Abstract
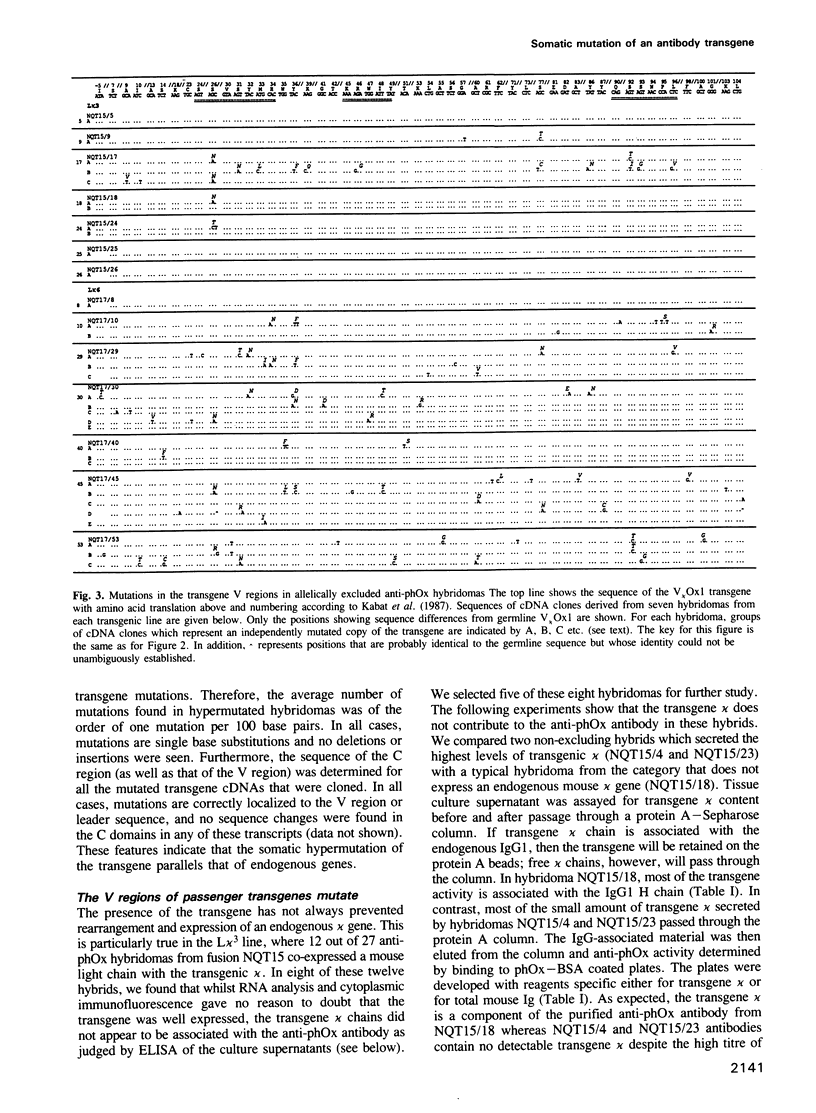
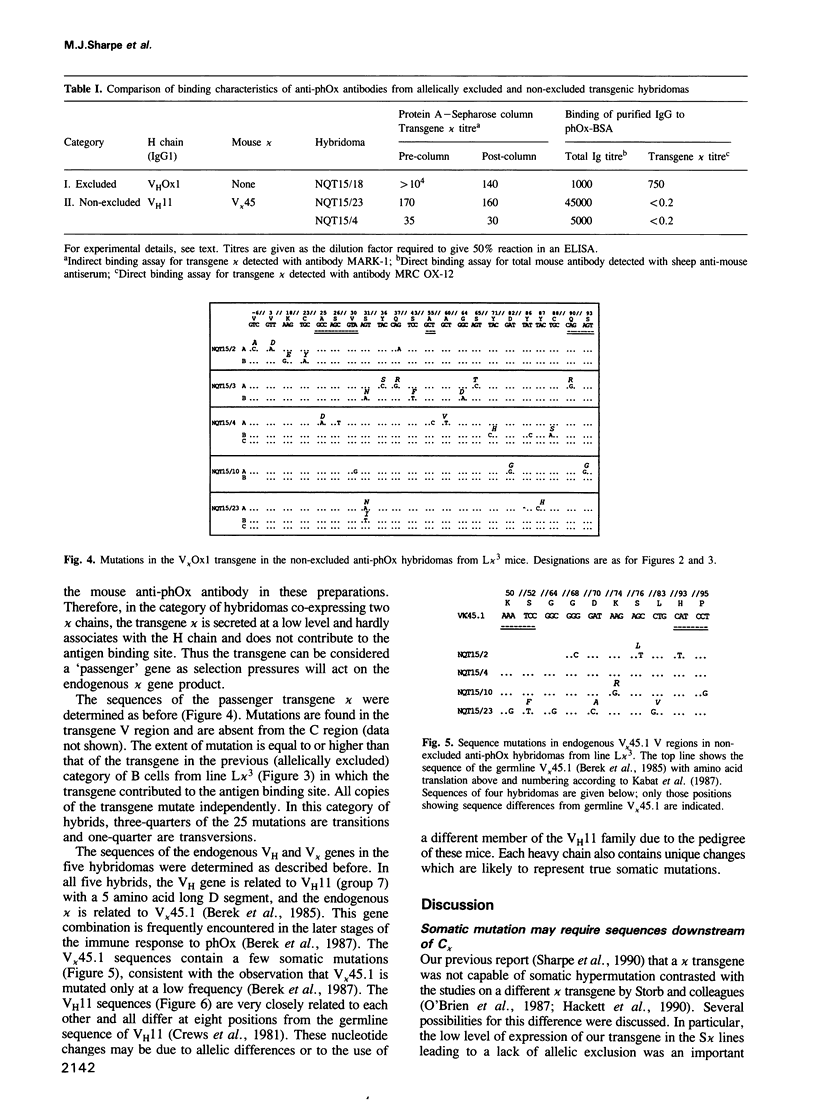
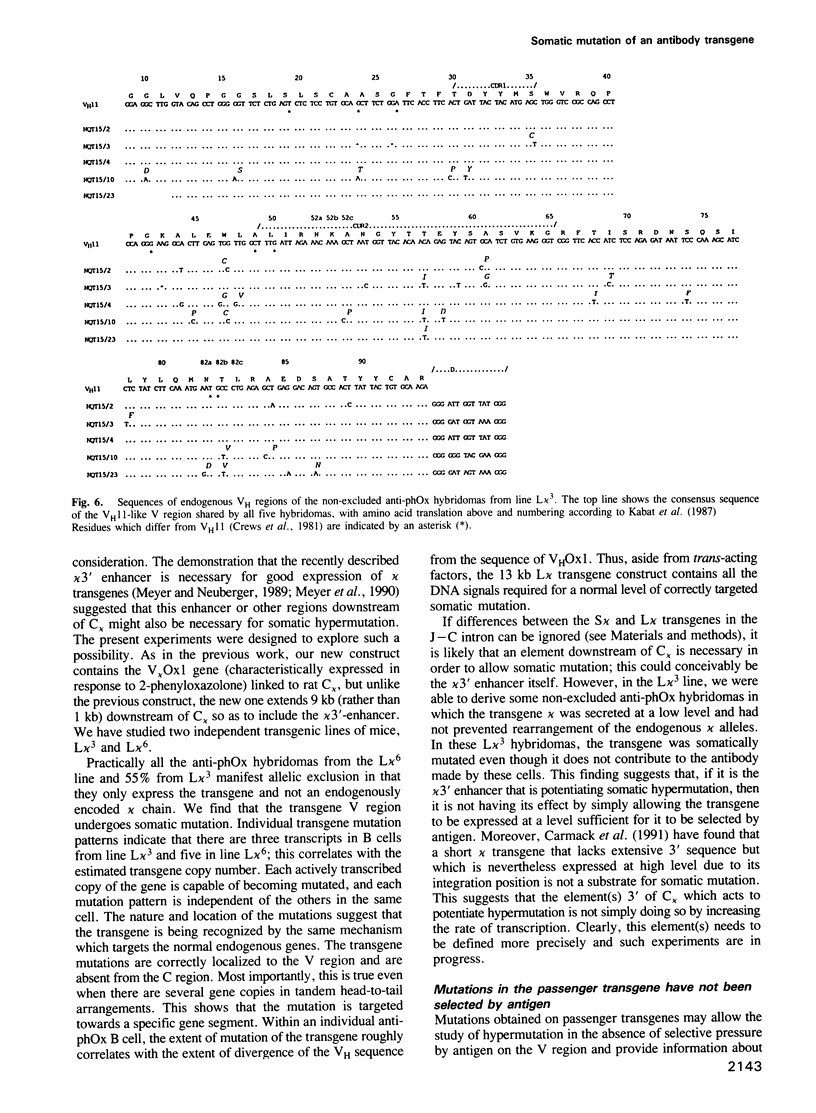
We have compared the pattern of somatic mutation in different immunoglobulin kappa transgenes and suggest that an element(s) located between 1 kb and 9 kb 3' of C kappa is necessary for somatic hypermutation of the antibody V gene. The sequences of transgenic and endogenous Ig V regions were determined in antigen-specific B cell hybridomas specific for 2-phenyloxazolone from independent lines of hyperimmunized transgenic mice. We analysed somatic mutation of the transgene both in hybridomas in which the transgenic kappa chain contributes to the antigen combining site as well as in hybridomas in which the transgene is a passenger with the expressed antibody being composed of endogenously-encoded heavy and light chains. In both cases, nucleotide changes in the transgene are correctly targeted to the V region and are absent from the C region. They accumulate at a similar rate to that in the endogenous Ig genes within the same cell and we find that, irrespective of whether or not the transgene kappa is directly selected by antigen, somatic mutation occurs at a similar rate and involves only single base substitutions. Furthermore, the pattern of mutations in passenger transgenes gives information about the intrinsic sequence specificities of the somatic hypermutation mechanism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzari P. M., Spinelli S., Mariuzza R. A., Boulot G., Poljak R. J., Jarvis J. M., Milstein C. Three-dimensional structure determination of an anti-2-phenyloxazolone antibody: the role of somatic mutation and heavy/light chain pairing in the maturation of an immune response. EMBO J. 1990 Dec;9(12):3807–3814. doi: 10.1002/j.1460-2075.1990.tb07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Berek C., Jarvis J. M., Milstein C. Activation of memory and virgin B cell clones in hyperimmune animals. Eur J Immunol. 1987 Aug;17(8):1121–1129. doi: 10.1002/eji.1830170808. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. The dynamic nature of the antibody repertoire. Immunol Rev. 1988 Oct;105:5–26. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Blier P. R., Bothwell A. L. The immune response to the hapten NP in C57BL/6 mice: insights into the structure of the B-cell repertoire. Immunol Rev. 1988 Oct;105:27–43. doi: 10.1111/j.1600-065x.1988.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Taylor L., Pollard J. W., Steele E. J. Distribution of mutations around rearranged heavy-chain antibody variable-region genes. Mol Cell Biol. 1990 Oct;10(10):5187–5196. doi: 10.1128/mcb.10.10.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin J. L., Berry J., Flaherty D., Dunnick W. Somatic evolution of diversity among anti-phosphocholine antibodies induced with Proteus morganii. J Immunol. 1987 May 1;138(9):3060–3068. [PubMed] [Google Scholar]
- Cotton R. G., Milstein C. Letter: Fusion of two immunoglobulin-producing myeloma cells. Nature. 1973 Jul 6;244(5410):42–43. doi: 10.1038/244042a0. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- De Préval C., Fougereau M. Specific interaction between VH and VL regions of human monoclonal immunoglobulins. J Mol Biol. 1976 Apr 15;102(3):657–678. doi: 10.1016/0022-2836(76)90340-5. [DOI] [PubMed] [Google Scholar]
- Durdik J., Gerstein R. M., Rath S., Robbins P. F., Nisonoff A., Selsing E. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2346–2350. doi: 10.1073/pnas.86.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Hackett J., Jr, Rogerson B. J., O'Brien R. L., Storb U. Analysis of somatic mutations in kappa transgenes. J Exp Med. 1990 Jul 1;172(1):131–137. doi: 10.1084/jem.172.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Manser T., Wysocki L. J., Margolies M. N., Gefter M. L. Evolution of antibody variable region structure during the immune response. Immunol Rev. 1987 Apr;96:141–162. doi: 10.1111/j.1600-065x.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Sharpe M. J., Surani M. A., Neuberger M. S. The importance of the 3'-enhancer region in immunoglobulin kappa gene expression. Nucleic Acids Res. 1990 Oct 11;18(19):5609–5615. doi: 10.1093/nar/18.19.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Cuello A. C. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983 Oct 6;305(5934):537–540. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Brinster R. L., Storb U. Somatic hypermutation of an immunoglobulin transgene in kappa transgenic mice. 1987 Mar 26-Apr 1Nature. 326(6111):405–409. doi: 10.1038/326405a0. [DOI] [PubMed] [Google Scholar]
- Orlandi R., Güssow D. H., Jones P. T., Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 May;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Pettersson S., Sharpe M. J., Gilmore D. R., Surani M. A., Neuberger M. S. Cellular selection leads to age-dependent and reversible down-regulation of transgenic immunoglobulin light chain genes. Int Immunol. 1989;1(5):509–516. doi: 10.1093/intimm/1.5.509. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Roes J., Hüppi K., Rajewsky K., Sablitzky F. V gene rearrangement is required to fully activate the hypermutation mechanism in B cells. J Immunol. 1989 Feb 1;142(3):1022–1026. [PubMed] [Google Scholar]
- Sharpe M. J., Neuberger M., Pannell R., Surani M. A., Milstein C. Lack of somatic mutation in a kappa light chain transgene. Eur J Immunol. 1990 Jun;20(6):1379–1385. doi: 10.1002/eji.1830200625. [DOI] [PubMed] [Google Scholar]