopsy samples of infants with food-protein-induced proctocolitis and neonatal transient eosinophilic colitis (Mori and others 2014). MCP-1 and GRO-alpha were also secreted by FIF at higher levels compared to controls, but not in IIF. These cytokines, expressed by several different cell types, including fibroblasts, are also consistent with a proinflammatory profile (Tettelbach and others 1993; Cohen and others 1996; Ajuebor and others 1998; Spoettl and others 2006; Deshmane and others 2009).
We examined the effects of different TLR activation on IL-8, IL-6, and IL-10 production by FIF and IIF using agonists. FIF were responsive to the TLR4 agonist, while IIF were predominantly more sensitive to stimulation with TLR5 and 2 agonists. Our prior analysis demonstrated that IL-8 production by fetal cells decreased by 50% with anti-TLR4 antibodies (Okogbule-Wonodi and others 2012), thus implicating other bacterial lipoproteins as participating in the activation of our fibroblast cells. The mature healthy host exhibits tolerance to abundant LPS and other bacterial antigens present in the intestinal lumen through down- and upregulation of pathways associated with pro- and anti-inflammatory signaling. Downregulation of interleukin 1 receptor-associated kinase 1 (IRAK-1) postnatally decreases TLR4 signaling in response to LPS (Lotz and others 2006). In another report, low-dose flagellin and LPS downregulated several Caco-2 proinflammatory, cell death, and apoptosis genes associated with IL-8 production (Li and others 2012). However, the immature intestinal mucosal barrier is more permeable and hyper-responsive to inciting stimuli (Nanthakumar and others 2000; van Elburg and others 2003; Claud and others 2004). We have demonstrated that fetal myofibroblasts respond similarly to LPS. Conversely, we speculate that fibroblasts from more mature tissues have counterinflammatory mechanisms present that dampen their response to the intraluminal levels of LPS that translocate to the lamina propria. We speculate that this immature immune response may explain why we did not detect the anti-inflammatory cytokine IL-10 in the supernatants of stimulated fetal cells. Furthermore, a longer incubation with the TLR agonists may be required for significant IL-10 production by both cell types (Edelson and others 1999).
In the present study, FIF cocultured with IFN-γ expressed more MHC II compared to unstimulated controls and coincubation with LPS had no added benefit on HLA-DR expression. Intestinal fibroblasts are uniquely situated in the lamina propria and therefore have the potential to facilitate interactions between luminal contents and intestinal epithelial and submucosal immune cells. The expression of HLA-DR on FIF suggests that they may behave like antigen-presenting cells and interact with lamina propria T cells and thus influence the adaptive immune response. Our results further support those of other investigators who demonstrated that intestinal and gastric myofibroblasts express class II MHC molecules and activate T cells in vitro (Saada and others 2006; Pinchuk and others 2013). IIF expressed constitutive levels of HLA-DR that trended to increase in the presence of IFN-γ. Similar to FIF, LPS did not appear to have an added effect on HLA-DR expression on the IFN-γ-stimulated cells. Overall, FIF were more responsive to IFN-γ and have propensity to activate an adaptive immune response.
In conclusion, this study further elucidates the role FIF may play in promoting intestinal inflammation. FIF produce a distinct cytokine profile that includes increased IL-8 and IL-6, in response to activation of TLR4 that promotes excessive inflammation. FIF also express class II MHC molecules, which may make them capable of antigen presentation and activation of the adaptive immune system. Taken together, our results indicate that FIF may contribute to the overall dysregulated inflammatory response in the immature intestine.
Acknowledgment
Funding support was provided by NIH grants DK 46461 and DK 067872.
Author Disclosure Statement
No competing financial interests exist.
References
- Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, Perretti M. 1998. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol 63(1):108–116 [DOI] [PubMed] [Google Scholar]
- Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. 2007. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther 114(1):94–106 [DOI] [PubMed] [Google Scholar]
- Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. 2006. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 117(4):e760–e768 [DOI] [PubMed] [Google Scholar]
- Bahrami B, Macfarlane S, Macfarlane GT. 2011. Induction of cytokine formation by human intestinal bacteria in gut epithelial cell lines. J Appl Microbiol 110(1):353–363 [DOI] [PubMed] [Google Scholar]
- Beltinger J, McKaig BC, Makh S, Stack WA, Hawkey CJ, Mahida YR. 1999. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol 277(2 Pt 1):C271–C279 [DOI] [PubMed] [Google Scholar]
- Benkoe T, Baumann S, Weninger M, Pones M, Reck C, Rebhandl W, Oehler R. 2013. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PloS One 8(3):e58720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani ML, De Lutiis MA, Toniato E, Conti F, Felaco P, Fulcheri M, Theoharides TC, Caraffa A, Antinolfi P, Conti P, Cuccurullo C, Ciampoli C, Felaco M, Orso C, Salini V, Cerulli G, Kempuraj D, Tete S, Shaik B. 2010. Impact of RANTES, MCP-1 and IL-8 in mast cells. J Biol Regul Homeost Agents 24(1):1–6 [PubMed] [Google Scholar]
- Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, Leung KT, Poon TC, Lee KH, Li K, Fok TF, Ng PC. 2012. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PloS One 7(5):e36977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Li Y, Wu J, Nie Y, Li L, Liu X, Charoude HN, Chen H. 2008. IL-8 induces imbalances between nitric oxide and endothelin-1, and also between plasminogen activator inhibitor-1 and tissue-type plasminogen activator in cultured endothelial cells. Cytokine 41(1):9–15 [DOI] [PubMed] [Google Scholar]
- Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. 2004. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci U S A 101(19):7404–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claud EC, Savidge T, Walker WA. 2003. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res 53(3):419–425 [DOI] [PubMed] [Google Scholar]
- Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, Goncalves LF, Gomez R. 1996. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol 35(1):23–29 [DOI] [PubMed] [Google Scholar]
- De LKT, Whiting CV, Bland PW. 2007. Proinflammatory cytokine synthesis by mucosal fibroblasts from mouse colitis is enhanced by interferon-gamma-mediated up-regulation of CD40 signalling. Clin Exp Immunol 147(2):313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6):313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A, Pender SL, Jackson CL, Prothero JD, Gordon JN, Picariello L, Rovedatti L, Docena G, Monteleone G, Rampton DS, Tonelli F, Corazza GR, MacDonald TT. 2007a. Functional modulation of Crohn's disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 133(1):137–149 [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Pickard KM, Gordon JN, Salvati V, Mazzarella G, Beattie RM, Vossenkaemper A, Rovedatti L, Leakey NA, Croft NM, Troncone R, Corazza GR, Stagg AJ, Monteleone G, MacDonald TT. 2007b. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology 133(4):1175–1187 [DOI] [PubMed] [Google Scholar]
- Dunican AL, Leuenroth SJ, Ayala A, Simms HH. 2000. CXC chemokine suppression of polymorphonuclear leukocytes apoptosis and preservation of function is oxidative stress independent. Shock 13(3):244–250 [DOI] [PubMed] [Google Scholar]
- Edelson MB, Bagwell CE, Rozycki HJ. 1999. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 103(4 Pt 1):766–771 [DOI] [PubMed] [Google Scholar]
- Fritsch C, Swietlicki EA, Lefebvre O, Kedinger M, Iordanov H, Levin MS, Rubin DC. 2002. Epimorphin expression in intestinal myofibroblasts induces epithelial morphogenesis. J Clin Invest 110(11):1629–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatomi O, Andoh A, Yagi Y, Ogawa A, Hata K, Shiomi H, Tani T, Takayanagi A, Shimizu N, Fujiyama Y. 2007. Matrix metalloproteinase-3 secretion from human pancreatic periacinar myofibroblasts in response to inflammatory mediators. Pancreas 34(1):126–132 [DOI] [PubMed] [Google Scholar]
- Joyce NC, Haire MF, Palade GE. 1987. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology 92(1):68–81 [DOI] [PubMed] [Google Scholar]
- Li N, Quidgley MC, Kobeissy FH, Joseph J, Neu J. 2012. Microbial cell components induced tolerance to flagellin-stimulated inflammation through Toll-like receptor pathways in intestinal epithelial cells. Cytokine 60(3):806–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. 2006. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 203(4):973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto R, Rodriguez-Diaz J, Collado MC, Salminen S, Isolauri E, Lehtonen L. 2013. Gross blood in stools of premature neonates, a clinical and microbiological follow-up study. Acta Paediatr 102(5):486–491 [DOI] [PubMed] [Google Scholar]
- McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. 1999. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol 276(5 Pt 1):G1087–G1093 [DOI] [PubMed] [Google Scholar]
- Mori M, Ohtsuka Y, Ishida A, Yamazaki S, Jimbo K, Inage E, Aoyagi Y, Kudo T, Suzuki R, Shimizu T. 2014. Outcome of infants presenting rectal bleeding: a retrospective study in a single institution. Pediatr Int 56(6):884–890 [DOI] [PubMed] [Google Scholar]
- Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. 2000. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A 97(11):6043–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okogbule-Wonodi AC, Li G, Anand B, Luzina IG, Atamas SP, Blanchard T. 2012. Human foetal intestinal fibroblasts are hyper-responsive to lipopolysaccharide stimulation. Dig Liver Dis 44(1):18–23 [DOI] [PubMed] [Google Scholar]
- Otte JM, Rosenberg IM, Podolsky DK. 2003. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 124(7):1866–1878 [DOI] [PubMed] [Google Scholar]
- Pietzcker J, Kluthea C, Klinghammer K, Buhrer C, Rustow B, Guthmann F. 2012. Developmental delay in hypoxia-induced HO-1 expression predisposes to gut injury. J Perinat Med 40(2):191–197 [DOI] [PubMed] [Google Scholar]
- Pinchuk IV, Morris KT, Nofchissey RA, Earley RB, Wu JY, Ma TY, Beswick EJ. 2013. Stromal cells induce Th17 during Helicobacter pylori infection and in the gastric tumor microenvironment. PloS One 8(1):e53798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. 1999. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 277(2 Pt 1):C183–C201 [DOI] [PubMed] [Google Scholar]
- Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. 2009. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Design 15(18):2095–2103 [DOI] [PubMed] [Google Scholar]
- Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Di Mari JF, Reyes VE, Powell DW. 2006. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol 177(9):5968–5979 [DOI] [PubMed] [Google Scholar]
- Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, Claud EC. 2011. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem 286(14):12123–12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoettl T, Hausmann M, Herlyn M, Gunckel M, Dirmeier A, Falk W, Herfarth H, Schoelmerich J, Rogler G. 2006. Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes. Clin Exp Immunol 145(1):190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelbach W, Nanney L, Ellis D, King L, Richmond A. 1993. Localization of MGSA/GRO protein in cutaneous lesions. J Cutan Pathol 20(3):259–266 [DOI] [PubMed] [Google Scholar]
- van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. 2003. Intestinal permeability in relation to birth weight and gestational and postnatal age. Archives of disease in childhood. Fetal Neonatal Ed 88(1):F52–F55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi RM, Lyon NH, Sun CC, Hebel JR, Hasday JD. 1997. Inflammatory cytokine mRNAs in surgical specimens of necrotizing enterocolitis and normal newborn intestine. Pediatr Pathol Lab Med 17(4):547–559 [PubMed] [Google Scholar]