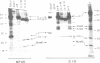Abstract
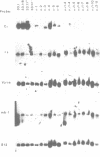
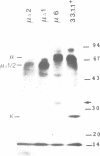
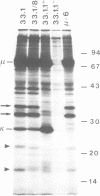
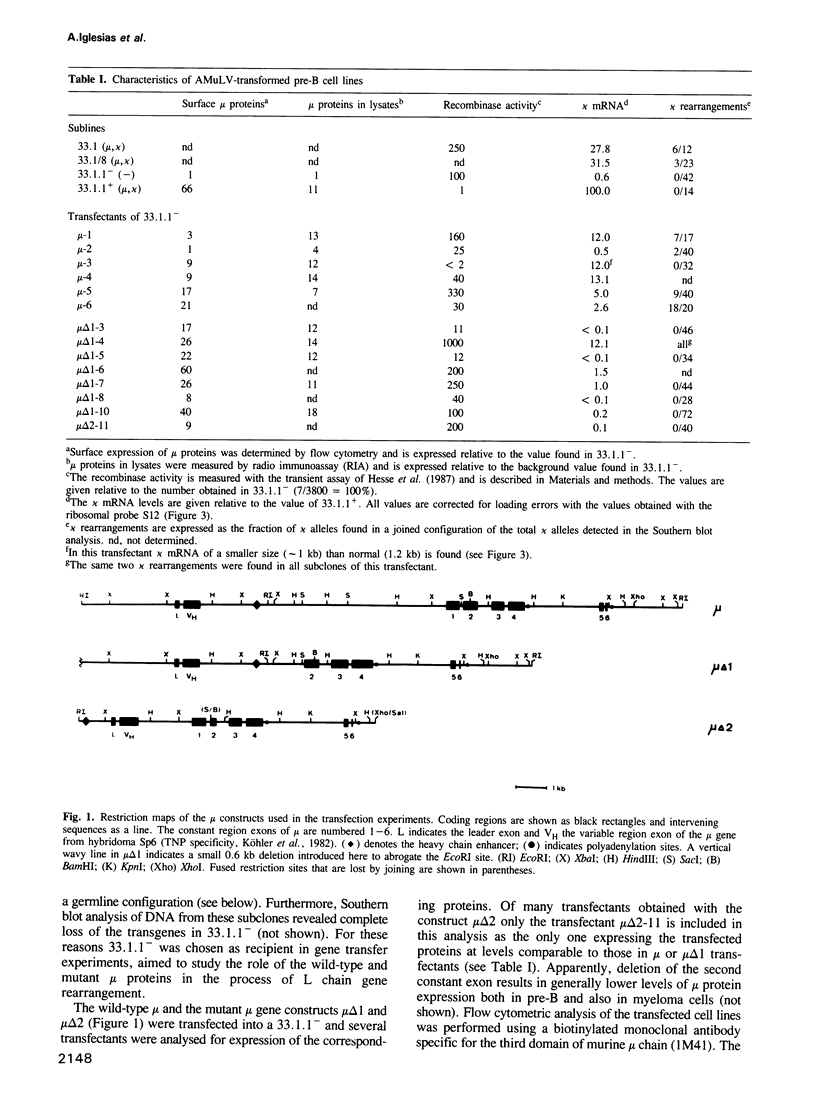
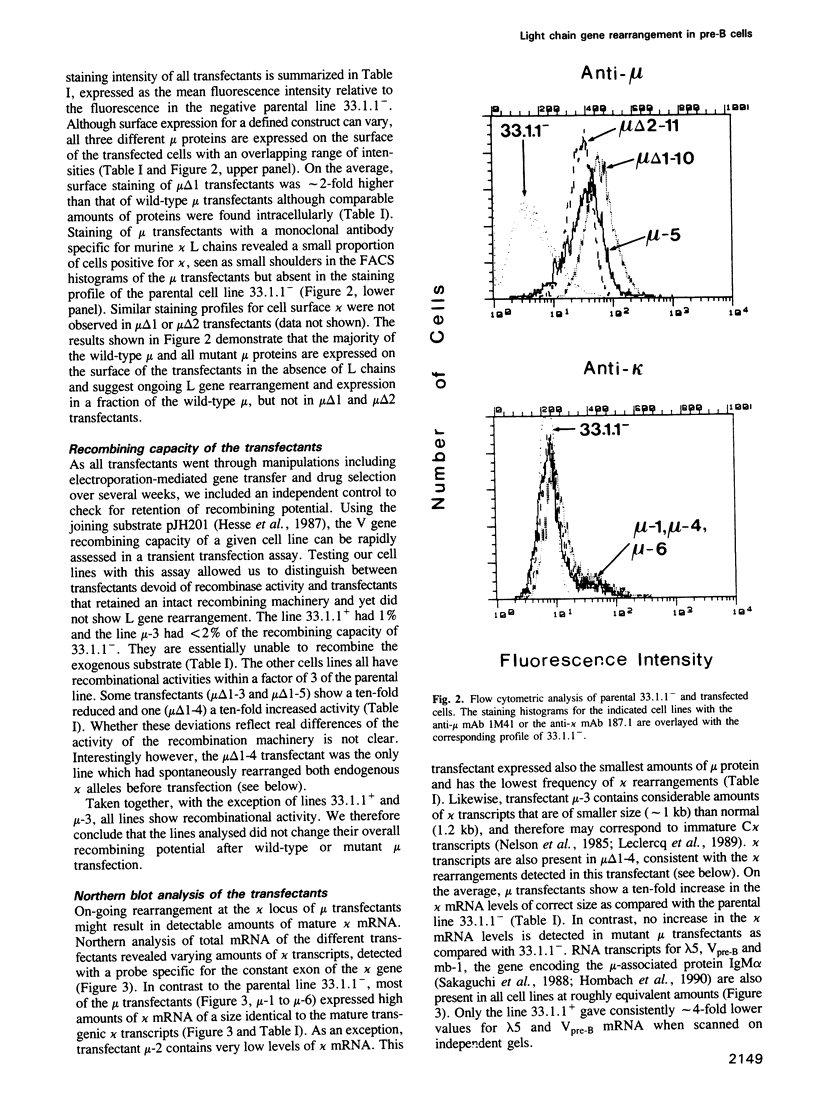
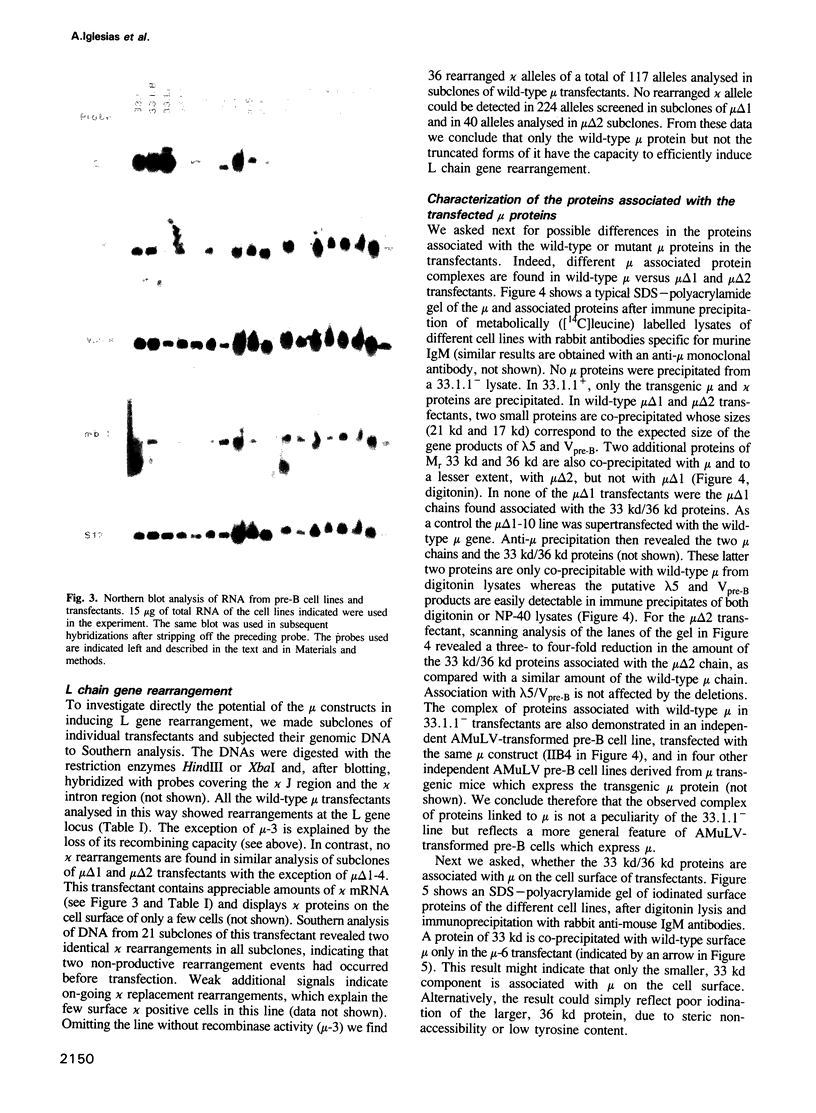
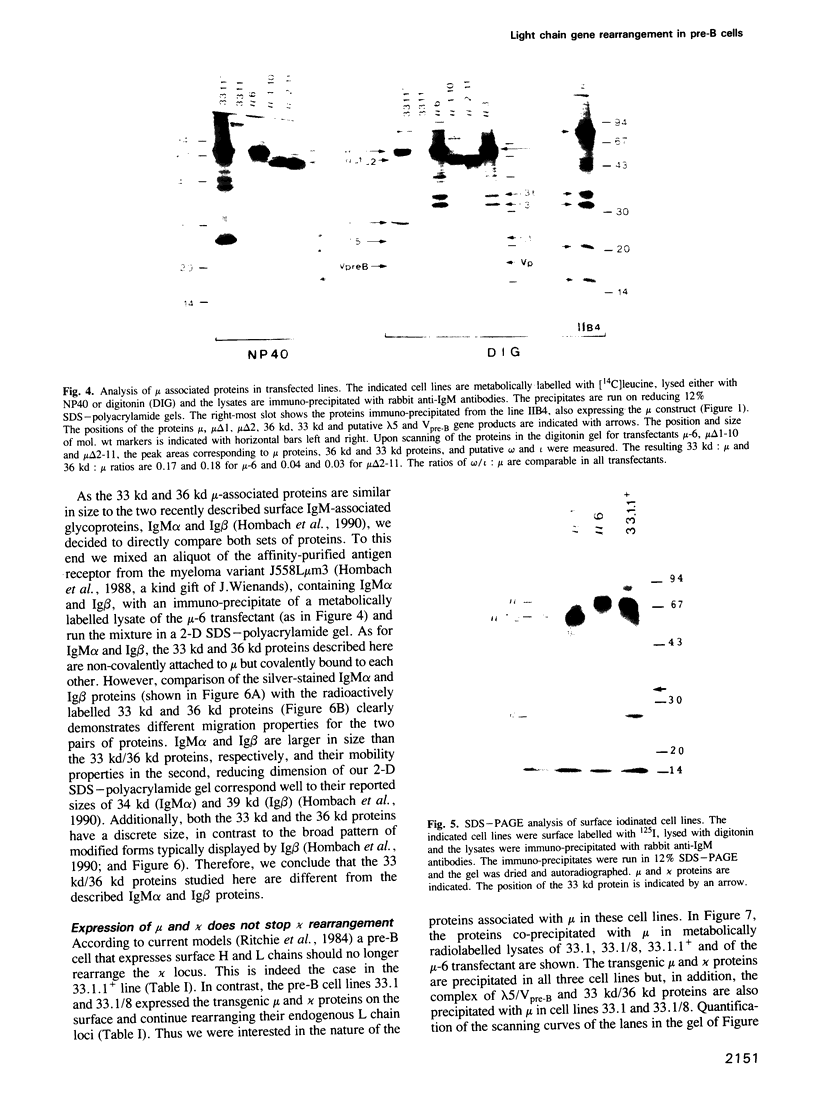
During B cell differentiation rearrangement of immunoglobulin (Ig) genes is partially regulated by the Ig proteins. Rearrangement of heavy (H) chain genes is inhibited, whilst that of light (L) chain genes is induced by the membrane form of the mu H chain. In order to analyse additional structural requirements of mu induced L chain gene rearrangement we transfected wild-type mu and mutant mu constructs lacking functional exons encoding the first or second constant domains into Abelson murine leukemia virus (AMuLV) transformed pre-B cells. All mu chains are expressed on the surface of the pre-B cell and all associate with omega and iota, two proteins forming a surrogate light chain, necessary for mu membrane expression. Nevertheless, only wild-type mu and not the mutant mu proteins promote L gene rearrangement. A heterodimer of proteins with Mr of 33 kd and 36 kd was found associated with wild-type but not with the mutant mu proteins. Continuous presence of mu is required for L chain gene recombination since loss of mu stopped and readdition of mu started L gene rearrangement. We propose that the protein complex composed of mu and the 33 kd/36 kd protein heterodimer is responsible for the activation of the L chain gene locus and its rearrangement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayane M., Nielsen P., Köhler G. Cloning and sequencing of mouse ribosomal protein S12 cDNA. Nucleic Acids Res. 1989 Aug 25;17(16):6722–6722. doi: 10.1093/nar/17.16.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Ferrier P., Malynn B. A., Pollock R. R., Covey L. R., Suh H. Y., Heinke L. B., Fulop G. M., Phillips R. A., Yancopoulos G. D. The effect of the scid mutation on mechanism and control of immunoglobulin heavy and light chain gene rearrangement. Curr Top Microbiol Immunol. 1989;152:85–94. doi: 10.1007/978-3-642-74974-2_12. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Malynn B. A., Pollock R. R., Ferrier P., Covey L. R., Fulop G. M., Phillips R. A., Yancopoulos G. D., Alt F. W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989 Mar;8(3):735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Haustein D. Effective radioiodination by lactoperoxidase and solubilisation of cell-surface proteins of cultured murine T lymphoma cells. J Immunol Methods. 1975 Apr;7(1):25–38. doi: 10.1016/0022-1759(75)90127-1. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Lamers M., Köhler G. Expression of immunoglobulin delta chain causes allelic exclusion in transgenic mice. Nature. 1987 Dec 3;330(6147):482–484. doi: 10.1038/330482a0. [DOI] [PubMed] [Google Scholar]
- Kerr W. G., Cooper M. D., Feng L., Burrows P. D., Hendershot L. M. Mu heavy chains can associate with a pseudo-light chain complex (psi L) in human pre-B cell lines. Int Immunol. 1989;1(4):355–361. doi: 10.1093/intimm/1.4.355. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Cooper M. D., Carroll A. J., Burrows P. D. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Potash M. J., Lehrach H., Shulman M. J. Deletions in immunoglobulin mu chains. EMBO J. 1982;1(5):555–563. doi: 10.1002/j.1460-2075.1982.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq L., Butkeraitis P., Reth M. A novel germ-line JK transcript starting immediately upstream of JK1. Nucleic Acids Res. 1989 Sep 12;17(17):6809–6819. doi: 10.1093/nar/17.17.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M., Potash M. J., Grützmann R., Heusser C., Shulman M., Köhler G., Melchers F. Monoclonal antibodies specific for murine IgM I. Characterization of antigenic determinants on the four constant domains of the mu heavy chain. Eur J Immunol. 1984 Jun;14(6):534–542. doi: 10.1002/eji.1830140610. [DOI] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane mu, but not by secreted mu heavy chains. J Exp Med. 1988 Oct 1;168(4):1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Maizel J. V., Jr, Leder P. The nucleotide sequence of a 5.5-kilobase DNA segment containing the mouse kappa immunoglobulin J and C region genes. J Biol Chem. 1981 May 25;256(10):5116–5120. [PubMed] [Google Scholar]
- Melchers F., Strasser A., Bauer S. R., Kudo A., Thalmann P., Rolink A. Cellular stages and molecular steps of murine B-cell development. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):183–189. doi: 10.1101/sqb.1989.054.01.023. [DOI] [PubMed] [Google Scholar]
- Misener V., Jongstra-Bilen J., Young A. J., Atkinson M. J., Wu G. E., Jongstra J. Association of Ig L chain-like protein lambda 5 with a 16-kilodalton protein in mouse pre-B cell lines is not dependent on the presence of Ig H chain protein. J Immunol. 1990 Aug 1;145(3):905–909. [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Kelley D. E., Perry R. P. Inducible transcription of the unrearranged kappa constant region locus is a common feature of pre-B cells and does not require DNA or protein synthesis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5305–5309. doi: 10.1073/pnas.82.16.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Campos-Torres J., Leder P. Allelic exclusion in transgenic mice carrying mutant human IgM genes. J Exp Med. 1988 Jun 1;167(6):1969–1974. doi: 10.1084/jem.167.6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. The omega and iota surrogate immunoglobulin light chains. Curr Top Microbiol Immunol. 1988;137:136–139. doi: 10.1007/978-3-642-50059-6_20. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Petrac E., Wiese P., Lobel L., Alt F. W. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987 Nov;6(11):3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K. A., Brinster R. L., Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in kappa transgenic mice. Nature. 1984 Dec 6;312(5994):517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. 1985 Mar 28-Apr 3Nature. 314(6009):330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Sitia R., Neuberger M., Alberini C., Bet P., Fra A., Valetti C., Williams G., Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990 Mar 9;60(5):781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Takemori T., Mizuguchi J., Miyazoe I., Nakanishi M., Shigemoto K., Kimoto H., Shirasawa T., Maruyama N., Taniguchi M. Two types of mu chain complexes are expressed during differentiation from pre-B to mature B cells. EMBO J. 1990 Aug;9(8):2493–2500. doi: 10.1002/j.1460-2075.1990.tb07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Wienands J., Hombach J., Radbruch A., Riesterer C., Reth M. Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 1990 Feb;9(2):449–455. doi: 10.1002/j.1460-2075.1990.tb08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Venkitaraman A. R., Gilmore D. J., Neuberger M. S. The sequence of the mu transmembrane segment determines the tissue specificity of the transport of immunoglobulin M to the cell surface. J Exp Med. 1990 Mar 1;171(3):947–952. doi: 10.1084/jem.171.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]