Abstract
Objective: To discuss the use of Grafix®, a commercially available, cryopreserved placental membrane, for the treatment of chronic/stalled wounds of different etiologies.
Approach: To describe the unique composition of Grafix, to provide an overview of the existing clinical evidence supporting the benefits of Grafix for wound treatment, and to share the experience of the South Shore Hospital Center for Wound Healing (Weymouth, MA) with Grafix for the treatment of nonhealing wounds.
Results: Clinical evidence supports the safety and efficacy of Grafix for the treatment of chronic/stalled wounds, including those that have failed other advanced treatment modalities.
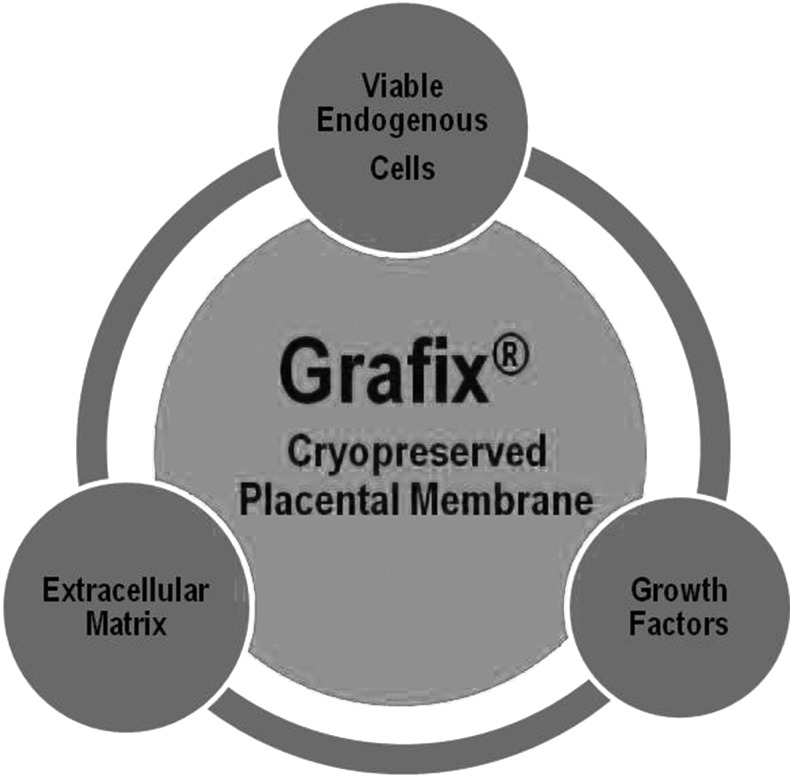
Innovation: Grafix is a cryopreserved placental membrane manufactured utilizing a novel technology that enables the preservation of all placental membrane components in their native state. Placental membranes have a unique composition of extracellular matrix, growth factors, and cells (including mesenchymal stem cells), which makes this tissue unique among other advanced biological wound treatment modalities.
Conclusion: Clinical evidences support the benefits of Grafix for head-to-toe wound treatment.

Gary W. Gibbons, MD, FACS
Introduction
Chronic wounds are wounds that fail to repair in a timely and orderly manner due to their inability to progress from the inflammatory phase into the proliferative phase and ultimately into the remodeling phase.1 Chronic wounds are estimated to affect more than 6.5 million patients in the United States alone, and more than $50 billion annually is spent.2,3 Some new economic data suggest that $15 billion is spent treating diabetic foot ulcers (DFUs), $18 billion on venous leg ulcers (VLUs), and $13 billion on pressure ulcers without even the inclusion of acute and traumatic wounds.4–8 One to two percent of the population in developed countries will experience a chronic wound due to increased life expectancy coupled with a rise in comorbidities—specifically diabetes, obesity, venous hypertension, and peripheral vascular disease.9
Various standardized treatment algorithms for the treatment of chronic wounds have been developed by the American Diabetes Association (ADA), the Wound Healing Society (WHS), the American Society of Plastic Surgeons, and other societies for different wound types.10–12 Early aggressive surgical debridement and control of infection, appropriate compression for venous ulcers, off-loading or complete pressure relief for diabetic foot and pressure ulcers, and timely revascularization are the most common approaches recommended by the Chronic Wound Care Guidelines, a publication of the WHS. However, the implementation of these guidelines is frequently fragmented or silo/specialty driven. For example, the evidence to support the use of off-loading devices for healing foot ulcers is clear. A total contact cast (TCC) is considered the gold standard for off-loading plantar foot ulcers; however, it is the preferred treatment in only 2% of centers in the United States.13–15 Closure rates, even when using appropriate standard of care (SOC) for chronic wounds, vary from 21% to 35% depending on the type of wound.16 SOC for VLUs, for instance, is often insufficient as evidenced by stalled healing and high recurrence rates.17 Wounds that do not heal with SOC place the patient at an increased risk for wound-related morbidities and mortality, as well as increased costs to the patient and the healthcare system.15 In such cases, physicians should consider advanced therapies that provide the components necessary for wound healing—cells, including mesenchymal stem cells (MSCs), growth factors, and extracellular matrix (ECM) proteins—as found in autologous skin grafts.18–20
Recently, literature has supported the early adoption of advanced care products for wounds that do not respond to SOC in a timely manner (4 weeks).21 These advanced products, such as human skin equivalents and topical recombinant growth factors, have been shown to reduce overall costs and prevent amputations.22,23 Skin substitutes have held the most promise for healing chronic wounds because they contain some components needed for wound healing, but they are deficient due to a lack of MSCs, which are present in normal healthy skin.24 The beneficial effect of MSCs on wound healing has been observed in a variety of animal models and in reported clinical cases. Specifically, MSCs have been successfully used to treat chronic wounds and stimulate the stalled healing processes.18,25,26
Recent studies have revealed that human placental membranes are a rich source of MSCs, collagen matrix, and growth factors, and they can support tissue regeneration and repair.27,28 Placental membranes have a long history of use for wound treatment, and their beneficial effects have been, in part, attributed to their anti-inflammatory, antimicrobial, and angiogenic activities.29–32 The membranes also create a moist environment, which is important for healing.33 Currently, there are more than 25 commercial placental products, but they are all devitalized. Grafix® (Osiris Therapeutics, Inc., Columbia, MD) is a cryopreserved, human placental membrane and the only commercially available placental membrane product to contain viable endogenous cells. Grafix is manufactured using a novel technology that facilitates the retention of all inherent components of the native tissue: three-dimensional extracellular matrix, inherent growth factors, and living cells (including epithelial cells, fibroblasts, and MSCs).18 Recent in vitro studies demonstrate that preservation of endogenous viable cells in placental membranes enhances the anti-inflammatory, antioxidant, chemoattractive, and angiogenic activities of placental membranes compared to devitalized membranes.34–36 In clinical studies, treatment with Grafix led to high wound closure rates for difficult-to-treat chronic wounds, which suggests that Grafix is a promising new wound treatment therapy.37,38 The purpose of this technology report is to overview the composition of Grafix and clinical evidence for the use of Grafix for stalled wounds.
Clinical Problem Addressed
Despite a variety of advanced wound care products available, nonhealing wounds remain a large and growing problem, particularly chronic wounds such as DFUs, VLUs, and pressure ulcers. Patients with nonhealing wounds have a poor quality of life and are at the greatest risk for wound-related morbidities.38 Treatment of such patients puts an enormous financial burden on our society. Grafix, a cryopreserved placental membrane allograft, is a new advanced wound care product that has recently become available for such patients. The unique composition of Grafix offers a safe and effective treatment modality for patients with nonhealing wounds.
Materials and Methods
Grafix technology
Grafix is a cryopreserved placental membrane in which the structural and cellular integrity of fresh placental membranes is preserved through an innovative technology. Grafix contains a collagen-rich matrix, growth factors, cytokines, and endogenous viable cells, including epithelial cells, fibroblasts, and MSCs (Fig. 1).18,34 Previous attempts to preserve viable endogenous cells in placental membranes have resulted in <50% cell viability post-thaw.40 The cryopreservation process used to manufacture Grafix has been shown to result in >80% cell viability post-thaw.34 The benefits of retaining viable endogenous cells in placental membranes have been demonstrated in a series of in vitro studies. Results show that preservation of viable cells enhances wound-relevant properties, such as anti-inflammatory and cell recruitment, of cryopreserved placental membranes compared to devitalized membranes.34,35 The cells and growth factors present in Grafix are integrally associated with a collagen-rich ECM. Current bioengineered or advanced wound care products are deficient in one or more of these components. The presence of MSCs is a unique characteristic of Grafix, which differentiates this product from other advanced wound care therapies. Although the contribution of MSCs to the biological activities of placental membranes remains to be fully elucidated, accumulated data demonstrate that MSCs play an important role in all phases of healing, and their presence supports the achievement of full wound closure.18,25,26 As such, the therapeutic application of MSCs has been shown to enhance and improve wound healing in clinical settings.18
Figure. 1.
Grafix®, a cryopreserved placental membrane, maintains all components of fresh placental tissue, which includes extracellular matrix, growth factors, and viable endogenous cells (epithelial cells, fibroblasts, and mesenchymal stem cells), in their native state.
Grafix regulatory status and intended use
Grafix is regulated by the FDA under 21 CFR Part 1271 Part 361 Human Cells, Tissues, and Cellular and Tissue-based Products (HCT/Ps). It is intended for use as a wound cover and can be used for the treatment of both acute and chronic wounds, including but not limited to DFUs, VLUs, pressure ulcers, burns, surgical incisions and dehiscence, pyoderma gangrenosum, and epidermolysis bullosa. Extensive donor screening and serological, bioburden, and sterility testing are performed on every lot to demonstrate suitability for transplantation. Each lot is additionally tested to confirm at least 70% cellular viability post-thaw.
Grafix storage, preparation, and application to the wound
Grafix is stored at −80°C before use and has a 2-year shelf life. Grafix is supplied in a cryogenic bag filled with a cryoprotectant solution. It is mounted on a plastic applicator for easy handling. Grafix should be thawed before application. Figure 2 shows the preparation and application procedure as per the manufacturer's recommendations. To apply, slightly bend the plastic applicator to remove the top solid cover, and slide Grafix from the perforated backing onto the wound bed using aseptic technique (Fig. 2). Grafix is flexible and conforms and adheres to complex anatomies. Thus, it does not require suturing or other methods of fixation. Before treatment with any wound care product, wounds are recommended to be appropriately cleaned and debrided. After applying Grafix, a nonadherent dressing should be placed over Grafix to fully cover both the membrane and the wound bed. Saline-moistened gauze (for dry wounds) or a foam dressing (for draining wounds) should be placed on top of the nonadherent dressing, followed by an appropriate compressive or off-loading outer dressing. Patients should use an appropriate off-loading shoe, boot, or walker. It is recommended to apply Grafix on a weekly basis.37
Figure. 2.
Preparation and application of Grafix. This preparation and application guide is provided by Osiris Therapeutics, Inc., the manufacturer of Grafix.
Grafix should not be applied to acute or chronic wounds with an active untreated infection and in patients with known hypersensitivity to gentamicin, vancomycin, or Amphotericin B. These three antibiotics are used during tissue processing for bioburden reduction. Although the antibiotics are removed from the tissue by rinsing, some residual amount may remain.
Clinical Results
Previously published clinical data
The safety and efficacy of Grafix have been demonstrated in two recently published clinical studies, the results of which are recently reported.37,38 One study was a multicenter, single-blinded, randomized controlled clinical trial conducted by Lavery et al. comparing the treatment of DFUs with SOC versus Grafix. The primary endpoint was the proportion of patients with complete wound closure after 12 weeks, and secondary endpoints included time to wound closure, number of adverse events, and wound closure in the crossover phase (open-label treatment for patients who failed to close with SOC).37 One unique, important trial design element was blinded imaging verification of wound closure. This was the first randomized controlled trial (RCT) for advanced wound care products, in which the primary endpoint was confirmed by third party, blinded wound care experts to further remove potential bias and to increase the reliability of the results. Lavery et al. have also stated that this is the only multicenter DFU trial to date that meets statistically significant prespecified interim analysis.37 Ninety-seven patients were enrolled in the trial: 50 were treated with Grafix & SOC and 47 received SOC alone. SOC included wound debridement of necrotic tissues, application of a nonadherent dressing (Adaptic®, Systagenix, Gatwick, UK), and either saline-moistened gauze or Allevyn® (Smith & Nephew, London, United Kingdom) for moderately draining wounds followed by an outer dressing. Patients received walking boots if the wound was on the sole of the foot or a postoperative shoe if the wound was on the dorsum of the foot or ankle. Patients were evaluated weekly, and Grafix was applied weekly. There were no statistically significant differences in patient demographics or baseline characteristics between the Grafix and SOC groups. Results revealed that treatment of DFUs with Grafix showed a statistical improvement in wound closure compared to SOC—62% versus 21.3%, respectively (p=0.0001). Relative improvement is a measure comparing the effect of the treatment arm to the control arm, which allows comparisons between different trials. In the clinical study by Lavery et al., the relative improvement was 191% compared with SOC—calculated as (% wound closure with Grafix−% wound closure with SOC) / % wound closure with SOC.37 A previously published analysis of RCTs shows that among cellular products, the highest relative improvement was 64% for Dermagraft (Organogenesis, Canton, MA) over the control group.41 Overall, TCC had the highest relative improvement of 81%.41 Therefore, to date, Grafix has the highest relative improvement among advanced wound care products with RCT data.
In addition, Grafix also demonstrated decreased complications associated with DFUs, particularly the rate of wound-related infection, which translated into decreased hospitalizations. There were significantly fewer patients with wound-related infections in the Grafix treatment arm compared to SOC (18.0% vs. 36.2%, p=0.044), which can be explained by faster wound closure in the Grafix arm (42 days vs. 69.5 days median time to wound closure for Grafix vs. SOC, p=0.019).37 In addition, it is known that placental membranes have antibacterial properties, which could contribute to the lower rate of infections that may be linked to these properties.29 In the Lavery et. al. trial, no immunological adverse events related to the use of allogeneic tissue containing viable cells were recorded.37 These data are in agreement with previous clinical reports in which placental tissue was used for wound treatment.40,42 The wound closure with Grafix was durable: 82.1% of patients remained closed after 12 weeks of follow-up. Patients in the control arm who failed to heal during the initial 12-week treatment period could cross over to receive Grafix in the open-label crossover phase of the trial (26 patients enrolled). After Grafix treatment for up to an additional 12 weeks, the probability of wound closure in the crossover phase was 67.8% with a mean time to closure of 42 days.37
The second study was a large, retrospective single-center study conducted by Regulski et al.38 Data for all wound care patients who received at least one Grafix application between April 2010 and March 2012 were collected retrospectively through chart review by the treating physicians at the Ocean County Foot and Ankle and the Wound Institute of New Jersey. At each visit, the physician would assess the wound and determine if the Grafix treatment was appropriate. Grafix was applied to patients whose wounds were present for 4 weeks or longer, and the underlying morbidity and infection were adequately treated. Grafix was applied directly to wounds that were prepared by sharp debridement followed by application of a non-adherent dressing, saline-moistened gauze, and dry gauze. Depending on the wound type, multilayer compressing bandaging (for VLUs) and/or off-loading devices, such as TCC, surgical shoe, or DH Offloading Walker® (Ossur Americas, Foothill Ranch, CA), were provided. Wounds were measured with a ruler, and the treating physician determined wound closure by 100% reepithelialization and no evidence of drainage.
This study consisted of 66 patients with 67 chronic wounds and demonstrated a 76.1% wound closure rate for all types of wounds. The types of wounds in this study included DFUs (n=27), VLUs (n=34), and other chronic wounds (n=6). The average wound size at baseline was 6.65±9.68 cm2, and the average wound duration before Grafix treatment was 38±70.8 weeks. Fifty patients (74.6%) had failed to heal using other advanced therapies, which included collagen dressings, acellular matrices, skin grafts, cellular skin substitutes, hyperbaric oxygen therapy, wound vacuum, and topical growth factors. After 12 weeks of care, 51 of the 67 wounds (76.1%) were healed: 23 of 27 (85.2%) DFUs, 23 of 34 (67.6%) VLUs, and 5 of 6 (83.3%) other wounds. The average time to closure in these wounds was 5.8±2.5 weeks with an average 3.2 applications for patients who healed. No adverse events attributed to the use of Grafix were reported for this study. No wound recurrences occurred during an average follow-up time of 20.4 months.38 This study demonstrates that Grafix shows benefits not only for DFUs but also for other types of chronic wounds. In addition, the patient population includes refractory or difficult-to-treat patients, which is usually excluded from controlled clinical trials. However, the absence of a control group is a limitation of the study due to its retrospective nature.
The South Shore Hospital Center for Wound Healing experience with Grafix
The South Shore Hospital Center for Wound Healing (Weymouth, Massachusetts) has a growing experience using Grafix in patients experiencing difficult-to-heal wounds, which have an adverse impact on their quality of life. The short form 36 health survey (SF-36) is used to assess patients' quality of life, and we observed that the scores for many patients with chronic wounds are comparable to those of cancer patients. Patients are selected to be treated with Grafix if their wound does not reduce by 50% after 4 weeks of treatment with proper off-loading and adequate vascular status or if wounds persist for >2 years. In addition, many of the wounds seen at the wound care center have failed other advanced wound therapies, including Apligraf® (Organogenesis, Canton, MA), and therefore, are also candidates for treatment with Grafix. To date, approximately 10–15 patients have been treated or continue to receive treatment with Grafix. Although it is recommended for Grafix to be applied weekly, we sometimes apply Grafix bi-weekly if the wound size is rapidly decreasing. Below is a presentation of three unique cases of nonhealing wounds of different etiologies treated with Grafix at the South Shore Hospital Center for Wound Healing.
Stage IV sacral wound case
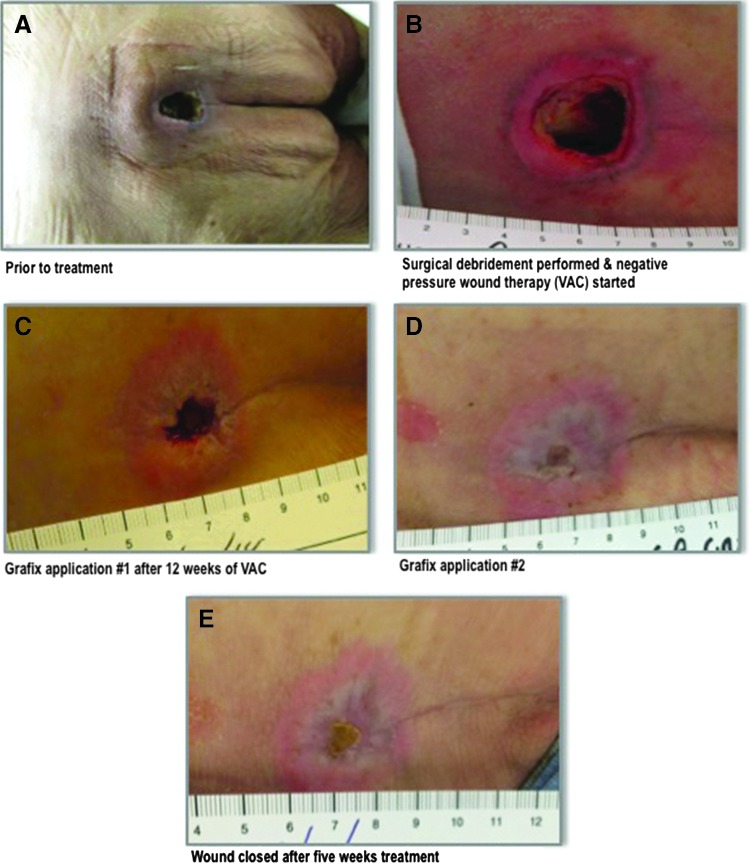
An 82-year-old male was transferred to the wound care clinic from a rehabilitation center where he had been admitted after sustaining a fall and developing pneumonia. The patient presented with a stage IV sacral wound of 3 months duration measuring 2.5×2.5×1.6 cm (Fig. 3A). Past medical history included Parkinson's disease and both urinary and bowel incontinence. A collagenase ointment was being applied at the time of admission to our clinic. Neither off-loading nor cushioning for the wound was previously prescribed.
Figure. 3.
(A) A 2.5×2.5×1.6 cm stage IV sacral wound persisting for 3 months in an 82-year-old male before treatment. (B) Wound was surgically debrided to a 2 cm depth. A negative pressure wound vacuum (VAC), off-loading cushion, and air alternating mattress were prescribed. (C) First Grafix applied after 12 weeks of VAC, which was discontinued at this time. (D) Three weeks after first Grafix application. (E) Complete wound closure was recorded after two Grafix applications over 5 weeks.
The patient was taken to the operating room where the wound was radically debrided to a depth of 2.0 cm (Fig. 3B). Cultures taken at this time were negative. Postoperatively, a negative pressure wound vacuum (VAC) (125 mm/Hg) was prescribed as well as an off-loading cushion and air alternating mattress.
After 12 weeks of VAC and proper off-loading, the wound showed a well granulating base that measured 0.8×0.8×0.3 cm (Fig. 3C). At this point, VAC was discontinued because the noise and its effect on patient mobility had a significant negative impact on the quality of life for the patient. Grafix was a convenient option for this patient, and we believed that Grafix would close this wound faster than VAC. The wound was primed to receive Grafix, and upon application, the patient was discharged to return home where proper off-loading and cushioning were continued.
After 3 weeks of follow-up care in the wound clinic, the wound measured 0.5×0.6×0.1 cm (Fig. 3D). The wound was cleansed and debrided of nonviable tissue, and a second application of Grafix was applied to the wound. The patient was maintained on proper off-loading and followed for the next 2 weeks. In conclusion, 4 months after initial presentation of this patient, while under the management of a multidisciplinary team dedicated to wound healing, the wound was completely reepithelialized after two applications of Grafix over a 5-week period (Fig. 3E). To date, 15 months post Grafix application, this patient's wound has remained closed restoring him with improved quality of life and function.
Diabetic foot ulcer case
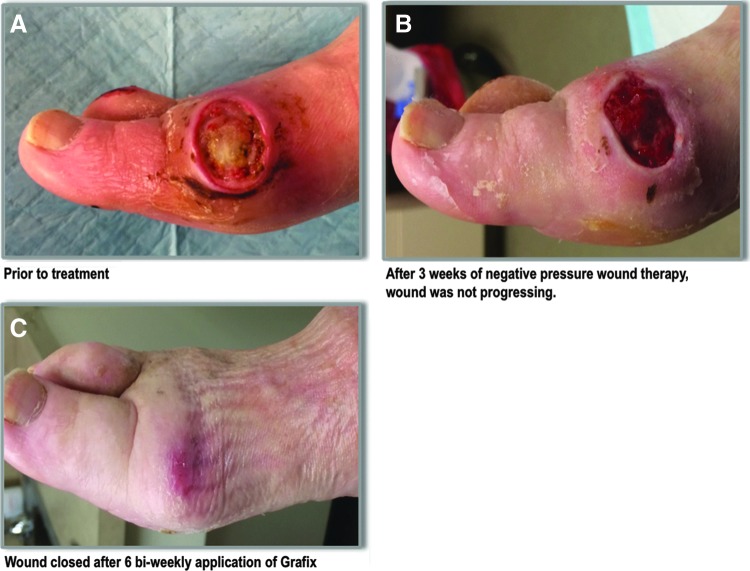
A 69-year-old insulin-dependent diabetic female presented to the wound care center with an ulceration on the medial aspect of the right first metatarsal head after wearing new shoes and cellulitis extending up the lower extremity was noted. The wound measured 1.5×2.9×0.3 cm and probed to capsule, but not into the joint (Fig. 4A). There was a mild peripheral arterial disease upon examination. The wound was aggressively debrided; radiographs were negative for osteomyelitis. Cultures were taken, the foot was off-loaded, and the patient was prescribed clindamycin and levaquin and instructed to return to the wound care center in 48 h. Upon return, it was noted that the cellulitis was responding to the antibiotic treatment. Wound cultures were positive for Streptococcus aureus and α-Streptococcus, and clindamycin therapy was continued. The wound was further debrided down to the exposed capsule, and negative pressure wound therapy (125 mm/Hg) was applied. The patient was followed up in the clinic for the following 3 weeks with serial debridements, negative pressure therapy, and off-loading (multilayer felt foam) to achieve optimal wound bed preparation.
Figure. 4.
(A) An ulceration on the medial aspect of the right first metatarsal head from wearing a new shoe in a 69-year-old insulin-dependent diabetic female before treatment. Cellulitis extending up the lower extremity was noted. The wound probed down to capsule, but not to the joint. (B) She received 3 weeks of continuous negative pressure wound therapy. The wound bed showed signs of granulation, but was not progressing to closure. (C) Bi-weekly applications of Grafix were started. After six applications, the wound was completely closed.
After 3 weeks of continued negative pressure wound therapy, the wound bed showed signs of granulation, but was not progressing to closure; therefore, the team elected to discontinue the negative pressure therapy and apply an advanced regenerative therapy (Fig. 4B). Grafix was selected as a treatment modality, and six bi-weekly applications of Grafix were performed. After six bi-weekly applications of Grafix, the wound was completely reepithelialized, and no further dressings were needed (Fig. 4C). To prevent re-ulceration, the patient's shoes were adjusted with appropriate inserts. Despite initial joint capsule exposure and infection, admission and potential readmissions were avoided by following an evidence-based algorithm recommended for DFU by ADA and WHS, including the use of advanced regenerative therapy to achieve closure.
Refractory (49-year persistence) shrapnel leg wound case
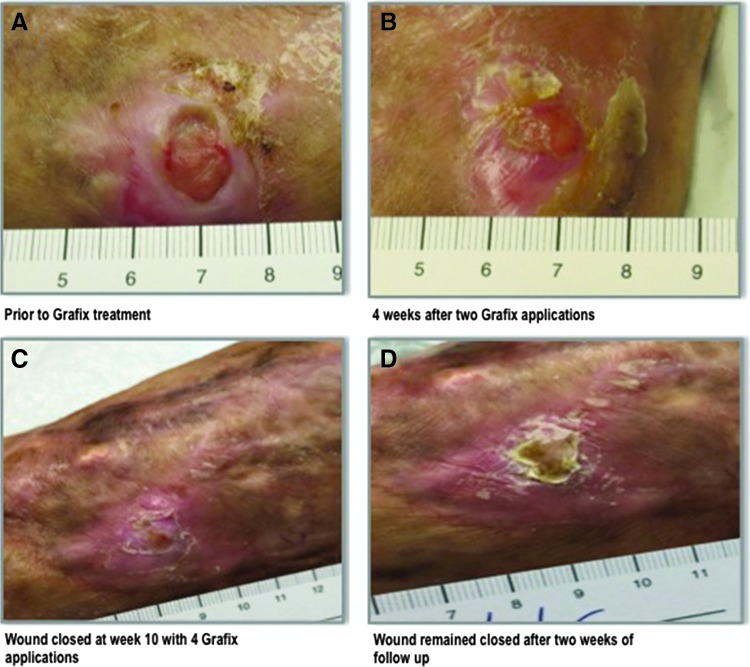
A 68-year-old Vietnam veteran sustained a shrapnel injury, open fractures, and a wound to the right lower leg in 1966. Since that time, he had been hospitalized for 13 months and had multiple surgeries, including debridement of osteomyelitis, fixations, muscle and skin flaps, and grafts. His leg wound never completely closed over the 49-year period and required periodic hospital admissions, IV antibiotics, and surgeries. In 2012, he had a series of surgeries, negative pressure therapy, 50 treatments of hyperbaric oxygen therapy, multiple skin grafts, and bioengineered skin applications without reaching complete sustained wound closure. In October 2014, he was admitted to the South Shore Hospital with significant wound infection and staphylococcal septicemia requiring 4 weeks of IV antibiotics. After that, he was re-admitted with severe generalized muscle weakness, fever, fatigue, and a diagnosis of polymyalgia, which was treated with a prednisone course tapered to 10 mg/day. He was discharged from the hospital in November 2014 with a referral to our wound center where the wound was debrided and biopsied. There was no remaining shrapnel and no indication of osteomyelitis, vascular disease, or chronic infection in the wound. The tissue near the wound was fibrotic, which created tension on the surrounding tissues and might be one of the reasons for nonhealing. The wound size was 1.1×1.2×0.1 cm and 1.1×1.2×0.2 cm pre- and postdebridement, respectively (Fig. 5A). After 2 weeks and negative results for the biopsy culture, the wound was debrided and the first application of Grafix was performed. The patient's visits were scheduled on a weekly basis. Four applications of Grafix were performed (Fig. 5B, C) after wound debridement: the first three applications were done biweekly and the last application was applied 3 weeks after application number 3. Complete wound closure was recorded 10 weeks after the first Grafix application, and to date, the wound has remained closed (Fig. 5D).
Figure. 5.
A 68-year-old Vietnam veteran who sustained shrapnel injury and open fractures resulting in a wound on the right lower leg in 1966. Over a period of 49 years, the wound was never completely closed. (A) 1.1×1.2×0.1 cm wound before Grafix application. (B) Four weeks post-treatment at the time of third Grafix application. (C) Wound closure was achieved after 10 weeks of treatment with four Grafix applications. (D) Wound remained closed during follow-up.
Discussion
The use of placental membranes for wound treatment has been reported for over 100 years as an allogeneic wound covering.43 Human placental membranes have been shown to have anti-inflammatory, antibacterial, and antiscarring properties, which are favorable for wound healing.29,30 Despite these benefits, the short shelf life of fresh tissue and the risk of disease transmission associated with insufficient time for testing have hindered its use.44 To overcome these limitations and utilize the beneficial properties of placental membranes, different preservation methods have been developed.
Recently, a new preservation technology was developed that retains all components found in fresh placental tissue in their native state.18 This novel technology is utilized to manufacture Grafix, a cryopreserved placental membrane, which is the only commercially available placental product to preserve viable endogenous cells, including MSCs. All other commercial placental membrane products are all devitalized or decellularized.45 The role of viable cells in placental membranes remains to be determined; however, accumulated data point to the importance of preserving complete tissue integrity, including viable cells, for the functionality of placental membranes.34–36
The preservation of endogenous MSCs within placental membranes sets Grafix apart from other advanced wound therapies that do not contain MSCs. Currently, there are no skin substitutes containing MSCs on the market. MSCs have been shown to enhance and improve wound healing in clinical settings and are especially important in the elderly as aging is associated with a reduced number and function of MSCs and a diminished healing response.18,47,48
Key Findings.
• Grafix is a human cryopreserved placental membrane in which all components of the native tissue are preserved: three-dimensional extracellular matrix, growth factors, and viable cells, including epithelial cells, fibroblasts, and MSCs.
• Clinical data indicate that Grafix can be beneficial for treatment of a broad variety of wounds, including nonhealing DFUs, VLUs, pressure ulcers, surgical dehiscence, wounds with exposed tendons and bone, and others.
• Grafix is regulated as a human cellular tissue product and can be used for the treatment of both acute and chronic wounds. It offers wound care providers a treatment modality for wounds of different etiologies.
The safety and efficacy of Grafix have been demonstrated in two clinical studies, one of which is a high-quality multicenter RCT.37,38 Lavery et al. showed that DFUs closed 4 weeks faster when treated with Grafix compared to SOC (42 days vs. 69.5 days for Grafix vs. SOC respectively, p=0.019).37 This correlated with a significantly lower rate of wound-related infections and fewer hospitalizations. In addition, in the crossover open-label phase of the trial, Grafix closed wounds in patients who failed SOC during the initial 12 weeks of treatment.37 These data point to potential cost effectiveness of Grafix compared to SOC. In addition, a multicenter, open-label, single-arm study to evaluate the safety and efficacy of Grafix for the treatment of complex diabetic foot wounds with exposed tendon and/or bone is ongoing.48 Data suggest that Grafix combined with SOC might be beneficial for the treatment of different types of wounds, regardless of their size, persistence, location, and etiology. Grafix shows effectiveness in closing wounds that have failed other advanced modalities and treatments. The South Shore Hospital Wound Care Center has also shown that a systematic approach for patient and wound evaluation should be applied, and thorough wound bed preparation is required before the application of any advanced wound modality for the achievement of wound closure.
Innovation
Grafix is a commercially available placental membrane product based on a unique tissue processing and cryopreservation technology that retains all placental membrane components, including viable endogenous cells, in their native state. Clinical data indicate that wounds of different etiologies, which have not responded to standard of care in a timely manner, can benefit from treatment with Grafix.
Abbreviations and Acronyms
- ADA
American Diabetic Association
- DFU
diabetic foot ulcer
- ECM
extracellular matrix
- HCT/Ps
human cells, tissues, and cellular- and tissue-based products
- MSC
mesenchymal stem cell
- RCT
randomized controlled trial
- SOC
standard of care
- TCC
total contact cast
- VAC
negative pressure wound vacuum
- VLU
venous leg ulcer
- WHS
Wound Healing Society
Acknowledgments and Funding Sources
This work was not funded by any organization and represents the author's personal analysis and experience.
Author Disclosure and Ghostwriting
The opinions expressed are those of the author and do not represent those of the South Shore Hospital Center for Wound Care and Hyperbaric Medicine. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Author
Dr. Gary W. Gibbons, MD, FACS, has focused his vascular surgical career on advancing the treatment of diabetic patients with lower extremity wounds and vascular disease. He is a pioneer of the Dorsalis Pedis Bypass and has helped to develop many of the currently used algorithms and multidisciplinary care models used today in the treatment of diabetic patients with lower extremity wound and/or ischemia. He is an international lecturer and has published hundreds of articles. Dr. Gibbons received his undergraduate with Summa Cum Laude honors from the University of Maine and received his medical degree from the University of Cincinnati School of Medicine in 1971. He completed his surgical residency and chief residency at the New England Deaconess Hospital, where he also completed a nutrition fellowship specializing in the nutritional complications of cardiac disease and surgery. Dr. Gibbons is the Medical Director of the South Shore Hospital Center for Wound Healing, a full-time clinical vascular surgery and wound healing/limb preservation practice. He has received accolades and national and international recognition, including the prestigious International Edward James Olmos Award for Advocacy in Amputation Prevention and the Georgetown Distinguished Achievement Award in Diabetic Limb Salvage.
References
- 1.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Medicine 2014;6:265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regene 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fife CE, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds 2012;24:10–17 [PubMed] [Google Scholar]
- 4.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Medical, drug, and work-loss costs of venous leg ulcers. Value Health 2013;16:A73. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services. Fed. Register “Proposed Rules”. May 3, 2007
- 6.Margolis DJ, Malay DS, Hoffstad OJ, et al. Incidence of Diabetic Foot Ulcer and Lower Extremity Amputation Among Medicare Beneficiaries, 2006 to 2008: Data Points #2. Rockville, MD: Data Points Publication Series, 2011 [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 8.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 9.Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence-based management strategies for treatment of chronic wounds. Eplasty 2009;9:e19. [PMC free article] [PubMed] [Google Scholar]
- 10.Barbul A, et al. Wound Care Guidelines of the Wound Healing Society. Wound Repair Regene 2006;14:645–711 [Google Scholar]
- 11.American Society of Plastic Surgeons. Evidence-based clinical practice guideline: chronic wounds of the lower extremity. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/evidence-practice/Evidence-based-Clinical-Practice-Guideline-Chronic-Wounds-of-the-Lower-Extremity.pdf (last accessed April23, 2015)
- 12.American Diabetes Association. Standards of medical care in diabetes-2015 Clinical diabetes: a publication of the American Diabetes Association 2015;33:1–93 [Google Scholar]
- 13.Burns J, Begg L. Optimizing the offloading properties of the total contact cast for plantar foot ulceration. Diabet Med 2011;28:179–185 [DOI] [PubMed] [Google Scholar]
- 14.Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care 2008;31:2118–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong DG, Isaac AL, Bevilacqua NJ, Wu SC. Offloading foot wounds in people with diabetes. Wounds 2014;26:13–20 [Google Scholar]
- 16.Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care 1999;22:692–695 [DOI] [PubMed] [Google Scholar]
- 17.Gibbons GW, Orgill DP, Serena TE, et al. A prospective, randomized, controlled trial comparing the effects of noncontact, low-frequency ultrasound to standard care in healing venous leg ulcers. Ostomy Wound Manage 2015;61:16–29 [PubMed] [Google Scholar]
- 18.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valencia IC, Falabella AF, Eaglstein WH. Skin grafting. Dermatol Clin 2000;18:521–532 [DOI] [PubMed] [Google Scholar]
- 20.Hong S, Alapure BV, Lu Y, Tian H, Wang Q. Immunohistological localization of endogenous unlabeled stem cells in wounded skin. J Histochem Cytochem 2014;62:276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder RJ, Kirsner RS, Warriner RA, 3rd, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010;56:S1–S24 [PubMed] [Google Scholar]
- 22.Steinberg J, Beusterien K, Plante K, et al. A cost analysis of a living skin equivalent in the treatment of diabetic foot ulcers. Wounds 2002;14:142–149 [Google Scholar]
- 23.Persson U, Willis M, Odegaard K, Apelqvist J. The cost-effectiveness of treating diabetic lower extremity ulcers with becaplermin (Regranex): a core model with an application using Swedish cost data. Value Health 2000;3 Suppl 1:39–46 [DOI] [PubMed] [Google Scholar]
- 24.Sellheyer K, Krahl D. [Cutaneous mesenchymal stem cells. Current status of research and potential clinical applications]. Hautarzt 2010;61:429–434 [DOI] [PubMed] [Google Scholar]
- 25.Ennis WJ, Sui A, Bartholomew A. Stem cells and healing: impact on inflammation. Adv Wound Care 2013;2:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin L, Peterson DA. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med 2013;2:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J, Jang I, Lee D, Seo Y, et al. A comparison of lyophilized amniotic membrane with cryopreserved amniotic membrane for the reconstruction of rabbit corneal epithelium. Biotechnol Bioprocess Eng 2005;10:262–269 [Google Scholar]
- 28.Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 2008;26:300–311 [DOI] [PubMed] [Google Scholar]
- 29.Robson MC, Krizek TJ, Koss N, Samburg JL. Amniotic membranes as a temporary wound dressing. Surg Gynecol Obstet 1973;136:904–906 [PubMed] [Google Scholar]
- 30.Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf 2004;2:177–187 [DOI] [PubMed] [Google Scholar]
- 31.Faulk WP, Matthews R, Stevens PJ, Bennett JP, Burgos H, Hsi BL. Human amnion as an adjunct in wound healing. Lancet 1980;1:1156–1158 [DOI] [PubMed] [Google Scholar]
- 32.Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J 1910;15:307 [Google Scholar]
- 33.Robson MC, Duke WF, Krizek TJ. Rapid bacterial screening in the treatment of civilian wounds. JSurg Research 1973;14:426–430 [DOI] [PubMed] [Google Scholar]
- 34.Duan-Arnold Y, Gyurdieva A, Johnson A, Uveges TE, Jacobstein DA, Danilkovitch A. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care 2015. Epub ahead of print, April 22, 2015. DOI: 10.1089/wound.2015.0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan-Arnold Y, Gyurdieva A, Johnson A, Jacobstein DA, Danilkovitch A. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care June 2015;4(6):329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan-Arnold Y, Uveges TE, Gyurdieva A, Johnson A, Danilkovitch A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care 2015. Epub ahead of print July 10, 2015. DOI: 10.1089/wound.2015.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix((R)) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 2014;11:554–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage 2013;59:38–43 [PubMed] [Google Scholar]
- 39.Vowden P. Hard-to-heal wounds made easy. Wounds Int 2011;2:1–6 [Google Scholar]
- 40.Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 2001;42:1539–1546 [PubMed] [Google Scholar]
- 41.Frykberg RG, Rogers LC. Emerging evidence on advanced wound care for diabetic foot ulcerations. Proceedings from the Superbones West Conference; October 21–24, 2010; Las Vegas, Nevada: Supplement to Podiatry Today, 2010 [Google Scholar]
- 42.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981;2:1003–1005 [DOI] [PubMed] [Google Scholar]
- 43.Sabella N. Use of fetal membranes in skin grafting. Med Rec NY 1913;83:478–480 [Google Scholar]
- 44.Adds PJ, Hunt CJ, Dart JK. Amniotic membrane grafts, “fresh” or frozen? A clinical and in vitro comparison. Br J Ophthalmol 2001;85:905–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brantley JN, Verla T. Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv Wound Care 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasper G, Mao L, Geissler S, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 2009;27:1288–1297 [DOI] [PubMed] [Google Scholar]
- 47.Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321–326 [DOI] [PubMed] [Google Scholar]
- 48.GrafixCORE®. Open-label study to evaluate the safety and efficacy of GrafixCORE® for complex diabetic foot wounds. https://clinicaltrials.gov/ct2/show/NCT02260609 (last accessed February27, 2015)