Abstract
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with repetitive mild traumatic brain injury. It is defined pathologically by the abnormal accumulation of tau in a unique pattern that is distinct from other tauopathies, including Alzheimer’s disease (AD). Although trauma has been suggested to increase amyloid β peptide (Aβ) levels, the extent of Aβ deposition in CTE has not been thoroughly characterized. We studied a heterogeneous cohort of deceased athletes and military veterans with neuropathologically diagnosed CTE (n = 114, mean age at death = 60) to test the hypothesis that Aβ deposition is altered in CTE and associated with more severe pathology and worse clinical outcomes. We found that Aβ deposition, either as diffuse or neuritic plaques, was present in 52 % of CTE subjects. Moreover, Aβ deposition in CTE occurred at an accelerated rate and with altered dynamics in CTE compared to a normal aging population (OR = 3.8, p < 0.001). We also found a clear pathological and clinical dichotomy between those CTE cases with Aβ plaques and those without. Aβ deposition was significantly associated with the presence of the APOE ε4 allele (p = 0.035), older age at symptom onset (p < 0.001), and older age at death (p < 0.001). In addition, when controlling for age, neuritic plaques were significantly associated with increased CTE tauopathy stage (β = 2.43, p = 0.018), co-morbid Lewy body disease (OR = 5.01, p = 0.009), and dementia (OR = 4.45, p = 0.012). A subset of subjects met the diagnostic criteria for both CTE and AD, and in these subjects both Aβ plaques and total levels of Aβ1–40 were increased at the depths of the cortical sulcus compared to the gyral crests. Overall, these findings suggest that Aβ deposition is altered and accelerated in a cohort of CTE subjects compared to normal aging and that Aβ is associated with both pathological and clinical progression of CTE independent of age.
Keywords: Chronic traumatic encephalopathy, Alzheimer’s disease, Beta-amyloid, Neurodegenerative disorders, Dementia, Tau
Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease likely caused by repetitive traumatic brain injury (RTBI). Many contact sports have now been linked to CTE, including American football, boxing, hockey, soccer, and rugby. In addition, we have found CTE in military personnel exposed to an explosive blast [10, 29]. The clinical features of CTE typically manifest years or decades after the initial RTBI and consist of impairments in mood (depression, suicidality, irritability), behavior (explosivity, violence, impulsivity), cognition (impaired memory, executive dysfunction, diminished concentration), and motor functioning (parkinsonism, dysarthria, gait changes, weakness) [32, 33, 46]. The reported clinical symptoms following RTBI can vary widely, and different subtypes have been identified in neuropathologically confirmed cases of CTE [46]. Research clinical diagnostic criteria have recently been proposed [32], but investigations to establish reliability and diagnostic accuracy in predicting underlying CTE pathological changes are ongoing [32]. In some cases the clinical features can overlap with and be clinically indistinguishable from those of Alzheimer’s disease (AD). An analysis of neuropathologically confirmed CTE without co-morbid disease demonstrated that in 11 % of subjects overall and in 40 % of demented subjects the clinical presentation is indistinguishable from AD. Moreover, as in AD, there is a spectrum of pathology in CTE, and some, but not all, subjects with pathological CTE will have dementia or CTE-related clinical symptoms [46]. A more complete understanding of the pathology may help explain and predict the clinical outcomes.
CTE is a tauopathy characterized by neurofibrillary tangles, tau-positive astrocytes, and tau-positive cell processes that preferentially involve the cortical sulci, medial temporal lobe, diencephalon, and brainstem. In CTE the tau pathology is characteristically perivascular, most pronounced at the sulcal depths, and preferentially involves the superficial cortical layers. This pattern of tau pathology is distinct from other tauopathies, including Alzheimer’s disease. Recently, the US National Institute of Neurological Disorders and Stroke convened a panel of expert neuropathologists to establish consensus criteria for CTE [42]. It was determined that the pathognomonic lesion in CTE was an abnormal perivascular accumulation of tau in neurons, astrocytes, and cell processes in an irregular pattern at the depths of the cortical sulci, and that CTE could be reliably distinguished from other tauopathies including AD, progressive supranuclear palsy, argyrophilic grain disease, corticobasal degeneration, Guamanian Parkinsonism Dementia Complex, and primary age-related tauopathy [4].
The role of amyloid β peptide (Aβ) in CTE has been controversial. At one point, Aβ deposition was thought to be a universal feature [39], but subsequent analyses found Aβ pathology within only a subset of individuals. In our reported cohort of 68 athletes and military veterans with CTE, 44 % had some deposition of primarily diffuse plaques, and 10 % met the criteria for AD [30]. Some epidemiologic evidence exists that suggests moderate to severe TBI is a risk factor for AD [13, 24, 27, 38] although most reports linking TBI and AD have relied on a clinical diagnosis of probable AD without neuropathological verification and a definitive link has yet to be established [17]. It has been shown that acutely following TBI, amyloid precursor protein (APP) and Aβ increase in tissue and CSF, and there can be rapid formation of diffuse Aβ plaques in the cortex [9, 16, 36, 40, 41]. Therefore, the release of Aβ into surrounding tissue following injury may be a basis for plaque formation [41, 43]. The relation of Aβ deposition in CTE to genetic factors, other pathological lesions, and to the clinical course has yet to be determined.
Here we set out to test the hypotheses that Aβ deposition is altered in CTE compared to a control population, Aβ accumulation in CTE differs from AD, and the presence of diffuse or neuritic plaques increases pathological severity and worsens clinical outcomes in CTE. To test these hypotheses we examined the brains of 114 participants with a history of RTBI and compared the pattern of neuropathological lesions in subjects with and without Aβ to each other, to the brains of individuals with neuropathologically confirmed AD without a known history of RTBI, and to the reported pathology of a large non-selected autopsy cohort of 2332 normal aging cases [2].
Materials and methods
Subjects
A total of 114 subjects were evaluated from Boston University’s Chronic Traumatic Encephalopathy Center including former athletes, military veterans, and civilians with a history of repetitive mild traumatic brain injury. Next of kin provided written consent for participation and brain donation. Institutional review board approval for brain donation was obtained through the Boston University Medical Center and the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA. Institutional review board approval for post-mortem clinical record review, interviews with family members, and neuropathological evaluation was obtained through Boston University Medical Center. The brains from an additional 319 subjects from the Boston University AD Center were included for comparative analysis.
Clinical assessment
Concussion and RTBI history, history of cognitive and behavioral changes and clinical status leading up to death were determined through post-mortem interviews with next of kin performed by physicians, neuropsychologists, and doctoral candidates trained to assess for RTBI, dementia, and neurodegenerative diseases (JM, TS, DHD, PHM, and RAS). Interviewers were blind to the results of the neuropathological examination at the time of interview, and the neuropathologists were blind to the results of the clinical interviews at the time of neuropathological examination and diagnosis. Informants were interviewed before receiving the results of the neuropathological examination. The interview was semi-structured and conducted by telephone. Medical record review was also performed (JM, TS, PK, DHD, PHM, TDS, ACM, and RAS). Recorded clinical measures included the presence or absence of CTE symptoms including mood, behavioral, or cognitive changes (reviewed in [32]), age of symptom onset, presence of symptoms associated with parkinsonism (e.g., tremor, bradykinesia, rigidity, gait disturbance, ataxia, dysarthria, dysphagia), and behavioral or cognitive variant of CTE [46]. We recorded dementia status using a conservative definition of dementia based on a diagnosis made during life. In the subset of AD subjects selected for ELISA and Aβ plaque burden, subjects and their informants were asked during life about a history of contact sports play or head injury including RTBI and those without such history were selected for comparison.
Pathological diagnoses
Chronic traumatic encephalopathy
The diagnosis and staging of CTE followed the definitions and criteria described previously [30] and adapted to include the following: (1) perivascular foci of hyperphosphorylated tau immunoreactive neurons, astrocytes, and cell processes; (2) irregular cortical distribution of hyperphosphorylated tau immunoreactive neurons and astrocytes with a predilection for the depths of cerebral sulci; and (3) hyperphosphorylated tau-positive neurons in the cerebral cortex located preferentially in the superficial layers. Supportive features included clusters of subpial and periventricular astrocytic tangles in the cerebral cortex, diencephalon, basal ganglia and brainstem. CTE stages varied from I to IV based on the extent and severity of tau pathology as previously described [30, 45]. Briefly, Stage I CTE is characterized by isolated perivascular foci of hyperphosphorylated tau within neurons, astrocytes, and cell processes present at the sulcal depths. In stage II the tau pathology extends to involve the superficial cortical layers into the gyral crest. In stage III there is additional involvement of the parietal and temporal lobes as well as medial temporal lobe tau pathology (i.e. hippocampus, amygdala, entorhinal cortex). By stage IV all cortical lobes are involved with severe tau pathology. In addition, there is abnormal tau accumulation within the diencephalon, brainstem, and cerebellum.
Alzheimer’s disease
Neuropathological diagnosis of AD required an intermediate or high degree of neuropathological changes as defined by the ABC score. Subjects with AD changes had a low degree of neuropathological change as defined by the ABC score [15, 34]. Neuritic plaques were assessed with Bielschowsky silver staining and using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scoring system for plaque pathology [31].
Lewy body disease
The presence or absence of Lewy bodies was determined by immunohistochemical staining for alpha-synuclein. The regions examined in each case included the olfactory bulb, medulla, substantia nigra, amygdala, hippocampus, and cingulate gyrus.
Immunohistochemistry
Human tissue was fixed in periodate-lysine-paraformaldehyde, and tissue blocks were paraffin-embedded and cut at 10 µm for immunohistochemistry. Antigen retrievals were performed by formic acid treatment for 2 min for Aβ antibodies. Sections were incubated at 4 °C overnight with antibodies to phosphorylated PHF-tau (AT8; Pierce Endogen, Rockford IL; 1:2000), Aβ1–40 (AB5074P; EMD Millipore, Billerica, MA; 1:2000), Aβ1–42 (AB5078P; EMD Milli-pore; 1:2000), alpha-synuclein (rabbit polyclonal; Chemi-con, Temecula, CA; 1:15,000), and pTDP-43 (pS409/410 mouse monoclonal; Cosmo Bio Co, Tokyo, Japan; 1:2000). For determination of Aβ deposition in the medial temporal lobe (section of the hippocampal formation, parahippocampal gyrus, transentorhinal region, and portions of occipito-temporal gyrus), the monoclonal anti-Aβ antibody (Clone 4G8; Covance, Dedham, MA, 1:100,000) was used. After three washes with PBS (pH 7.4), sections were treated with biotinylated secondary antibody and labeled with a 3-amino-9-ethylcarbazol HRP substrate kit (Vector Laboratories). Sections were counterstained with Gill’s Hematoxlin (Vector Laboratories H-3401) and then coverslipped with Permount mounting medium.
Quantitative ELISA measurement of Aβ and tau
The buffer conditions, protease inhibitors, and centrifiugation protocols have been reported previously [51]. Brieflvolume of 5 M Guanidine Hydrochloridey, a 4-mm tissue punch was used to isolate and remove gray matter from the gyral crests and sulcal depths of the middle frontal gyrus and neighboring sulci and superior temporal gyrus and sulcus. Brain tissue was homogenized in fivefold volume of 5 M Guanidine Hydrochloride/50 mM Tris–HCL, pH 8.0, with protease inhibitors (Thermo Scientific, 78439) and phosphatase inhibitors (Sigma, P5726 and P0044). Tissue was homogenized using a mechanical homogenizer for 25 strokes followed by ultrasonic disruption on ice. The homogenates were shaken at room temperature overnight. Samples were diluted tenfold with 0.4 % Block Ace (Dainippon Pharmaceuticals Co, Japan) and centrifuged at 14,000 rpm for 15 min at 4 °C. Enzyme-linked immunosorbent assay (ELISA) was performed for Aβ1–38, 1–40, and 1–42 using a multiplex plate from Meso Scale Discovery (MSD, Rockville, MD, USA) as well as for levels of phosphorylated tau (Thr231) following the manufacturer’s instruction.
Aβ plaque deposition was determined using Aβ1–40 and Aβ1–42 immunostaining and quantified as reported previously [37]. Briefy, digital images were captured at 200 × magnification and a threshold optical density was obtained, which discriminated staining from background. The amyloid burden was defined as the total percentage of cortical surface area covered by either Aβ1–40 or Aβ1–42 deposition over three sections and was calculated for the bottom third of the sulcus (depth of the sulcus) and the top third of the gyrus (gyral crest).
APOE genotyping
DNA was extracted from brain tissue samples using a Qiagen QIAamp DNA extraction kit (Qiagen, Valencia, CA, USA). Two single nucleotide polymorphisms (National Center for Biotechnology Information SNPs rs429358 and rs7412) were examined using TaqMan assays (Applied Biosystems, Foster City, CA, USA). Allelic discrimination was automated using the manufacturer’s software. Positive controls, consisting of DNA of each of the 6 possible APOE genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, ε4/ε4), were included on each plate and genotyped with restriction isotyping.
Statistical methods
Both SPSS version 20.0 (IBM Inc., Chicago, IL, USA) and SAS version 9.3 (SAS Institute, Cary, NC, USA) were utilized for statistical analyses. Significance was set a priori at α = 0.05. Neuropathologically confirmed CTE tauopathy cases were divided into two subgroups based on the presence or absence of Aβ plaques and further distinguishing between diffuse and neuritic types. Group comparisons were based on demographic (e.g., age at death), clinical (e.g., symptom onset, presentation subtype), genotype (APOE), exposure (e.g., sport, RTBI history), and lesion (e.g., Aβ, LBD, TPD-43) characteristics. Spearman’s rank order correlation was used to determine the statistical association between CTE stage and all linear variables of interest. For non-linear independent variables, the Wilcoxon– Mann–Whitney U test was used for independent variables with only two groups (e.g., presence of neuritic plaques). A two-sample Chi square test weighted by sample size and a logistic regression model were used to compare the frequency of Aβ deposition in the medial temporal lobe by age in our CTE cohort to a published non-selected community-based autopsy series of 2332 subjects [2]. The Chi square statistical method is nonparametric and, therefore, makes no assumptions about the population distribution. Linear regression analysis was performed to determine the relationship of Aβ deposition and age at death and CTE stage. The interaction effect between age and Aβ pathology in predicting CTE stage was calculated when appropriate. Logistic regression models were used to generate odds ratios (OR) while adjusting for age and CTE stage when appropriate.
Results
Beta-amyloid pathology in CTE, AD, and aging
We first set out to determine whether the frequency of plaques differed in our cohort of CTE versus AD and normal aging. Beta-amyloid plaques can be either diffuse or neuritic as defined by the presence of abnormal tau-positive neurites. In our cohort of 114 subjects with CTE (mean age at death = 60 years, all men), we found Aβ deposition in the form of diffuse plaques in 52 % with Aβ immunostaining and neuritic plaques (CERAD > 0) in 36 % using Bielschowsky silver staining. The percent of cases with both diffuse and neuritic Aβ deposition increased with CTE stage (Table 1). When compared to AD subjects from the Boston University’s AD Center, stage IV CTE had a similar frequency of diffuse Aβ plaques. However, there were significantly fewer neuritic plaques in stage IV CTE compared to AD (Table 1, Z = −9.94, p < 0.001).
Table 1.
Frequency of Aβ deposition in CTE
| n | Mean age at death (years) | APOE ε4 % (+/−)a | DP freq (%) | NP freq (%) | |
|---|---|---|---|---|---|
| CTE (all) | 114 | 60 | 33 % (27/55) | 52 | 36 |
| Stage I | 13 | 35 | 33 % (3/6) | 15 | 0 |
| Stage II | 30 | 52 | 32 % (7/15) | 33 | 20 |
| Stage III | 34 | 62 | 23 % (6/20) | 44 | 27 |
| Stage IV | 37 | 75 | 44 % (11/14) | 87 | 70 |
| AD | 319 | 80 | 59 % (13/9) | 84 | 100 |
CTE Chronic traumatic encephalopathy, DP diffuse plaques, NP neuritic plaques, AD Alzheimer’s disease
% of both ε4 hetereozytes and homozygotes out of those cases with genotypes (n = 82)
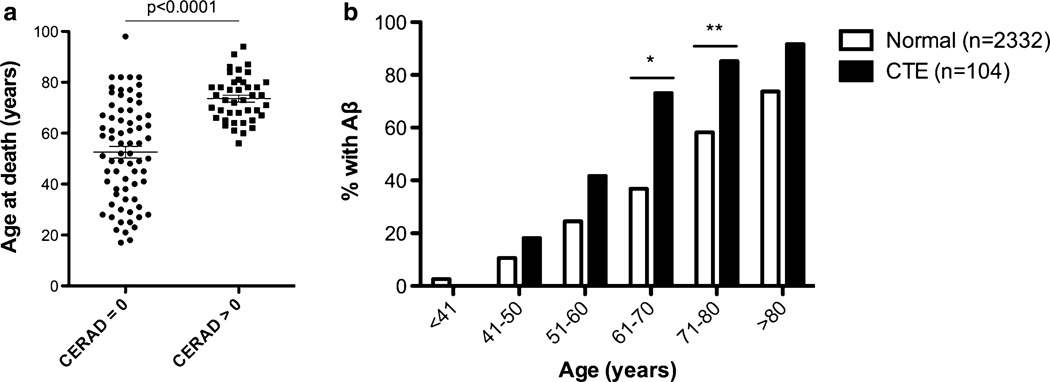
The mean age at death was significantly higher in CTE subjects with Aβ plaques compared to those without (Fig. 1a; Table 3). To evaluate the age-dependent increase of Aβ deposition in our CTE cohort and to determine whether it differs from normal aging, we grouped the age at death of our subjects into decades and plotted the frequency of cases positive for Aβ deposition in the medial temporal lobe (available in n = 104 subjects) as assessed by the anti-Aβ antibody 4G8 (Fig. 1b). Aβ pathology first appeared in CTE cases aged 41–50, with a frequency that was significantly higher in later decades (Fig. 1b). The odds of developing Aβ pathology in CTE increased 2.7 fold for every decade (p < 0.0001). A clear age-dependent increase in Aβ plaque deposition was also reported in the medial temporal lobe using identical methods in a non-selected community-based autopsy series of 2332 subjects [2]. When compared to this normal aging population, the frequency of Aβ deposition in our CTE cohort was higher in CTE subjects in the decades between the 50 and 90s and significantly higher for CTE subjects in their 60s (χ2 = 13.7, p < 0.001) and 70s (χ2 = 7.76, p = 0.005), and the overall risk of developing plaque pathology was higher for the CTE group (Fig. 1b). In fact, the odds of developing plaque pathology were 3.8 times higher in CTE than in normal aging (p < 0.0001). To test the hypothesis that Aβ plaque frequency in CTE and normal aging are not derived from a common distribution we performed a weighted two-sample Chi square test. Importantly, this demonstrated a distinct distribution of Aβ by age in the CTE cohort compared to normal aging (χ2 = 721, p < 0.0001). This suggests that Aβ deposition in CTE is not simply accelerated aging, but rather a distinct process with altered dynamics.
Fig. 1.
The presence of neuritic Aβ plaques (NPs, CERAD > 0) was associated with age at death and was accelerated in CTE. a Subjects with neuritic plaques were significantly older at death than those without neuritic plaques (CERAD = 0). b When compared to the gradual age-dependent increase in the frequency of Aβ plaques in the medial temporal lobe of a non-selected autopsy series [2], the frequency of Aβ plaques in CTE is significantly higher in subjects in their 60 s (*χ2 = 13.7, p < 0.001) and 70 s (**χ2 = 7.76, p = 0.005)
Table 3.
Clinical, exposure, and pathological findings in CTE with and without Aβ deposition
| CTE |
CTE with DPs |
CTE with NPs |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | n | Mean ± SEM | p* | n | Mean ± SEM | p* | |
| Age at death (year) | 55 | 48.2 ± 2.6 | 59 | 71.6 ± 1.4 | <0.001 | 41 | 73.6 ± 1.4 | <0.001 |
| Athletic exposure (year) | 51 | 15.0 ± 1.0 | 46 | 15.9 ± 0.9 | 0.500 | 31 | 16.7 ± 1.2 | 0.22 |
| Concussion no. | 48 | 11.5 ± 3.0 | 41 | 24.2 ± 6.6 | 0.071 | 28 | 17.5 ± 5.4 | 0.99 |
| Age at Sx onset (year) | 43 | 36.2 ± 2.3 | 42 | 54.2 ± 2.3 | <0.001 | 30 | 56.4 ± 2.7 | <0.001 |
| CTE | CTE with DPs |
CTE with NPs |
||||||
| Freq (+/−) | Freq (+/−) | p** | p^ | Freq (+/−) | p** | p^ | ||
| CTE Sx | 76 % (37/12) | 90 % (43/5) | 0.059 | 0.419 | 94 % (31/2) | 0.028 | 0.164 | |
| Dementia | 20 % (10/39) | 73 % (35/13) | <0.001 | 0.013 | 82 % (27/6) | <0.001 | 0.012 | |
| Cognitive variant | 58 % (23/17) | 76 % (31/10) | 0.067 | 0.382 | 76 % (22/7) | 0.15 | 0.597 | |
| Parkinsonism | 2.1 % (1/48) | 25 % (12/36) | 0.001 | 0.050 | 27 % (9/24) | 0.010 | 0.113 | |
| LBD pathology | 3.7 % (2/52) | 35 % (19/35) | <0.001 | 0.019 | 42 % (16/22) | <0.001 | 0.009 | |
| TDP-43 pathology | 67 % (37/18) | 84 % (48/9) | 0.030 | 0.810 | 93 % (38/3) | 0.003 | 0.097 | |
For statistical tests, CTE with DPs compared to CTE without DPs (labeled CTE in table) and CTE with NPs compared to CTE without NPs (data not shown)
DP diffuse plaques, NP neuritic plaques
Student’s t test,
Pearson χ2 test,
logistic regression controlling for age, significant p values are in bold
APOE ε4 allele is enriched in CTE subjects with Aβ
We next set out to determine whether the proportion of APOE ε4 was enriched in CTE cases overall and in those with Aβ versus those without. First, we tested the hypothesis that the proportion of ε4 alleles is elevated in our CTE cohort overall. Although there were more ε4 alleles in the overall CTE cohort (20 %) when compared to an age-matched normative sample (15 % from [28]), the difference was not quite significant (Table 2, Z = 1.48, p = 0.069, z test). Similar to what was reported previously in a subset of these cases [46], there was a significantly greater proportion of ε4 homozygotes (Z = 1.94, p = 0.026) and a non-significant increase in heterozygotes in the overall CTE cohort compared to the normative sample. To test the hypothesis that the ε4 proportion is elevated in CTE subjects with Aβ, but not in those without, we compared the proportions of these different groups to a control population [28]. In CTE subjects without either diffuse or neuritic plaques, the proportion of ε4 alleles (14 %) was not significantly different from population norms (15 %). However, the proportion of ε4 alleles was significantly greater than a control population in CTE subjects with diffuse plaques (27 %, Z = 2.92, p = 0.002) or neuritic plaques (25 %, Z = 2.01, p = 0.022). We also examined the other APOE allele frequencies. In CTE subjects with diffuse plaques there was a decrease in the number of ε3 alleles (65 %) compared to the control population (77 %, Z = −2.51, p = 0.012). Differences in the ε2 allele frequency were not detected (Table 2). Finally, to directly test the hypothesis that the ε4 allele is associated with Aβ in our CTE cohort, we performed a Fisher’s exact test and found that ε4 was significantly associated with the presence of diffuse plaques (p = 0.035), but did not quite reach Significance for neuritic plaques (p = 0.086).
Table 2.
APOE allele frequencies in CTE
| Group | ε2 allele count n (%) |
p value (Z score)* | ε3 allele count n (%) |
p value (Z score)* | ε4 allele count n (%) |
p value (Z score)** |
|---|---|---|---|---|---|---|
| CTE overall | 12 (7.3 %) | 0.726 (−0.348) | 120 (73 %) | 0.298 (−1.04) | 32 (20 %) | 0.069 (1.48) |
| CTE without Aβ | 5 (6.4 %) | 0.596 (−0.534) | 64 (82 %) | 0.258 (1.13) | 11 (14 %) | 0.386 (−0.294) |
| CTE with DPs | 6 (7.0 %) | 0.711 (−0.368) | 56 (65 %) | 0.012 (−2.51) | 23 (27 %) | 0.002 (2.92) |
| CTE with NPs | 3 (5.4 %) | 0.459 (−0.742) | 39 (70 %) | 0.219 (−1.23) | 14 (25 %) | 0.022 (2.01) |
| Population norms, ages 42–70 years [28] |
758 (8.1 %) | 7205 (77 %) | 1439 (15 %) |
APOE apolipoprotein E gene, CTE chronic traumatic encephalopathy, DPs diffuse plaques, NPs neuritic plaques; significant p values in bold (Z test versus population norms)
Two-tailed,
one-tailed
Sulcal versus gyral Aβ levels
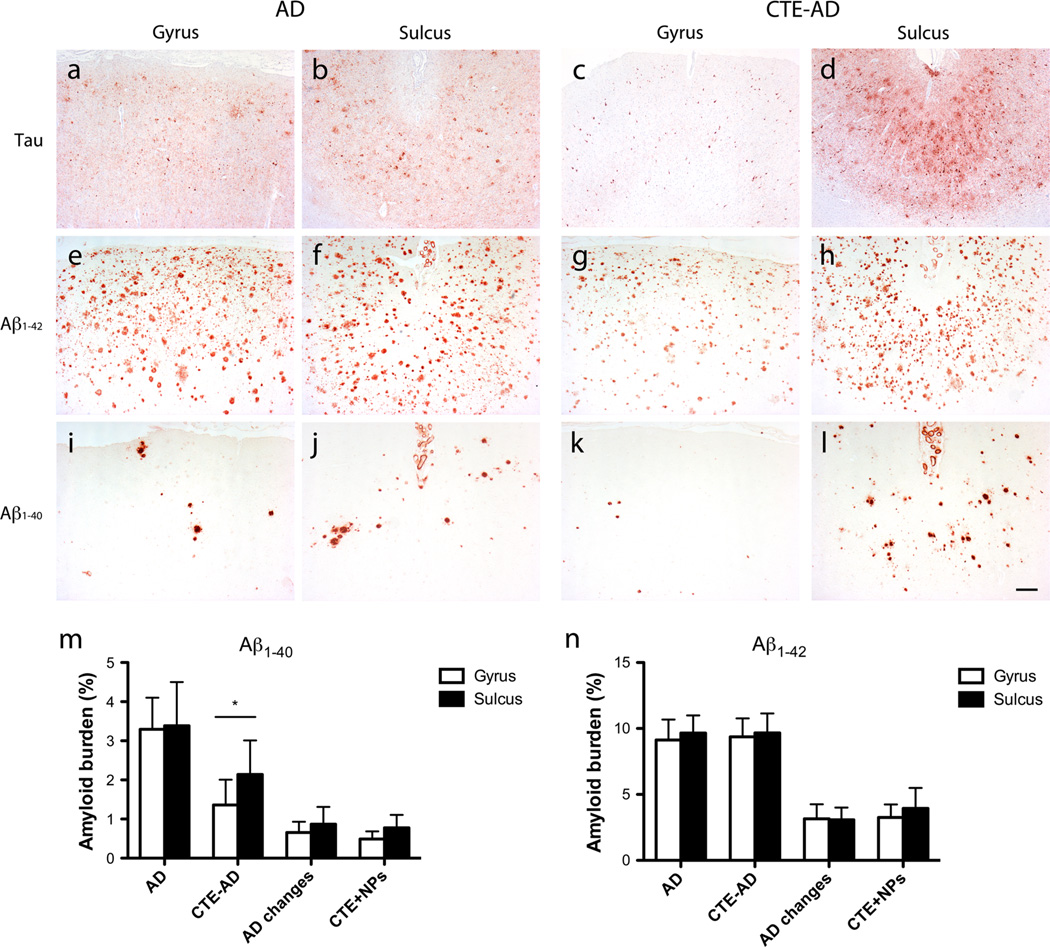
If traumatic injury influences the deposition of Aβ, we would hypothesize that more Aβ would be present at the sulcal depths due to the putative stress concentration there [45]. Therefore, we next examined a subset of cases that met the neuropathological criteria for both AD and CTE (CTE-AD; Supplementary Table 1) for differences in Aβ levels at the sulcal depths of the middle frontal sulcus versus the gyral crests. We also examined subjects with CTE and neuritic plaques that did not reach the full criteria for an AD diagnosis (CTE + NPs). These groups were compared to subjects without any history of RTBI or professional contact sports play, including those with pathologically diagnosed AD, and to those with neuritic plaques that did not have AD (AD changes; Supplementary Table 1). In subjects with only AD, there was no difference in Aβ1–40 or Aβ1–42 plaque deposition between the sulcus and the gyrus (Fig. 2). However, in CTE-AD subjects, the Aβ1–40 plaque burden was significantly greater in the sulcus compared to the gyral crest (t = 2.21, p = 0.029, Student’s t test), but this difference was not present for Aβ1–42. The Aβ1–42 plaque burden was similar between AD and CTE-AD subjects. In contrast, there was a significantly lower Aβ1–40 plaque burden in the gyral crest in CTE-AD compared to AD subjects (Fig. 2, t = −2.80, p = 0.013). Subjects with AD changes and subjects with CTE + NPs had similar plaque burden levels for both Aβ1–40 and Aβ1–42 in both the gyrus and the sulcus, and all were significantly less compared to the AD subject groups (p < 0.05, Student’s t test).
Fig. 2.
Aβ and tau pathologies were concentrated within the sulcal depths as compared to the gyral crests of the dorsolateral frontal cortex in CTE-AD. a–d Immunostaining for tau showed a greater accumulation of tau-positive neurons, astrocytes, and cell processes in the sulcus of CTE-AD subjects (d) as compared to the gyrus (c), but no difference in AD subjects (a, b). e–l Immunostaining for Aβ1-40 and Aβ1–42 showed no difference between the gyrus (e, i) and sulcus (f, j) of AD subjects. However, there were a greater number of plaques in the sulcus of subjects with CTE-AD (h, l) as compared to the gyrus (g, k) although the difference in Aβ1–40 was most pronounced (k, l). Scale bar 200 µm. (m, n) Quantitation of the percent amyloid burden showed significantly increased Aβ1–40 (m), but not Aβ1–42 (n) within the sulcus compared to the gyrus in the dorsolateral frontal cortex of CTE-AD subjects (*p = 0.029, Student’s t test). There was no difference between sulcal or gyral plaque burden for Aβ1–40 or Aβ1–42 in subjects with AD, AD changes, or CTE with neuritic plaques (CTE+NPs)
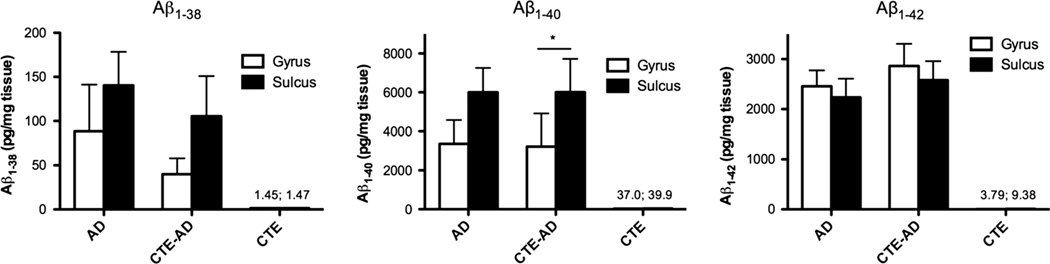
To determine whether total levels of various species of Aβ were elevated in the sulcus compared to the gyrus in subjects with CTE compared to those without, we next used ELISA to measure the total levels of Aβ1–38, Aβ1–40, and Aβ1–42 isolated from the sulcal depths of the middle frontal cortex and compared them to levels in the gyral crests. Similar to the data on Aβ plaque burden, we found significantly more Aβ1–40 in the sulcus compared to the gyrus in CTE-AD subjects (paired t = 1.965, p = 0.045) and no difference between sulcal and gyral levels of Aβ1–42 (Fig. 3). Surprisingly, we also found a trend of elevated Aβ1–40 levels in the middle frontal sulcus compared to the gyrus of AD subjects without a history of RTBI; however, this did not reach Significance (t = 2.16, p = 0.059, paired t test). As expected, subjects with CTE without neuritic plaques had very low levels of Aβ1–38, Aβ1–40, and Aβ1–42 (Fig. 3).
Fig. 3.
Total levels of Aβ in subjects with AD, CTE-AD, or CTE alone as measured by ELISA. Subjects with both CTE and AD (CTE-AD) had a significantly higher Aβ1–40 burden within the sulcus compared to the gyrus (*p = 0.045, paired t test). There was no difference between sulcal or gyral Aβ1–42 levels in subjects with AD or CTE-AD. Subjects with CTE alone had low levels of Aβ1–38, Aβ1–40, and Aβ1–42 that were not significantly different between sulci and gyri
Effect of Aβ on CTE tauopathy
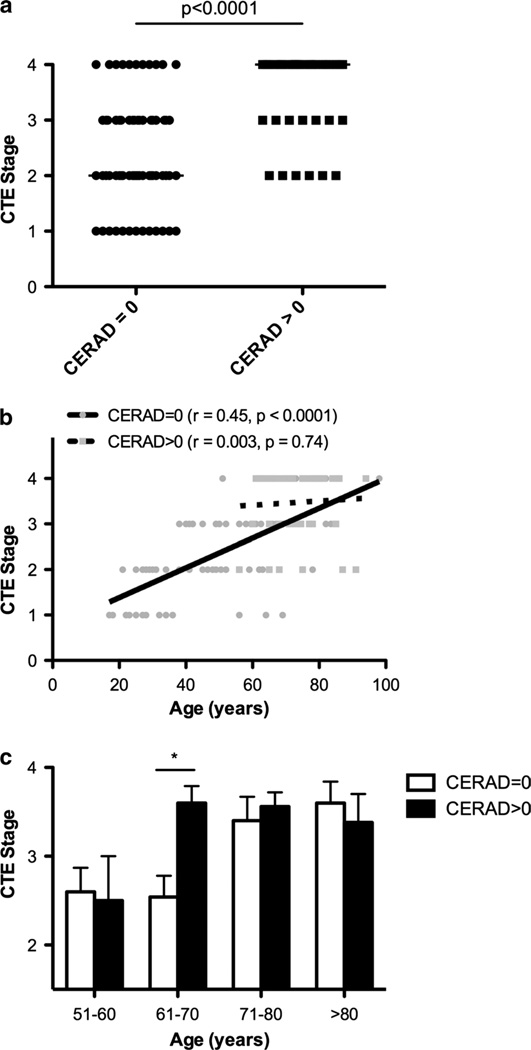
To test the hypothesis that the development of Aβ pathology relates to an increased severity and progression of CTE tauopathy, several regression analyses were employed. The CTE stage is a measure of the degree and distribution of tau pathology [30] and was significantly increased in subjects with co-morbid neuritic plaque pathology (Fig. 4a). In the overall CTE cohort, age was also significantly associated with CTE stage. Therefore, we also tested for an interaction between neuritic plaques and age on CTE stage. The overall linear regression model showed that CTE stage was significantly predicted by neuritic plaques (β = 2.43, p = 0.018) when adjusted for age (β = 0.029, p = 0.001) and the two-way interaction (age × neuritic plaque, β = −0.028, p = 0.047). Taken together, Aβ pathology and age accounted for almost half of the total variance in the progressive development of CTE stage (r2 = 0.47) although the contribution of age to the development of CTE tau pathology had a small effect (β coefficient <0.25). Figure 4b illustrates the nature of this interaction by separately plotting the regression of age and CTE stage in subjects with neuritic plaques versus those without. Notably, there was a significant association between age at death and CTE stage in subjects lacking neuritic plaques (β = 0.032, p < 0.001), but not in CTE subjects with neuritic plaques (β = 0.004, p = 0.740). The slope of the linear regression line between age and CTE stage was significantly greater in CTE subjects without neuritic plaques compared to those with neuritic plaques (F = 4.147, p = 0.044). This demonstrates that the presence of neuritic plaques modifies the relationship between age and CTE stage. When broken down by decade, CTE stage was significantly increased in subjects in their 60s with neuritic plaques, but not younger subjects (Fig. 4c). After age 70, the average CTE stage plateaued to near its maximum level for subjects both with and without NPs. To examine this relationship even further, we employed binary logistic regression and found the odds of a case progressing to the most severe stage of CTE, stage IV tauopathy, was 3.90 times higher in subjects presenting with co-morbid neuritic plaques compared to those without (p = 0.008) while adjusting for age. Overall, these data demonstrate that the presence of neuritic plaques was associated with more severe CTE-related tauopathy independent of age.
Fig. 4.
CTE subjects with neuritic Aβ plaques (CERAD > 0) had an accelerated tauopathy. a The median CTE stage was significantly greater in CTE subjects with neuritic plaques (*p < 0.001, Mann– Whitney U test). bScatter plots of CTE tau pathology (stage) versus age are shown with separate regression lines for subjects without neuritic plaques (CERAD = 0) and those with neuritic plaques (CERAD > 0). There was a significant correlation between age and CTE stage in subjects without neuritic plaques, but not in CTE subjects with neuritic plaques. The slope of the regression line between age and CTE stage was significantly greater (p = 0.044) in CTE subjects without compared to those with neuritic plaques. c The increase in CTE stage in subjects with neuritic plaques was significant in the 7th decade of life (*p < 0.002, Mann–Whitney U test), while older subjects had similarly elevated CTE stages
Quantitative levels of tau phosphorylated at threonine 231 (ptau231) were also measured by ELISA in the middle frontal cortex in a subset of subjects (Supplementary Table 1). ptau231 was significantly increased in CTE subjects with neuritic plaques compared to those without (Supplementary Figure 1, t = 2.10, p = 0.045) and was further increased in CTE-AD subjects. CTE-AD subjects had similar levels of ptau231 as AD subjects despite an average age at death almost a decade earlier (70 years in CTE-AD versus 79 years in AD). This further supports the hypothesis that Aβ deposition is associated with more severe tau pathology in CTE.
Clinical and mTBI exposure associations
We next set out to determine whether the presence of Aβ was associated with worse clinical outcomes. First, we found no significant difference between the number of years of athletic exposure. Subjects with diffuse or neuritic plaques did have an increased number of reported concussions compared to CTE subjects without Aβ, but this difference was not significant (Table 3). Both the age at death and the age at symptom onset were significantly greater in CTE subjects with Aβ (either diffuse or neuritic plaques) compared to those without Aβ (Table 3, p < 0.001).
There was a significantly greater frequency of dementia in subjects with Aβ compared to those without. A logistic regression analysis controlling for age shows that dementia remained significantly enriched in the CTE with Aβ subgroups (Table 3). In fact, the odds of developing dementia were 3.9 times higher in persons with diffuse plaque pathology (OR = 3.93, p = 0.013) while controlling for age (p < 0.001) and 4.5 times higher in persons with neuritic pathology (OR = 4.45, p = 0.012) while controlling for age (p < 0.001). In addition, the proportion of subjects with CTE clinical symptoms was greater in those subjects with either diffuse or neuritic plaques (neuritic: p = 0.028, Pearson χ2 test), although this difference was not significant when controlling for age (Table 3).
Two distinct clinical presentations of CTE have been reported: some subjects present with cognitive symptoms (cognitive variant) and others present with behavioral or mood symptoms (behavioral-mood variant) [46]. The frequency of the cognitive variant was increased in CTE subjects with Aβ, but this difference did not reach significance (Table 3).
Motor symptoms including parkinsonism have been reported in a subset of CTE subjects. Moreover, Lewy body disease has been associated with Aβ deposition [11]. We, therefore, tested whether Aβ is associated with Lewy body disease and parkinsonism in our CTE cohort. We found a significantly higher frequency of both Lewy body disease pathology and parkinsonism in CTE subjects with Aβ compared to those without. A logistic regression analysis controlling for age shows that both Lewy body pathology (neuritic: OR = 5.01, p = 0.009; diffuse: OR = 7.09, p = 0.019) and parkinsonism (diffuse: OR = 8.86, p = 0.050) are significantly enriched in the CTE with Aβ subgroups (Table 3).
The CTE cohort is a heterogeneous group with multiple comorbidities, including a history of substance abuse (present in 44 % of subjects, n = 94) and symptoms of depression (65 %). To test whether these comorbidities may influence the association of Aβ with dementia, parkinsonism, and Lewy body disease, we performed a logistic regression analysis controlling for them. Aβ deposition was still significantly associated with dementia, parkinsonism, and Lewy body disease, but neither a history of substance abuse nor symptoms of depression were significantly associated (data not shown). Furthermore, to test whether these comorbidities are associated with Aβ deposition in the CTE cohort, we performed a logistic regression analysis with the presence of diffuse plaques as the dependent variable and found a positive association with age (β = 0.081, p < 0.001), but a non-significant negative association with a history of substance abuse (β = −0.762, p = 0.148) and symptoms of depression (β = −0.088, p = 0.874). Results were similar for neuritic plaques suggesting that substance abuse and depression do not influence Aβ deposition in the CTE cohort.
Because forces associated with RTBI likely differ with the type of exposure, we hypothesized that Aβ deposition would also differ with the type of exposure. Thus we compared Aβ plaque deposition across CTE subjects with different exposure histories, specifically boxing, American football, hockey, and military combat (Table 4). Overall, we found that boxers in our series had a significantly greater CTE stage when compared to football players, hockey players, or military veterans (Table 4, Mann–Whitney U test). The boxers also had the greatest frequency of neuritic plaques (50 %) compared to football players (34 %), hockey players (40 %), and military veterans (33 %), although the difference was not significant. Similarly, boxers had a greater, but non-significant, frequency of diffuse plaques compared to football or hockey players or military veterans (Table 4).
Table 4.
CTE Stage and frequency of Aβ deposition by sport or military history
| n | Mean age at death (years) | CTE stage mean ± SEM | p value* | APOE ε4 % (+/−)a | DP freq (%) | NP freq (%) | |
|---|---|---|---|---|---|---|---|
| Boxing | 10 | 67 | 3.80 ± 0.13 | – | 29 % (2/5) | 70 | 50 |
| Football | 88 | 61 | 2.78 ± 0.11 | 0.002 | 35 % (22/41) | 51 | 34 |
| Hockey | 5 | 57 | 2.60 ± 0.40 | 0.012 | 40 % (2/3) | 40 | 40 |
| Military | 9 | 55 | 2.22 ± 0.12 | 0.002 | 20 % (1/4) | 44 | 33 |
DP diffuse plaques, NP neuritic plaques
Mann–Whitney U test compared to boxing, significant p values are in bold
% of both ε4 heterozygotes and homozygotes out of those cases with genotypes
Discussion
Here we show that Aβ plaque deposition was present in 52 % of all subjects with pathologically diagnosed CTE in our series (mean age at death = 60 years). We find that Aβ deposition occurred at a younger age and at an accelerated rate in our cohort of subjects with a RTBI history and a neuropathological diagnosis of CTE when compared to a community-based autopsy series [2] (Fig. 1b). In addition, there were elevated levels of Aβ1–40 within the sulcus compared to the gyrus in subjects with CTE-AD. When Aβ plaques were present in CTE, they were significantly associated with more severe tau and Lewy body pathology and worse clinical outcome independent of the effect of age.
While it is possible that the presence of Aβ simply represents the development of co-morbid Alzheimer’s disease pathology, Aβ deposition occurred at an accelerated rate in subjects with a neuropathological diagnosis of CTE when compared to a community-based cohort (Fig. 1b), which suggests that exposure to RTBI or the presence of hyper-phosphorylated tau might be a modifying factor in Aβ accumulation. In fact, a weighted two-sample Chi square test demonstrated that the age-dependent distribution of Aβ was significantly different in our CTE cohort compared to normal aging (χ2 = 721, p < 0.001), suggesting that RTBI is not simply accelerating an aging process, but also may be altering the normal dynamics of Aβ deposition. All the subjects in our CTE cohort were men, which is not the case in the normal aging cohort. However, men develop Aβ pathology at a significantly slower rate than women [2], suggesting that the actual increase in frequency of Aβ deposition in CTE may be greater than what we report. There was a high frequency of substance abuse history (44 %) and symptoms of depression (65 %) in the CTE cohort. However, neither of these comorbidities was significantly associated with Aβ deposition, suggesting that other factors drive plaque formation in CTE. Moreover, the pattern of Aβ deposition was altered in CTE-AD subjects with elevated levels of Aβ1–40 at the sulcal depths compared to the gyral crests. This sulcal predilection of Aβ corresponds to regions of axonal injury in gyrencephalic animals after acceleration–deceleration injury [44], which may be a result of stress concentration at the sulcal depths following trauma [3]. Altogether, these findings suggest that repetitive mild traumatic injury may accelerate and alter Aβ accumulation and deposition.
The presence of Aβ was associated with disease progression in CTE. Subjects with neuritic plaques had a significantly increased stage of CTE tauopathy even when controlling for age. Although an association between neuritic plaques and advanced tau pathology might be expected given that neuritic plaques require tau-positive neurites and are associated with neurofibrillary tangles in AD, the pattern of tau pathology associated with the various CTE stages is unique, and an association between Aβ deposition and tauopathy severity in CTE has not previously been shown. We further found that levels of ptau231 in CTE were increased in the presence of Aβ. Along with this increase in tau pathology, there was a significantly higher frequency of dementia in the presence of Aβ with the age-adjusted odds of developing dementia in CTE 4.5 times higher in persons with Aβ pathology than without. Taken together, this suggests that CTE with Aβ pathology is a distinct subtype of CTE—one that has a more aggressive pathology and clinical course.
Deposition of Aβ is strongly associated with Lewy body disease, the formation of alpha-synuclein inclusions, and higher levels of insoluble alpha-synuclein [11, 20]. In vitro studies have shown that Aβ promotes the formation of alpha-synuclein oligomers and polymers as well as inclusions in transfiected cell lines [25]. In our CTE cohort we found a significant association of Aβ with LBD even when controlling for age. Moreover, the age-adjusted odds for developing parkinsonism was 8.9 times higher in CTE subjects with diffuse Aβ deposition compared to those without Aβ. Thus, Aβ may lead to Lewy body pathology and par-kinsonism in addition to causing a more severe tauopathy in CTE.
The ε4 allele of APOE is a common genetic factor that appears to render individuals susceptible to Aβ deposition. The APOE ε4 allele is a major risk factor for Alzheimer’s disease with homozygotes possessing a more than tenfold greater risk for developing AD dementia and heterozygotes a threefold greater risk [8, 26]. In clinical control populations, the ε4 allele is associated with biomarkers indicating greater Aβ deposition [35, 50]. Moreover, the ε4 allele is associated with increased Aβ levels following TBI [6], and several studies have demonstrated worse clinical outcomes in ε4 carriers following a traumatic brain injury or concussion [19, 21, 23, 48]. Here we show that the APOE ε4 allele was significantly associated with Aβ deposition in CTE. In fact, when subjects with Aβ were excluded, there was no significant difference in the proportion of ε4 alleles between CTE and population norms although our sample size was small. Although additional effects of the ε4 allele cannot be ruled out, it may be that the ε4 allele increases the likelihood of Aβ accumulation and deposition following RTBI and this, in turn, worsens the pathological and clinical outcomes.
Although CTE is a distinct disease, there are similarities to AD. Trauma has long been thought to be a risk factor for developing AD, and moderate to severe TBI has been shown to acutely increase Aβ levels and plaque formation [16, 36]. An increased percentage of Thiofavin-S-positive plaques has been shown in subjects many years after single TBI compared to age-matched controls, suggesting that even a single TBI may accelerate and alter Aβ deposition [18]. Axonal injury is a proposed source of elevated Aβ in acute and moderate to severe TBI, and we found increased Aβ at the depths of sulci in CTE where there is also evidence of significant axonal injury in animal models of mild TBI. This suggests that the axonal injury provoked by mild TBI might also alter the chronic deposition of Aβ in CTE. We found increased Aβ1–40 at the sulcal depths compared to the gyral crests, but the level of Aβ1–42 was unchanged in subjects with both CTE and Alzheimer’s disease. The reason for this difference is unclear. Neuritic plaques are composed of both Aβ1–40 and Aβ1–42 while diffuse plaques are composed of Aβ1–42 [5, 12, 14]. Therefore, the increase in Aβ1–40 may result in more neuritic plaques in CTE.
Abundant genetic and experimental evidence suggests that Aβ can lead to the abnormal phosphorylation and accumulation of tau [1]. Our finding that the deposition of Aβ in CTE was associated with more severe tau pathology supports the hypothesis that Aβ can accelerate a tauopathy. These findings have important implications for diagnosing CTE before death. The interpretation of blood and cerebrospinal fluid biomarkers and positron emission tomography (PET) neuroimaging using both amyloid and tau ligands will need to consider the possibility of Aβ pathology in persons older than 40 with CTE (see Fig. 1).
There also may be differences in tau and amyloid pathology depending on the type of traumatic exposure. Studies in boxers using anti-Aβ immunohistochemistry with formic acid pretreatment demonstrated diffuse cortical Aβ plaques in 27 of 28 (96.4 %) subjects [39, 49]. In contrast to the original reports of CTE in boxers, the majority of cases in our cohort were football players. The nature, frequency, and intensity of traumatic impacts in different sports may lead to differences in CTE pathology. Here we show that boxers had a significantly greater CTE stage compared to football players, hockey players, or military veterans (Table 4). There was also a trend toward greater frequency of Aβ deposition in boxers although the difference was not significant, which may be due to the small sample size.
There are several limitations to our study. This is an autopsy-based study involving a heterogeneous cohort of individuals with a history of RTBI that came largely by self or family referral. The study subjects may not be representative of the entire RTBI population. Moreover, the clinical histories are retrospectively obtained and are subject to bias. Our subjects lived in the United States, and although the prevalence of AD does not vary much between the USA and European countries [47], it is unknown how differences in ethnicity may affect Aβ deposition. Ethnic differences have been shown to affect ε4 allele frequency. For instance, a recent study showed ε4 frequency increased with latitude [22]. We compared our cohort to a large meta-analysis that included data from multiple countries [28], and other US-based studies found similar ε4 allele frequencies [7, 50], supporting the use of this control population. Future longitudinal, prospective studies are needed to address the frequency and type of pathologies present in population-based cohorts and to obtain less restrictive clinical histories and evaluations. Better quantitation of subject exposure to RTBI and correlation to in vivo biomarkers as well as neuropathological diagnoses at autopsy will be critical to clearly understand the role of RTBI on Aβ aggregation and deposition and CTE pathogenesis.
Overall, our work suggests that CTE with Aβ pathology is a distinct pathological subtype of CTE that shows a greater degree of tauopathy, a greater likelihood of LBD, and has a worse clinical outcome compared with CTE without Aβ. RTBI may alter and accelerate the deposition of Aβ in some individuals, and Aβ, in turn, may accelerate tau and Lewy body pathologies and worsen the clinical course in CTE. It remains to be determined whether individuals susceptible to Aβ deposition can be identified in life with biomarkers, what genetic factors, in addition to APOE ε4, may influence susceptibility to Aβ deposition, and what minimum burden of head trauma is necessary for induction of CTE and modulation of Aβ deposition.
Supplementary Material
Acknowledgments
We gratefully acknowledge the use of resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA) as well as all the individuals whose participation and contributions made this work possible. This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Clinical Sciences Research and Development Merit Award; Alzheimer’s Association (NIRG-305779); Veterans Affairs Biorepository (CSP 501); Translational Research Center for Traumatic Brain Injury and Stress Disorders (TRACTS) Veterans Affairs Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B6796-C); National Institute of Neurological Disorders and Stroke, National Institute of Biomedical Imaging and Bioengineering (U01NS086659-01); National Institute of Aging Boston University AD Center (P30AG13846; supplement 0572063345-5); Department of Defense Peer Reviewed Alzheimer’s Research Program (DoD-PRARP #13267017); Sports Legacy Institute. This work was also supported by unrestricted gifts from the Andlinger Foundation and WWE.
Abbreviations
- AD
Alzheimer’s disease
- CTE
Chronic traumatic encephalopathy
- CTE-AD
Chronic traumatic encephalopathy with AD
- CERAD
Consortium to establish a registry of AD
- Aβ
Amyloid β peptide
- APP
Amyloid precursor protein
- NP
Neuritic plaques
- DP
Diffuse plaques
- RTBI
Repetitive traumatic brain injury
- ELISA
Enzyme-linked immunosorbent assay
- OR
Odds ratio
- ptau231
Tau phosphorylated at threonine 231
- PET
Positron emission tomography
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-015-1435-y) contains supplementary material, which is available to authorized users.
Conflict of interest Other than the stated grants the authors have no conficts of interest to disclose.
References
- 1.Bloom GS. Amyloid-β and tau. JAMA Neurol. 2014;71:505. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 3.Cloots RJH, Gervaise HMT, van Dommelen JAW, Geers MGD. Biomechanics of traumatic brain injury: influences of the morphologic heterogeneities of the cerebral cortex. Ann Biomed Eng. 2008;36:1203–1215. doi: 10.1007/s10439-008-9510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings BJ, Satou T, Head E, et al. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: evidence from cats and dogs. Neurobiol Aging. 1996;17:653–659. doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 6.DeKosky ST, Abrahamson EE, Ciallella JR, et al. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch Neurol. 2007;64:541–544. doi: 10.1001/archneur.64.4.541. [DOI] [PubMed] [Google Scholar]
- 7.Falcone GJ, Radmanesh F, Brouwers HB, et al. APOE ε variants increase risk of warfarin-related intracerebral hemorrhage. Neurology. 2014;83:1139–1146. doi: 10.1212/WNL.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandy S, DeKosky ST. APOE 4 status and traumatic brain injury on the gridiron or the battlefield. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004274. 134ed4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentleman SM, Greenberg BD, Savage MJ, et al. A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport. 1997;8:1519–1522. doi: 10.1097/00001756-199704140-00039. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003716. 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomperts SN, Locascio JJ, Marquie M, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27:965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güntert A, Döbeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Heyman A, Wilkinson WE, Stafford JA, et al. Alzheimer’s disease: a study of epidemiological aspects. Ann Neurol. 1984;15:335–341. doi: 10.1002/ana.410150406. [DOI] [PubMed] [Google Scholar]
- 14.Howlett DR, Hortobágyi T, Francis PT. Clusterin associates specifically with Aβ40 in Alzheimer’s disease brain tissue. Brain Pathol. 2013;23:623–632. doi: 10.1111/bpa.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomovic MD, Uryu K, Abrahamson EE, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson VE, Stewart W, Smith DH. Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan BD, Relkin NR, Ravdin LD, et al. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- 20.Kalaitzakis ME, Pearce RKB. The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 2009;118:587–598. doi: 10.1007/s00401-009-0597-x. [DOI] [PubMed] [Google Scholar]
- 21.Katsnelson A. Gene tests for brain injury still far from the football feld. Nat Med. 2011;17:638. doi: 10.1038/nm0611-638. [DOI] [PubMed] [Google Scholar]
- 22.Kern S, Mehlig K, Kern J, et al. The distribution of apoli-poprotein E genotype over the adult lifespan and in relation to country of birth. Am J Epidemiol. 2015;181:214–217. doi: 10.1093/aje/kwu442. [DOI] [PubMed] [Google Scholar]
- 23.Kutner KC, Erlanger DM, Tsai J, et al. Lower cognitive performance of older football players possessing apolipoprotein E epsilon4. Neurosurgery. 2000;47:651–657. doi: 10.1097/00006123-200009000-00026. (discussion 657-8) [DOI] [PubMed] [Google Scholar]
- 24.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neuro-degenerative causes of death among retired National Football League players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masliah E, Rockenstein E, Veinbergs I, et al. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal defcits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 27.Mayeux R, Ottman R, Tang MX, et al. Genetic susceptibility and head injury as risk factors for Alzheimer’s disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993;33:494–501. doi: 10.1002/ana.410330513. [DOI] [PubMed] [Google Scholar]
- 28.McKay GJ, Silvestri G, Chakravarthy U, et al. Variations in apolipoprotein E frequency with age in a pooled analysis of a large group of older people. Am J Epidemiol. 2011;173:1357–1364. doi: 10.1093/aje/kwr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10:S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 32.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6:68. doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montenigro PH, Corp DT, Stein TD, et al. Chronic traumatic encephalopathy: historical origins and current perspective. Annu Rev Clin Psychol. 2015 doi: 10.1146/annurev-clinpsy-032814-112814. [DOI] [PubMed] [Google Scholar]
- 34.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2011;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mormino EC, Betensky RA, Hedden T, et al. Amyloid and APOE 4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsson A, Csajbok L, Ost M, et al. Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fuid after severe traumatic brain injury. J Neurol. 2004;251:870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Nievas BG, Stein TD, Tai H-C, et al. Dissecting phe-notypic traits linked to human resilience to Alzheimer’s pathology. Brain. 2013;136:2510–2526. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 39.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatr. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts GW, Gentleman SM, Lynch A, et al. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatr. 1994;57:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts GW, Gentleman SM, Lynch A, Graham DI. Beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 42.Shen H. Researchers seek definition of head-trauma disorder. Nature. 2015;518:466–467. doi: 10.1038/518466a. [DOI] [PubMed] [Google Scholar]
- 43.Smith DH, Chen X-H, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 44.Smith DH, Chen XH, Xu BN, et al. Characterization of diffuse axonal pathology and selective hippocampal damage following inertial brain trauma in the pig. J Neuropathol Exp Neurol. 1997;56:822–834. [PubMed] [Google Scholar]
- 45.Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther. 2014;6:4. doi: 10.1186/alzrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takizawa C, Thompson PL, van Walsem A, et al. Epide-miological and economic burden of Alzheimer’s disease: a systematic literature review of data across Europe and the United States of America. J Alzheimers Dis. 2015;43:1271–1284. doi: 10.3233/JAD-141134. [DOI] [PubMed] [Google Scholar]
- 48.Terrell TR, Bostick RM, Abramson R, et al. APOE, APOE promoter, and tau genotypes and risk for concussion in college athletes. Clin J Sport Med. 2008;18:10–17. doi: 10.1097/JSM.0b013e31815c1d4c. [DOI] [PubMed] [Google Scholar]
- 49.Tokuda T, Ikeda S, Yanagisawa N, et al. Re-examination of ex-boxers’ brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- 50.Wirth M, Villeneuve S, La Joie R, et al. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J Neurosci. 2014;34:8612–8617. doi: 10.1523/JNEUROSCI.4612-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia W, Yang T, Shankar G, et al. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch Neurol. 2009;66:190–199. doi: 10.1001/archneurol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.