Abstract
Background
Immune dysregulation associated with mercury has been suggested, although data in the general population are lacking. Chronic exposure to low levels of methylmercury (organic) and inorganic mercury is common, such as through fish consumption and dental amalgams.
Objective
We examined associations between mercury biomarkers and antinuclear antibody (ANA) positivity and titer strength.
Methods
Among females 16–49 years of age (n = 1,352) from the National Health and Nutrition Examination Survey (NHANES) 1999–2004, we examined cross-sectional associations between mercury and ANAs (indirect immunofluorescence; cutoff ≥ 1:80). Three biomarkers of mercury exposure were used: hair (available 1999–2000) and total blood (1999–2004) predominantly represented methylmercury, and urine (1999–2002) represented inorganic mercury. Survey statistics were used. Multivariable modeling adjusted for several covariates, including age and omega-3 fatty acids.
Results
Sixteen percent of females were ANA positive; 96% of ANA positives had a nuclear speckled staining pattern. Geometric mean (geometric SD) mercury concentrations were 0.22 (0.03) ppm in hair, 0.92 (0.05) μg/L blood, and 0.62 (0.04) μg/L urine. Hair and blood, but not urinary, mercury were associated with ANA positivity (sample sizes 452, 1,352, and 804, respectively), after adjusting for confounders: for hair, odds ratio (OR) = 4.10 (95% CI: 1.66, 10.13); for blood, OR = 2.32 (95% CI: 1.07, 5.03) comparing highest versus lowest quantiles. Magnitudes of association were strongest for high-titer (≥ 1:1,280) ANA: hair, OR = 11.41 (95% CI: 1.60, 81.23); blood, OR = 5.93 (95% CI: 1.57, 22.47).
Conclusions
Methylmercury, at low levels generally considered safe, was associated with subclinical autoimmunity among reproductive-age females. Autoantibodies may predate clinical disease by years; thus, methylmercury exposure may be relevant to future autoimmune disease risk.
Citation
Somers EC, Ganser MA, Warren JS, Basu N, Wang L, Zick SM, Park SK. 2015. Mercury exposure and antinuclear antibodies among females of reproductive age in the United States: NHANES. Environ Health Perspect 123:792–798; http://dx.doi.org/10.1289/ehp.1408751
Introduction
Autoimmune disorders, although individually rare, are collectively estimated to afflict 7.6–9.4% of Americans (Cooper et al. 2009) and are among the 10 leading causes of death among women (Thomas et al. 2010; Walsh and Rau 2000). Almost all autoimmune diseases have a strong preponderance among females, with female to male ratios of up to 9:1 and onset often occurring during mid-adulthood (Cooper and Stroehla 2003; Somers et al. 2007, 2014).
Autoimmunity, which can include autoantibody formation, represents a breakdown of tolerance against self-antigens (Lleo et al. 2010). Self-reactive lymphocytes may occur in healthy individuals, and in the absence of related pathology, autoimmunity represents pre- or subclinical immune dysregulation. Thus, the term “autoimmunity” should be distinguished from autoimmune disease, because it does not denote clinical or symptomatic disease. Data are sparse regarding the prognostic significance of preclinical autoimmunity or the “conversion” rate to particular disorders, although autoantibodies may precede autoimmune diagnoses by several years (Arbuckle et al. 2003) and nearly all autoimmune diseases are characterized by circulating autoantibodies (Scofield 2004). Antinuclear antibodies (ANAs) are highly sensitive for a variety of autoimmune conditions, including systemic lupus erythematosus (SLE), scleroderma, and Sjögren’s syndrome. Estimates of ANA prevalence in individuals without autoimmune disease vary widely (1–24%) (Fritzler et al. 1985; Rosenberg et al. 1999) due to differing methodologies and population characteristics. ANA prevalence of approximately 13% has been reported in key studies using a 1:80 titer cutoff (Satoh et al. 2012; Tan et al. 1997) based on an immunofluorescence assay, the method recommended by the American College of Rheumatology as the gold standard for ANA testing (Meroni and Schur 2010).
Mercury is a ubiquitous and persistent toxicant with pleiotropic effects, and it is currently ranked as a top three priority pollutant by the U.S. Agency for Toxic Substances and Disease Registry (2011). Consumption of seafood, particularly of large species, is a common source of organic mercury (methylmercury) exposure (Mergler et al. 2007). It has been estimated that in the United States each year, approximately 8% of mothers and 0.6 million newborns have mercury concentrations exceeding levels considered by regulatory agencies to be safe (Trasande et al. 2005). Immunotoxic effects, including autoantibody production, have been clearly demonstrated in murine models in response to both organic and inorganic mercury (Pollard et al. 2010). In humans, occupational mercury exposure (predominantly inorganic and elemental species) among miners has been associated with increased risk of autoimmunity (Gardner et al. 2010; Silva et al. 2004), and an increased risk of SLE has been reported among dental professionals (Cooper et al. 2004). However, immune effects associated with low levels of exposure to each type of mercury in the general population are not well characterized (Mergler et al. 2007).
Because the biologic effects, sources, and patterns of exposure to organic and inorganic mercury are expected to differ, it is important to examine both species. Biomarkers of mercury exposure in humans include hair mercury, representing predominantly organic (methyl) mercury; total blood mercury, a combination of organic and inorganic mercury; and urinary mercury, a marker predominantly of inorganic/elemental mercury. In the U.S. National Health and Nutrition Examination Survey (NHANES), hair mercury was measured in adult females (16–49 years of age) but not in males.
Using NHANES data, we explored the associations between three types of biomarkers of mercury exposure and the presence, strength, and patterns of ANAs in a representative sample of reproductive-age females from the U.S. population.
Methods
Study population. NHANES is conducted by the Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) (CDC/NCHS 2015b). It uses a stratified, multistage probability cluster design, with oversampling of selected subpopulations, to obtain a representative sample of the civilian, noninstitutionalized U.S. population. NHANES protocols were approved by the NCHS Institutional Review Board, and informed consent was obtained. In our study we used data from three cycles of continuous NHANES data (1999–2004) (CDC/NCHS 2015a). Participation rates were 76% for 1999–2000, 80% for 2001–2002, and 76% for 2003–2004 (CDC/NCHS 2013). The eligible population for our analysis included female participants 16–49 years of age who completed a physical examination with biospecimen collection for ANA and mercury assessment.
From a total of 5,984 females 16–49 years of age in NHANES 1999–2004, 1,932 were included in the one-third subsample with ANA assessment, of whom 1,354 had available ANA data. Hair mercury was available for one cycle (1999–2000), total blood mercury for three (1999–2004), and urinary mercury for two (1999–2002). For hair, blood, and urinary mercury, respectively, samples sizes were 452, 1,352, and 804 (after excluding 16, 2, and 29 persons with missing data).
ANAs. As detailed elsewhere (CDC/NCHS 2012), standard methodology for ANA screening was used, involving indirect immunofluorescence with HEp-2 substrate for detection of IgG antibodies to cellular antigens. Titers to which fluorescence remained positive (serial dilution range, 1:80–1:1,280) and staining patterns were determined for positive specimens. ANA patterns refer to indirect immunofluorescence patterns (e.g., speckled, nucleolar, homogeneous) reflecting the anatomic distribution of intracellular antigens, and thus different nuclear components. A variety of different ANAs can give rise to a given pattern. Follow-up immunoprecipitation was used for identification of specific antigens from a standard panel.
Mercury exposure assessment. Three types of biomarkers for mercury exposure were used: hair (organic), total blood (organic and inorganic), and urine (predominantly inorganic/elemental). One-centimeter hair segments were utilized (approximating exposure during the preceding 2.5 months). Standard methodology for mercury measurement was used, as described elsewhere (CDC/NCHS 2005, 2007). In brief, for hair, cold vapor atomic fluorescence spectrometry following analyte extraction was used. For blood and urine, flow-injection cold vapor atomic absorption spectrometry (PerkinElmer Flow Injection Mercury System-400) was used in NHANES 1999–2002, and inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer ELAN 6100) was used in 2003–2004. Limits of detection (LODs) for hair mercury varied by batch and ranged between 0.011 and 0.027 ppm (method detection limit); 6% of the females in our study had hair mercury levels below the LOD. LODs for total blood mercury varied according to cycle and batch, ranging between 0.14 and 0.2 μg/L. Of the females in our study, 7.4%, 6.5%, and 7.5% had total blood mercury levels below the LOD for the three cycles, respectively. We did not separately investigate the inorganic fraction of blood mercury because of the large proportion < LOD (97.4%, 95.1%, and 77.2% for the three cycles). The LOD for urinary mercury was 0.14 μg/L, with 13.3% and 14.3% of the participants having urinary mercury levels < LOD for the two cycles, respectively.
Other variables. Sociodemographic data were collected by self-administered questionnaires. Body mass index (BMI), calculated as kilograms of body weight divided by height in meters squared, was included due to the role of obesity in chronic inflammation. Serum cotinine (nanograms per milliliter), a marker of active and passive smoking, was measured by isotope dilution–high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry; tobacco exposure has been linked to increased risk of autoimmune diseases (Costenbader and Karlson 2006). C-reactive protein (CRP), a nonspecific inflammatory marker, was quantified (milligrams per deciliter) by latex-enhanced nephelometry. Nutrient data were estimated based on a multiple pass, computer-assisted dietary interview of food and beverage consumption, with recall assessment of individual foods consumed in the previous 24 hr. We derived data on selenium (micrograms), eicosapentaenoic acid (20:5 n-3), and docosahexaenoic acid (22:6 n-3), all found in seafood; omega-3 fatty acid intake was calculated as eicosapentaenoic acid plus docosahexaenoic acid. Selenium potentially mitigates effects of mercury (Cuvin-Aralar and Furness 1991), and omega-3s have anti-inflammatory effects (Simopoulos 2002). Among participants who underwent the dietary interview, weekly seafood intake was estimated based on recall of fish/shellfish consumption in the previous 30 days. Serum polychlorinated biphenyls (PCBs) were measured by high-resolution gas chromatography/isotope dilution high-resolution mass spectrometry (CDC 2006). A summary measure for coplanar (dioxin-like) polychlorinated biphenyls (cPCBs), which included congeners with suspected immunotoxicity (Wolff et al. 1997), was calculated as the sum of the products of the concentration of each serum lipid–adjusted congener (PCBs 81, 105, 118, 126, 156, 157, 167, 169) and its corresponding 2005 World Health Organization-defined toxic equivalency factor (TEF) (Van den Berg et al. 2006). An alternate PCB summary measure was the sum of the lipid-adjusted values for the four most prevalent PCB congeners (118, 138, 153, 180) (Laden et al. 2010); three of these are noncoplanar and without defined TEFs to take into account. To address the potential for drug-induced autoimmunity, we assessed use within the past month of four prescription medications that have been implicated in this phenomenon (procainamide, hydralazine, carbamazepine, and minocycline) (Schoonen et al. 2010).
Statistical analysis. To account for the complex, stratified, and multistage cluster sampling design, analyses were conducted using the survey packages of Stata (v.12; StataCorp) and R (v.2.11.1; R Foundation) to obtain appropriate estimates and standard errors. ANAs were measured in a one-third subsample, and we constructed and applied weights to our subsample according to NCHS analytic guidelines (Johnson et al. 2013). Values below the LOD for laboratory assays were handled as the LOD divided by the square root of 2. Hair and blood mercury were log-transformed due to their skewed nature, or handled as quantiles based on distributions in the study population. Two-sample t-tests for survey data and the Rao-Scott chi-square test were used for continuous and categorical data, respectively. p-Values < 0.05 were considered significant. Crude models included mercury as the independent variable; separate models were performed for each source of mercury (hair, blood, urine). Multivariable logistic regression was utilized to estimate adjusted odds ratios (ORs) for ANA positivity in association with mercury exposure. Model A included age, race/ethnicity, education, serum cotinine, and selenium; an indicator for the NHANES cycle was included in models combining data across cycles to account for potential methodological differences between cycles. Models B and C were further adjusted for omega-3 fatty acids and seafood intake, respectively, which have been suggested to negatively confound health effects of mercury (Budtz-Jørgensen et al. 2007; Guallar et al. 2002). Multivariable urinary mercury models adjusted for urinary creatinine to account for dilution of spot urine specimens. We performed sensitivity analyses adding BMI and CRP in Models A–C as potential confounders for all mercury types. Separate sensitivity analyses were performed, including the coplanar and prevalent PCB measures. For urinary mercury, we also conducted models excluding persons with impaired renal function [glomerular filtration rate (GFR) < 60 mL/min/1.73 m2] to account for potential reverse causation whereby chronic kidney disease may increase urinary mercury excretion. Piecewise continuous models were constructed, and linearity with the log-odds of ANA was examined by predicted probability plots with natural cubic splines with four degrees of freedom (three for hair). Multinomial logistic regression was utilized to examine ANA titer strength as the outcome, with negative ANA (< 1:80) as the base outcome, and low/moderate titer (1:80–1:640) and high titer (≥ 1:1,280) as the other outcome levels.
Results
Participant characteristics. Characteristics of the study population, according to ANA positivity, are summarized in Table 1. Sociodemographics were largely similar for ANA-positive and -negative persons, although in the first cycle there was a larger proportion of non-Hispanic whites and Mexican Americans among ANA positives (p = 0.08). In the combined three-cycle population, education level differed between ANA-positive and -negative groups, with a higher proportion of ANA-positive adults having less than a high school education (p = 0.04). Among ANA-positive compared with negative participants, hair and total blood mercury levels in cycle 1 were higher (p = 0.03 and p = 0.06, respectively), and blood mercury in the three-cycle population was nonsignificantly higher.
Table 1.
Participant characteristics according to antinuclear antibody (ANA) positivity among females 16–49 years of age in the general U.S. population (NHANES).
| Characteristic | NHANES 1999–2000 | NHANES 1999–2004 | ||||
|---|---|---|---|---|---|---|
| ANA positivea(n = 56) | ANA negativea(n = 396) | p‑Value | ANA positivea(n = 213) | ANA negativea(n = 1,139) | p‑Value | |
| Age (years) | 31.7 ± 2.8 | 32.7 ± 0.7 | 0.71 | 33.7 ± 0.02 | 33.6 ± 0.06 | 0.14 |
| Race/ethnicity | 0.08 | 0.25 | ||||
| White, non-Hispanic | 15 (74.2) | 135 (64.1) | 85 (71.5) | 458 (64.4) | ||
| Black, non-Hispanic | 12 (10.7) | 73 (10.7) | 48 (12.9) | 256 (12.9) | ||
| Mexican American | 26 (12.3) | 136 (7.3) | 63 (8.7) | 306 (9.0) | ||
| Other Hispanic | 2 (2.6) | 32 (9.9) | 13 (5.1) | 65 (6.5) | ||
| Others | 1 (0.2) | 20 (8.1) | 4 (1.8) | 54 (7.1) | ||
| Education | 0.28 | 0.04 | ||||
| < High school | 9 (18.1) | 74 (14.8) | 48 (17.2) | 227 (14.7) | ||
| High school graduate/GED | 15 (34.3) | 61 (20.2) | 38 (21.9) | 198 (22.1) | ||
| Some college/graduate school | 16 (43.3) | 134 (52.7) | 88 (55.8) | 446 (55.1) | ||
| Youth (16–19 years) | 16 (4.3) | 127 (12.3) | 39 (5.1) | 268 (8.2) | ||
| BMI (kg/m2) | 26.6 ± 1.1 | 27.3 ± 0.5 | 0.57 | 26.6 ± 0.1 | 27.5 ± 0.2 | 0.11 |
| Smokingb | 0.77 | 0.32 | ||||
| Never | 27 (60.7) | 171 (57.1) | 111 (61.2) | 529 (55.6) | ||
| Former | 7 (17.4) | 40 (15.0) | 28 (15.1) | 130 (15.3) | ||
| Current | 5 (21.9) | 58 (28.0) | 34 (23.8) | 212 (29.2) | ||
| Serum cotinine (ng/mL)c | 0.68 ± 0.43 | 0.75 ± 0.25 | 0.87 | 0.32 ± 0.11 | 0.62 ± 0.11 | 0.07 |
| C-reactive protein (mg/dL)c | 0.13 ± 0.03 | 0.18 ± 0.02 | 0.30 | 0.14 ± 0.00 | 0.18 ± 0.02 | 0.25 |
| < 1 mg/dL | 51 (84.5) | 336 (89.3) | 0.54 | 193 (92.1) | 978 (87.4) | 0.57 |
| ≥ 1 mg/dL | 5 (15.6) | 60 (10.7) | 20 (7.9) | 161 (12.6) | ||
| Selenium, dietary (μg)c | 87.9 ± 9.2 | 78.7 ± 4.0 | 0.30 | 85.5 ± 2.3 | 82.8 ± 1.6 | 0.52 |
| Omega-3, dietary (g)c | 0.02 ± 0.01 | 0.03 ± 0.003 | 0.57 | 0.02 ± 0.003 | 0.02 ± 0.001 | 0.57 |
| Hair Hg (ppm)c | 0.27 ± 0.04 | 0.21 ± 0.03 | 0.03 | NA | NA | NA |
| Total blood Hg (μg/L)c | 1.31 ± 0.17 | 1.01 ± 0.12 | 0.06 | 0.97 ± 0.00 | 0.91 ± 0.05 | 0.43 |
| Urinary Hg (μg/L)c | 0.80 ± 0.16 | 0.65 ± 0.08 | 0.34 | 0.67 ± 0.03d | 0.62 ± 0.05d | 0.56 |
| ANA titer | NA | NA | ||||
| Negative (≤ 1:40) | NA | 396 (100) | NA | 1,139 (100) | ||
| 1:80 | 0 | NA | 0 | NA | ||
| 1:160 | 2 (1.2) | NA | 3 (0.4) | NA | ||
| 1:320 | 16 (21.3) | NA | 42 (17.6) | NA | ||
| 1:640 | 14 (18.1) | NA | 64 (32.8) | NA | ||
| ≥ 1:1,280 | 24 (59.5) | NA | 104 (49.2) | NA | ||
| ANA patterne | NA | NA | ||||
| Nuclear (all) | 52 (98.9) | NA | 208 (99.7) | NA | ||
| Speckled | 50 (94.4) | NA | 203 (96.3) | NA | ||
| Nucleolar | 4 (3.1) | NA | 14 (5.1) | NA | ||
| Homogeneous | 1 (4.4) | NA | 3 (2.3) | NA | ||
| Abbreviations: Hg, mercury; NA, not applicable. aValues are mean ± SD or n (%); means and percentages are weighted. bSmoking data were available for ages ≥ 20 years (n = 308 for first cycle; n = 1,044 for 3 cycles). cGeometric mean ± geometric SD. dData for NHANES 1999–2002 (n = 804; data were unavailable for 2003–2004). eThree major types of nuclear staining patterns were tabulated, and more than one ANA pattern is possible; 3.1% (cycle 1) and 5.1% (cycles 1–3) of participants had more than one pattern. | ||||||
ANAs and mercury. The weighted proportion of participants with ANA positivity was 12% for cycle 1 and 16% for cycles 1–3. Among ANA positives, the speckled pattern was predominant (> 94%; Table 1). The geometric means (geometric SDs) for mercury corresponding to all participants included in Table 1 for whom data were available were as follows: hair, 0.22 (0.03) ppm; total blood, 0.92 (0.05) μg/L; urine, 0.62 (0.04) μg/L. Correlations between the sources of mercury in cycle 1 were as follows: hair and total blood (r = 0.69; p < 0.01), hair and urine (r = 0.34; p < 0.01), total blood and urine (r = 0.41; p < 0.01). Among the females in this study, 12.8% had a total blood mercury level > 3.5 μg/L, the reference dose extrapolated from the U.S. Environmental Protection Agency cord blood mercury reference dose of 5.8 μg/L (Mahaffey et al. 2004; Stern and Smith 2003).
Based on multivariable logistic regression (Table 2), we detected positive and statistically significant associations [confidence intervals (CIs) > 1] between ANA positivity and hair and total blood mercury, but not urinary mercury. From the multivariable models incorporating omega-3 fatty acids (Model B), the adjusted OR for ANA positivity comparing females in the highest versus lowest tertile of hair mercury was 4.10 (95% CI: 1.66, 10.13), and for the highest versus lowest quartile of blood mercury was 2.32 (95% CI: 1.07, 5.03).
Table 2.
Association between mercury (Hg) exposure and antinuclear antibody (ANA) positivity among females 16–49 years of age in the general U.S. population (NHANES).
| Hg exposure | ANA positiven (%)a | Crude modelbOR (95% CI) | Model AcOR (95% CI) | Model BdOR (95% CI) | Model CeOR (95% CI) |
|---|---|---|---|---|---|
| Hair Hg (ppm)f | |||||
| Tertile 1 (< 0.11) | 14 (8) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (0.11–0.27) | 22 (12) | 1.58 (0.27, 9.33) | 2.45 (0.47, 12.82) | 2.70 (0.57, 12.80) | 2.28 (0.40, 12.94) |
| Tertile 3 (0.271–5.96) | 20 (14) | 1.83 (0.54, 6.16) | 4.01 (1.57, 10.28) | 4.10 (1.66, 10.13) | 3.75 (1.06, 13.28) |
| Total blood Hg (μg/L)g | |||||
| Quartile 1 (< 0.4) | 30 (10) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Quartile 2 (0.4–0.8) | 71 (19) | 2.18 (1.05, 4.52) | 2.25 (1.08, 4.68) | 2.25 (1.09, 4.66) | 2.27 (1.06, 4.83) |
| Quartile 3 (0.9–1.5) | 51 (16) | 1.72 (0.82, 3.59) | 2.04 (0.94, 4.46) | 2.03 (0.95, 4.33) | 2.14 (0.89, 5.12) |
| Quartile 4 (1.6–32.8) | 61 (17) | 1.84 (0.88, 3.87) | 2.33 (1.05, 5.19) | 2.32 (1.07, 5.03) | 2.51 (1.04, 6.03) |
| Urinary Hg (μg/L)h,i | |||||
| Quartile 1 (< 0.0029) | 28 (12) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Quartile 2 (0.0029–0.0063) | 30 (10) | 0.73 (0.35, 1.53) | 0.88 (0.42, 1.87) | 0.88 (0.40, 1.94) | 0.89 (0.42, 1.88) |
| Quartile 3 (0.0063–0.0135) | 28 (17) | 1.30 (0.55, 3.09) | 1.36 (0.58, 3.20) | 1.36 (0.58, 3.20) | 1.40 (0.60, 3.25) |
| Quartile 4 (0.0137–0.8873) | 33 (12) | 0.90 (0.41, 1.96) | 1.18 (0.49, 2.82) | 1.18 (0.49, 2.83) | 1.20 (0.50, 2.90) |
| aWeighted percent. bCrude models included Hg as the independent variable; separate models were performed for each source of Hg (hair, blood, urine). cAdjusted for age, race/ethnicity, education, serum cotinine, selenium, and indicator for NHANES cycle for multicycle models. dModel A + further adjusted for omega-3 fatty acids. eModel A + further adjusted for total seafood intake. fNHANES 1999–2000, one cycle (n = 452). gNHANES 1999–2004, three cycles (n = 1,352). hNHANES 1999–2002, two cycles (n = 804). iIncluded all participants with urinary Hg data, irrespective of availability of hair or blood data; all urinary Hg models (including crude) adjusted for urinary creatinine. | |||||
We performed sensitivity analyses including BMI and CRP as covariates in the logistic regression models for all mercury types and found no substantive changes in results. Likewise, in separate sensitivity analyses incorporating each PCB summary measure, the mercury associations with ANA positivity remained similar (see Supplemental Material, Table S1). Of the eight study participants (0.78 weighted percent) reporting utilization of a prescription drug associated with drug-induced autoimmunity (four using carbamazepine, four using minocycline), none were ANA positive. In the urinary mercury sensitivity analyses excluding participants with GFR < 60 mL/min/1.73 m2, there were no substantive changes in results.
Spline regression models showed a nonlinear dose–response relationship for log-transformed hair and total mercury (Figure 1). To examine mercury as a continuous variable, we fit adjusted piecewise logistic regression models, with cut points based on visual inspection of the spline graphs. The dose–response relationship for both hair and total blood mercury increased in a statistically significant fashion within the lower ranges of mercury exposure (through –1 ppm log hair mercury and 0 μg/L log total blood mercury) and then plateaued.
Figure 1.
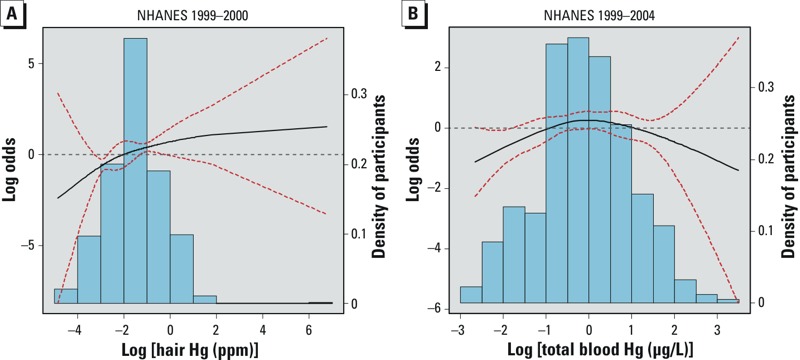
Associations of antinuclear antibody (ANA) positivity with log-transformed hair and total blood mercury (Hg), adjusted for Model B covariates. (A) Hair Hg (1999–2000; n = 452). (B) Total blood Hg (1999–2004; n = 1,352). Solid black lines represent the smoothing trends estimated from the natural spline with 3 degrees of freedom (df) for hair Hg and 4 df for total blood Hg (knots at 25th, 50th, and 75th percentiles); red dotted lines represent 95% CIs; and bars represent the weighted density distribution for Hg. The dose–response relationship for both hair and total blood Hg increased in a statistically significant fashion within the lower ranges of Hg exposure.
We also evaluated strength of ANA titer as an outcome. For both hair and total blood mercury, compared with the lowest mercury quantile, the upper quantiles contained a substantially higher proportion of individuals with high-titer ANA (≥ 1:1,280; Figure 2). Consistent with the logistic regression models, results from multinomial logistic regression (see Supplemental Material, Table S2) demonstrated a significant association between hair and total blood mercury, but not urinary mercury, and ANA positivity (data for urinary mercury not shown). Further, magnitudes of association were strongest for high-titer ANA (≥ 1:1,280), where adjusted ORs were > 10 for hair mercury and > 4 for total blood mercury.
Figure 2.
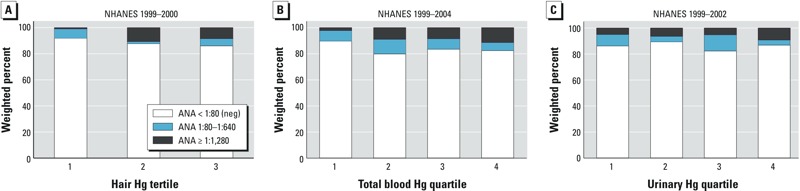
Weighted proportions of antinuclear antibody (ANA) positivity and titer categories according to mercury (Hg) exposure quantiles. (A) Hair Hg (1999–2000; n = 452). (B) Total blood Hg (1999–2004; n = 1,352). (C) Urinary Hg (1999–2002; n = 804).
Discussion
In this population-based study, we found that mercury exposure was associated with increased risk of high-titer ANA positivity among reproductive-age females in the general U.S. population. Specifically, this association appears to be driven by organic (methyl) mercury, the predominant species in hair and total blood. Notably, a dose–response relationship was observed for low methylmercury exposure levels (< 0.37 ppm hair mercury; < 1 μg/L total blood mercury), in the range generally considered safe for women of childbearing potential by regulatory agencies (Mergler et al. 2007). The predominant nuclear staining pattern of speckled found in our population is a marker of autoimmunity with a wide variety of clinical associations, including SLE, mixed connective tissue disease, Sjögren’s syndrome, and idiopathic inflammatory myopathies (Morehead 2008). The methylmercury association was robust across models, whereas other suspected risk factors in the multivariable models, including age and smoking, were not associated with ANA risk.
Our findings are compatible with murine data demonstrating development of autoimmunity in response to methylmercury exposure in genetically susceptible strains (Häggqvist et al. 2005; Hultman and Hansson-Georgiadis 1999). Results from human studies have been inconsistent regarding the relationship between organic mercury and autoimmunity. An ecologic study of two Brazilian riverine communities with high fish consumption found a suggestion of higher ANA prevalence in the community with higher average hair mercury levels (8 ppm vs. 6.4 ppm) (Silva et al. 2004). A further study in a riverine Brazilian community failed to detect an association (Alves et al. 2006); the mean hair mercury level in that study was 34.5 ppm, thus it is possible that a dose effect between methylmercury and ANA positivity could have been obscured if, as our data suggest, the response plateaued at a low exposure threshold. A study of females 12–85 years of age from one cycle of NHANES (2003–2004) failed to detect a significant association between total blood mercury and ANA positivity, although the nonlinear nature of association that we observed was not addressed in their analyses (Gallagher et al. 2013). Further, they did not report the titer for defining ANA positivity, and in their smaller sample (632 compared with 1,352 in our blood mercury analyses) statistical power may have been inadequate to detect an association.
Our study focused on females 16–49 years of age. It is well recognized that females have a higher risk of autoimmune diseases (Cooper et al. 2009; Somers et al. 2007, 2013, 2014), and that risk among females may also correlate with reproductive stage. Moreover, estrogenic hormones may promote autoimmunity (Somers and Richardson 2014). Mercury metabolism may also contrast between sexes, and differences in mercury excretion and distribution have been observed between sexes in mouse models (Hirayama and Yasutake 1986; Hirayama et al. 1987), as well as immunotoxic effects at lower internal doses in females (Nielsen and Hultman 2002).
Oxidative stress has been shown to contribute to the induction of autoimmune phenotypes in animal models, such as through epigenetic mechanisms converting normal helper T cells to autoreactive lymphocytes sufficient to cause lupus in the absence of added antigen (Somers and Richardson 2014). Mercury induces oxidative stress through sulfydryl reactivity and depletion of cellular antioxidants (Ercal et al. 2001). In human T cells treated with methylmercury, reductions in intracellular glutathione (GSH) concentration, glutathione S-transferase activity, and mitochondrial transmembrane potential have been observed, followed by generation of reactive oxygen species; intracellular GSH depletion has further been linked to susceptibility of T cells to undergo methylmercury-induced apoptosis (Shenker et al. 1999).
It is unclear why we did not find evidence linking inorganic mercury to ANAs because inorganic mercury has been more thoroughly linked to autoimmunity in animal models (Vas and Monestier 2008) and industrially exposed human populations (Cooper et al. 2004; Gardner et al. 2010; Silva et al. 2004). However, the higher doses in such studies limit their generalizability. Indeed, median urinary mercury levels were > 3.7 μg/L in a pair of studies in a Brazilian gold-mining population (Gardner et al. 2010; Silva et al. 2004) (compared with our median of 0.64 μg/L). Another distinction is that these studies used an ANA titer of ≥ 1:10 as detectable as well as a restricted dilution range (to 1:320); a more robust approach would be to employ higher titration levels to better assess strength of ANA positivity. Mechanisms of and degree of immunotoxicity may differ according to level of inorganic mercury. For instance, mercuric chloride at high concentrations (40 μM) has been associated with nonapoptotic cell death, rapid cellular permeabilization (Pollard et al. 1997), and modification of the nucleolar antigen fibrillarin from a 34-kDa non-disulfide–bonded form to a 32-kDa disulfide–bonded form. It is conceivable that structurally altered fibrillarin would be more immunogenic than its native form by unveiling of cryptic epitope(s), and together with cellular necrosis and permeabilization, could be more readily accessible to the immune system. At lower concentrations, fibrillarin migrated at both 32-kDa and the predominant 34-kDa forms, and greater cellular viability was maintained (Pollard et al. 1997). In contrast to inorganic species, organic mercury is lipophilic and more readily crosses cellular membranes, but it may demethylate intracellularly to inorganic mercury (Clarkson and Magos 2006), which may ultimately be more immunotoxic. It is plausible that subcytotoxic levels of organic mercury, such as those in our study, over long periods might yield higher intracellular doses of inorganic mercury and more efficient access to the nuclear environment than would occur with direct exposure to similarly low levels of inorganic mercury.
In our study, we found that speckled patterns predominated (96% of ANA positives). A variety of speckled ANA patterns can be seen by indirect immunofluorescence. Antigen specificities include U1-SnRNP (small nuclear ribonucleoproteins), Sm (Smith), U2-snRNP, U4/U6-snRNP, SSA/Ro, SSB/La, and other less common antigens (Bradwell et al. 2003). In contrast, the nucleolar pattern has primarily been reported in association with inorganic and methylmercury, with specificity of autoantibody formation to fibrillarin/U3RNP demonstrated in murine models in response to mercuric chloride (Hultman et al. 1989; Reuter et al. 1989). A proposed mechanism is that inorganic mercury cross-links with free sulfhydryls on two cysteines of fibrillarin, resulting in physiochemical protein modification (Pollard et al. 1997). Although anti-fibrillarin antibody formation is best recognized in response to inorganic mercury, anti-chromatin and anti-histone antibody formation have also been demonstrated (Hultman et al. 1996). For all three types of autoantibodies, the response varies according to mouse strain, underscoring the relevance of genetic susceptibility. Hultman et al. (1996) demonstrated that antibodies to fibrillarin and chromatin tended to persist several months following cessation of mercuric chloride treatment, whereas anti-histone antibodies resolved more rapidly. We found only 14 cases with the nucleolar pattern, none of which demonstrated anti-fibrillarin antigenicity upon immunoprecipitation. Only 3 cases had a nuclear homogeneous pattern, which would be compatible with histone or chromatin antigens. In humans, the nucleolar pattern, and particularly anti-fibrillarin antibodies, are associated with scleroderma, especially among blacks and males (Arnett et al. 1996). The rarity of scleroderma (prevalence ~ 27.6/100,000 adults) (Mayes et al. 2003) and its associated autoantibodies make it unlikely that our study would have adequate power to detect an association with these specific autoantibodies. However, it is difficult to explain why the speckled pattern was prominent in our study but not in the animal literature or human occupational studies. Whether organic mercury preferentially targets different nuclear antigens than does inorganic mercury, or whether an alternate biologic pathway is relevant to low compared with high doses of either species, remains to be elucidated.
Fish consumption is an exposure route common to both methylmercury and essential nutrients that may have beneficial impact on the immune system. Both organic and inorganic mercury are suggested to increase the production of prostaglandin E2 and phospholipase A2 (Mazerik et al. 2007), leading to release of arachidonic acid. Omega-3 fatty acids are an alternative substrate to arachidonic acid for cyclooxygenase and lipoxygenase enzymes, and they induce a series of anti-inflammatory eicosanoids (Simopoulos 2002). Thus, we incorporated omega-3 fatty acids into our modeling because of the potential for negative confounding (Choi et al. 2008). There was indeed a modest increase in the magnitudes of association for hair mercury when adjusting for omega-3 intake. PCBs are persistent toxicants with suggested immune effects (Gallagher et al. 2013; Heilmann et al. 2010) and fish consumption as an exposure route. The hair and total blood mercury associations were not appreciably altered with inclusion of PCBs in the models.
Limitations of our study include its cross-sectional nature, precluding the ability to determine the pattern and chronicity of mercury exposure, and persistence of ANA positivity or future risk of overt disease. However, the study of risk factors for preclinical disease is an important tool for dissecting the etiology of complex diseases with long latencies (Cooper 2009). Further, certain combinations of autoimmune diseases tend to co-occur within individuals and families (Cooper et al. 2009; Somers et al. 2006, 2009) to an extent inadequately explained by genetic background. The identification of shared environmental factors for immune dysregulation relevant to a variety of autoimmune phenotypes is an important goal (Dietert et al. 2010). The nonspecific nature of the ANA patterns documented here supports the premise of organic mercury as a risk factor for multiple autoimmune conditions. Future research is necessary to evaluate whether our study findings extend to other populations, including males and persons outside of the 16- to 49-year-age range.
Conclusions
We provide evidence for the first time, to our knowledge, that low levels of methylmercury exposure are linked to subclinical autoimmunity among females of reproductive age in the general population. Because autoantibody development is a marker of immune dysregulation and may predate clinical autoimmune diagnoses by several years, the prospect that organic mercury acts as an early but potentially modifiable trigger relevant to a spectrum of autoimmune conditions warrants more intense investigation.
Supplemental Material
Acknowledgments
We thank H. Hu (University of Toronto) and B. Richardson (University of Michigan) for their critical review of the manuscript.
Footnotes
This work was supported by grants K01ES019909 and P30ES017885 from the National Institute of Environmental Health Sciences, National Institutes of Health (NIH), and by grant UL1RR024986 from the National Center for Research Resources, NIH. E.C.S. was supported in part by an Arthritis Foundation Health Professional New Investigator Award.
E.C.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare they have no actual or potential competing financial interests.
References
- Agency for Toxic Substances and Disease Registry. 2013 ATSDR Substance Priority List. 2013. Available: http://www.atsdr.cdc.gov/SPL/index.html [accessed 15 January 2015]
- Alves MFA, Fraiji NA, Barbosa AC, De Lima DSN, Souza JR, Dórea JG, et al. 2006Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int J Environ Health Res 16255–262.; 10.1080/09603120600734147 [DOI] [PubMed] [Google Scholar]
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. 2003Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 3491526–1533.; 10.1056/NEJMoa021933 [DOI] [PubMed] [Google Scholar]
- Arnett FC, Reveille JD, Goldstein R, Pollard KM, Leaird K, Smith EA, et al. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum. 1996;39:1151–1160. doi: 10.1002/art.1780390712. [DOI] [PubMed] [Google Scholar]
- Bradwell AR, Hughes RG, Harden EL. Birmingham, UK: Binding Site; 2003. Atlas of HEp-2 Patterns. 2nd ed. [Google Scholar]
- Budtz-Jørgensen E, Grandjean P, Weihe P.2007Separation of risks and benefits of seafood intake. Environ Health Perspect 115323–327.; 10.1289/ehp.9738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Laboratory Procedure Manual: PCBs and Persistent Pesticides. 2006. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l28_c_met_ PCBs_and_Persistent_Pesticides.pdf [accessed 15 January 2015]
- CDC/NCHS (Centers for Disease Control and Prevention, National Center for Health Statistics). National Health and Nutrition Examination Survey. 1999 - 2000 Data Documentation, Codebook, and Frequencies: Hair Mercury (LAB22). 2005. Available: http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/LAB22.htm [accessed 15 January 2015]
- CDC/NCHS (Centers for Disease Control and Prevention, National Center for Health Statistics). National Health and Nutrition Examination Survey. 1999 - 2000 Data Documentation, Codebook, and Frequencies: Nutritional Biochemistries (LAB06). 2007. Available: http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/LAB06.htm [accessed 15 January 2015]
- CDC/NCHS (Centers for Disease Control and Prevention, National Center for Health Statistics). National Health and Nutrition Examination Survey. 1999 - 2000 Data Documentation, Codebook, and Frequencies: Immunofluorescence and Immunoprecipitation Analyses of Autoantibodies (NHANES Surplus Sera) (SSANA_A). 2012. Available: http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/SSANA_A.htm [accessed 15 January 2015]
- CDC/NCHS (Centers for Disease Control and Prevention, National Center for Health Statistics). NHANES Response Rates and Population Totals. 2013. Available: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm [accessed 15 January 2015]
- CDC/NCHS (Centers for Disease Control and Prevention, National Center for Health Statistics). Continuous NHANES Data, Questionnaires and Related Documentation. 2015a. Available: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm [accessed 15 January 2015]
- CDC/NCHS (Centers for Disease Control and Prevention, National Center for Health Statistics). National Health and Nutrition Examination Survey Homepage. 2015b. Available: http://www.cdc.gov/nchs/nhanes.htm [accessed 15 January 2015]
- Choi AL, Cordier S, Weihe P, Grandjean P.2008Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol 38877–893.; 10.1080/10408440802273164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L.2006The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36609–662.; 10.1080/10408440600845619 [DOI] [PubMed] [Google Scholar]
- Cooper GS.2009Unraveling the etiology of systemic autoimmune diseases: peering into the preclinical phase of disease. J Rheumatol 361853–1855.; 10.3899/jrheum.090682 [DOI] [PubMed] [Google Scholar]
- Cooper GS, Bynum MLK, Somers EC.2009Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun 33197–207.; 10.1016/j.jaut.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31:1928–1933. [PubMed] [Google Scholar]
- Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- Cuvin-Aralar MLA, Furness RW.1991Mercury and selenium interaction: a review. Ecotoxicol Environ Saf 21348–364.; 10.1016/0147-6513(91)90074-Y [DOI] [PubMed] [Google Scholar]
- Dietert RR, DeWitt JC, Germolec DR, Zelikoff JT.2010Breaking patterns of environmentally influenced disease for health risk reduction: immune perspectives. Environ Health Perspect 1181091–1099.; 10.1289/ehp.1001971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Fritzler MJ, Pauls JD, Kinsella TD, Bowen TJ. Antinuclear, anticytoplasmic, and anti-Sjogren’s syndrome antigen A (SS-A/Ro) antibodies in female blood donors. Clin Immunol Immunopathol. 1985;36:120–128. doi: 10.1016/0090-1229(85)90045-5. [DOI] [PubMed] [Google Scholar]
- Gallagher CM, McElroy AE, Smith DM, Golightly MG, Meliker JR.2013Polychlorinated biphenyls, mercury, and antinuclear antibody positivity, NHANES 2003–2004. Int J Hyg Environ Health 216721–727.; 10.1016/j.ijheh.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK.2010Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ Res 110345–354.; 10.1016/j.envres.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gómez-Aracena J, et al. 2002Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 3471747–1754.; 10.1056/NEJMoa020157 [DOI] [PubMed] [Google Scholar]
- Häggqvist B, Havarinasab S, Björn E, Hultman P.2005The immunosuppressive effect of methylmercury does not preclude development of autoimmunity in genetically susceptible mice. Toxicology 208149–164.; 10.1016/j.tox.2004.11.020 [DOI] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P.2010Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect 1181434–1438.; 10.1289/ehp.1001975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K, Yasutake A.1986Sex and age differences in mercury distribution and excretion in methylmercury-administered mice. J Toxicol Environ Health 1849–60.; 10.1080/15287398609530847 [DOI] [PubMed] [Google Scholar]
- Hirayama K, Yasutake A, Inoue M. Effect of sex hormones on the fate of methylmercury and on glutathione metabolism in mice. Biochem Pharmacol. 1987;36:1919–1924. doi: 10.1016/0006-2952(87)90489-8. [DOI] [PubMed] [Google Scholar]
- Hultman P, Eneström S, Pollard KM, Tan EM. Anti-fibrillarin autoantibodies in mercury-treated mice. Clin Exp Immunol. 1989;78:470–477. [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Hansson-Georgiadis H.1999Methyl mercury-induced autoimmunity in mice. Toxicol Appl Pharmacol 154203–211.; 10.1006/taap.1998.8576 [DOI] [PubMed] [Google Scholar]
- Hultman P, Turley SJ, Eneström S, Lindh U, Pollard KM. Murine genotype influences the specificity, magnitude and persistence of murine mercury-induced autoimmunity. J Autoimmun. 1996;9:139–149. doi: 10.1006/jaut.1996.0017. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013:1–24. [PubMed] [Google Scholar]
- Laden F, Bertrand KA, Altshul L, Aster JC, Korrick SA, Sagiv SK.2010Plasma organochlorine levels and risk of non-Hodgkin lymphoma in the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev 191381–1384.; 10.1158/1055-9965.EPI-10-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A, Invernizzi P, Gao B, Podda M, Gershwin ME.2010Definition of human autoimmunity—autoantibodies versus autoimmune disease. Autoimmun Rev 9A259–A266.; 10.1016/j.autrev.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC.2004Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect 112562–570.; 10.1289/ehp.6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. 2003Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 482246–2255.; 10.1002/art.11073 [DOI] [PubMed] [Google Scholar]
- Mazerik JN, Mikkilineni H, Kuppusamy VA, Steinhour E, Peltz A, Marsh CB, et al. 2007Mercury activates phospholipase A2 and induces formation of arachidonic acid metabolites in vascular endothelial cells. Toxicol Mech Methods 17541–557.; 10.1080/15376510701380505 [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, et al. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Meroni PL, Schur PH.2010ANA screening: an old test with new recommendations. Ann Rheum Dis 691420–1422.; 10.1136/ard.2009.127100 [DOI] [PubMed] [Google Scholar]
- Morehead K. In: Primer on the Rheumatic Diseases (Klippel JH, Stone J, Crofford L, White P, eds) New York: Springer; 2008. Evaluation of the patient. B. Laboratory assessment; pp. 15–20. [Google Scholar]
- Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002;110(suppl 5):877–881. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Hultman P, Kono DH.2010Toxicology of autoimmune diseases. Chem Res Toxicol 23455–466.; 10.1021/tx9003787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Lee DK, Casiano CA, Bluthner M, Johnston MM, Tan EM. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. J Immunol. 1997;158:3521–3528. [PubMed] [Google Scholar]
- Reuter R, Tessars G, Vohr HW, Gleichmann E, Lührmann R. Mercuric chloride induces autoantibodies against U3 small nuclear ribonucleoprotein in susceptible mice. Proc Natl Acad Sci USA. 1989;86:237–241. doi: 10.1073/pnas.86.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AM, Semchuk KM, McDuffie HH, Ledingham DL, Cordeiro DM, Cessna AJ, et al. Prevalence of antinuclear antibodies in a rural population. J Toxicol Environ Health A. 1999;57:225–236. doi: 10.1080/009841099157674. [DOI] [PubMed] [Google Scholar]
- Satoh M, Chan EKL, Ho LA, Rose KM, Parks CG, Cohn RD, et al. 2012Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 642319–2327.; 10.1002/art.34380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonen WM, Thomas SL, Somers EC, Smeeth L, Kim J, Evans S, et al. 2010Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol 70588–596.; 10.1111/j.1365-2125.2010.03733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield RH.2004Autoantibodies as predictors of disease. Lancet 3631544–1546.; 10.1016/S0140-6736(04)16154-0 [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Guo TL, O I, Shapiro IM.1999Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol 15723–35.; 10.1006/taap.1999.8652 [DOI] [PubMed] [Google Scholar]
- Silva IA, Nyland JF, Gorman A, Perisse A, Ventura AM, Santos ECO, et al. 2004Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in Amazon populations in Brazil: a cross-sectional study. Environ Health 311; 10.1186/1476-069X-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Somers EC, Antonsen S, Pedersen L, Sorensen HT.2013Parental history of lupus and rheumatoid arthritis and risk in offspring in a nationwide cohort study: does sex matter? Ann Rheum Dis 72525–529.; 10.1136/annrheumdis-2011-201165 [DOI] [PubMed] [Google Scholar]
- Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. 2014Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 66369–378.; 10.1002/art.38238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers EC, Richardson BC.2014Environmental exposures, epigenetic changes and the risk of lupus. Lupus 23568–576.; 10.1177/0961203313499419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers EC, Thomas SL, Smeeth L, Hall AJ.2006Autoimmune diseases co-occurring within individuals and within families: a systematic review. Epidemiology 17202–217.; 10.1097/01.ede.0000193605.93416.df [DOI] [PubMed] [Google Scholar]
- Somers EC, Thomas SL, Smeeth L, Hall AJ.2009Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am J Epidemiol 169749–755.; 10.1093/aje/kwn408 [DOI] [PubMed] [Google Scholar]
- Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ.2007Incidence of systemic lupus erythematosus in the United Kingdom, 1990–1999. Arthritis Rheum 57612–618.; 10.1002/art.22683 [DOI] [PubMed] [Google Scholar]
- Stern AH, Smith AE.2003An assessment of the cord blood:maternal blood methylmercury ratio: implications for risk assessment. Environ Health Perspect 1111465–1470.; 10.1289/ehp.6187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Griffiths C, Smeeth L, Rooney C, Hall AJ.2010Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am J Public Health 1002279–2287.; 10.2105/AJPH.2009.180273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Landrigan PJ, Schechter C.2005Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ Health Perspect 113590–596.; 10.1289/ehp.7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. 2006The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93223–241.; 10.1093/toxsci/kfl055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas J, Monestier M.2008Immunology of mercury. Ann NY Acad Sci 1143240–267.; 10.1196/annals.1443.022 [DOI] [PubMed] [Google Scholar]
- Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90:1463–1466. doi: 10.2105/ajph.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies [Letter]. Environ Health Perspect. 1997;105:13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


