Abstract
Background
Atopic sensitization to aeroallergens in early life has been shown to be a strong risk factor for developing persisting asthma in young children with recurrent wheeze.
Objective
The aim of this study was to assess the yield of skin prick test (SPT) compared to allergen specific serum IgE testing (sIgE) at identifying aeroallergen sensitization in atopic children < 4 years of age.
Methods
Concordance between SPT (Greer Laboratories, ComforTen™) and allergen specific sIgE (Immulite 2000™) for 7 common aeroallergens was analyzed in forty atopic inner-city children, 18–48 months of age (mean 36 +/− 9 months) with recurrent wheezing, family history of asthma and/or eczema.
Results
In 80% of children one or more allergen sensitizations would have been missed if only SPT had been performed, and in 38% of children one or more sensitizations would have been missed if only serum IgE testing had been performed. Agreement and between SPT and sIgE test was fair for most allergens (kappa between −0.04 and 0.50), as was correlation between sIgE levels and SPT grade (rho between 0.21 and 0.55). Children with high total sIgE (≥300 kU/l) were more likely to have sIgE positive tests with negative corresponding skin test (p=0.025).
Conclusions
Our study showed significant discordance between allergen specific SPT and sIgE testing results for common aeroallergens, suggesting that both SPT and sIgE testing should be done when diagnosing allergic sensitization in young children at high risk of asthma.
Introduction
Large epidemiologic studies have shown that sensitization to aeroallergens before 4 years of age is a risk factor for developing asthma in young children with recurrent wheeze [1–3]. In addition, sensitization to aeroallergens in young children at risk for asthma and allergy based on parental history was recently shown to increase the risk for rhinovirus-induced wheezing [4]. These observations suggest that early intervention to prevent or decrease allergic sensitization may help reduce asthma in atopic children, hence the importance of correctly identifying atopic sensitization.
Allergen sensitization is typically diagnosed by either skin testing or serum IgE testing for specific allergens and either method has typically been used in large epidemiologic studies [1, 3, 4]. Discrepancies of about 20% between skin and serum testing have been found in adults with rhinitis [5]. The current American Academy of Pediatrics (AAP) [6] as well as the current asthma management guidelines set forth by the National Lung and Heart Institute (NHLBI) [7] recommend to use skin or serum IgE testing to diagnose allergic sensitization in infants or children. Potential discrepancies in sensitivity between these two methods have been acknowledged [6]. To our knowledge, no study analyzed how these two methods of detecting aeroallergen specific IgE correlate in highly atopic children <4 years of age. The aim of this study was to assess the yield of skin prick test (SPT) compared to allergen specific serum IgE testing (sIgE) at identifying aeroallergen sensitization (=presence of allergen specific IgE) in a population of inner-city, atopic children aged < 4 years with history of wheezing.
Methods
Subjects
Forty children receiving care at an inner city-hospital based pediatric asthma center in the Bronx, New York, who were enrolled in an ongoing, randomized, prospective interventional clinical trial to evaluate the efficacy of subcutaneous immunotherapy in reducing asthma morbidity (clinicaltrial.gov identifier NCT01028560) were included in this analysis. All parents of participating children had provided written consent and the study was approved by the local Institutional Review Board. Children were eligible if they had physician diagnosed or parent-reported wheezing with significant asthma symptoms on > 1 occasion, were atopic based on having a positive skin or serum IgE test to an environmental allergen, and had major risk factors of developing asthma including a history of eczema or one parent with history of asthma [8]. Skin testing and serum IgE testing were done at baseline prior to initiating any study intervention.
Allergy testing
All children first underwent skin testing on their upper back using the ComforTen skin prick device (Hollister Stier Laboratories, LLC) loaded with allergen extracts (in 50% glycerin), one negative control (non-extract containing diluent with 50% glycerin) and one positive control (histamine base 6 mg/ml) purchased from Greer Laboratories, Inc., NC. Aeroallergen extracts used for skin testing included: grass pollen, ragweed pollen, dust mite, roach, mouse, cat, dog (table 1.) as well as tree pollen (“Eastern 10-tree pollen mix” 1:20 w/v, containing Red/River Birch, Red oak, American elm, Sugar/Hard maple, American beech, White ash, Shagbark hickory, Eastern cottonwood, American/Eastern sycamore, Sweet gum) and mold mix (“Mold mix#3”, containing Alternaria alternata, Aspergillus niger, Cladosporium sphaerospermum, Penicillum notatum). All personnel conducting skin testing were trained by one board certified allergist. Skin test results were recorded according to grades, defined as the maximum wheal diameter above the wheal diameter of the glycerin control: grade 0= glycerin control, grade 1 = 2 to <4 mm, grade 2= 4 to < 7 mm, grade 3= 7 to 10 mm, grade 4>10 mm/+ pseudopods. Flare size did not contribute to the grading system, because they were often difficult to demarcate on some of the subjects who had darker skin. Parents had been instructed to discontinue oral antihistamines at least 7 days prior to skin testing. Only children with any positive result on their aeroallergen skin test whose parents agreed to participate in the associated clinical immunotherapy trial went on to have serum IgE testing, typically within the next 4 weeks.
Table 1.
Comparison between extracts used for skin prick testing and in-vitro allergen specific sIgE testing
| Extracts used for skin testing Greer Laboratories |
Antigens used for serum IgE testing Immulite® 2000 3gAllergy™ system |
|
|---|---|---|
| Northern grass pollen mix 100.000 BAU/ml | ||
| Timothy grass | Timothy grass (G6) | |
| Kentucky Blue/June | n/a | |
| Orchard | n/a | |
| Redtop | n/a | |
| Sweet Vernal | n/a | |
| Giant and Short ragweed mix 1:20 w/v, (AgE 125 U/ml) | Common (=short) Ragweed (W1) | |
| Dermatophagoides pteronyssinus 10.000 AU/ml | Dermatophagoides farinae (D2) | |
| Cockroach mix (American and German) 1:20 w/v | Cockroach (I6) | |
| Mouse epithelia 1:20 w/v | Mouse urine (E72) | |
| Cat hair 10.000 BAU/ml | Cat dander-epithelium (E1) | |
| Dog epithelia, mixed breeds 1:20 w/v | Dog dander (E5) | |
Serum IgE (sIgE) testing was done in the hospital laboratory using the Immulite® 2000 3gAllergy™ system (Siemens AG). The Immulite® system measures allergen specific IgE between 0.1 kU/l and 100 kU/l. Hence a level of sIgE 0.1kU/l or greater was considered “positive”. Even though we also analyzed concordance of SPT and sIgE testing using the tradition cut-off level of 0.35kU/l, we decided to focus in the presented analysis on the detection cut-off as offered by the Immunlite 2000 and other modern methods of sIgE detection available to date, because the goal of our analysis was to compare the yield of detection of allergen specific IgE by these two methods.
For the comparative analysis between skin prick testing and serum IgE testing we excluded tree pollen and mold, because no serum testing was done for mold and because for tree pollen the skin test extract contained a tree pollen mixture that was only partially cross-reactive with the tree pollen mixture used for serum IgE testing. All aeroallergens used for serum testing and skin testing that were included in our comparative analysis are listed in table 1.
Statistical analysis
Descriptive statistics were applied to all variables. Continuous variables of non-parametric distribution were described as the median and range, and parametrically distributed variables were described as the mean and SD. Spearman rank correlation was tested to describe the correlation (rho) between sIgE level and grade of SPT result. Chi square tests (or 2-tailed Fisher’s exact test where appropriate) or logistic regression was performed when proportions were compared between 2 groups. The agreement between skin test and serum sIgE test was tested using kappa statistics. The data was analyzed using MS office excel 2010 and STATA SE11 software.
Results
Demographic variables
Table 2 summarizes the children’s demographic characteristics. The majority of children had more than 5 wheezing episodes, a history of eczema, concomitant food allergies and high total serum IgE levels, indicating a high risk for persisting asthma. All participants had Medicaid health insurance, consistent with a lower socioeconomic status.
Table 2.
Children’s demographic and clinical characteristics (n=40)
| Mean age in montds (+/−SD) | 36.5 (+/−9.4) | |
| Male gender (%) | 83 % | |
| Race (%) | ||
| Hispanic | 63 % | |
| African-American | 30 % | |
| Other | 7 % | |
| History of clinical food allergy | 62 % | |
| Eczema ever (%) | 92 % | |
| Eczema currently (%) | 70 % | |
| Number of wheezing episodes and/or asthma symptoms since birth (%) | ||
| 2–4 | 30 % | |
| 5–15 | 43 % | |
| >15 | 27 % | |
| Total serum IgE kU/l (%) | ||
| 0–50 kU/l | 13 % | |
| >50–100 kU/l | 3 % | |
| >100–300 kU/l | 30 % | |
| >300–1,000 kU/l | 26 % | |
| >1,000–2,000 kU/l | 13 % | |
| > 2,000 kU/l | 16 % | |
| Number of aeroallergen sensitizations per child based on SPT1 | 3.1 (+/−1.7) | |
| Number of aeroallergen sensitizations per child based on sIgE1,3 | 4.3 (+/−2.2) | |
| Number of food allergen sensitizations per child based on SPT2 | 1.5 (+/−1.8) | |
| SPT grade of histamine control | ||
| 1 | 3 % | |
| 2 | 70 % | |
| 3 | 18 % | |
| 4 | 10 % | |
Aeroallergens tested: tree pollen, grass pollen, ragweed pollen, dust mite, roach, cat, mouse, dog; (Mean+/−SD)
Food allergens tested: milk, egg, soy, wheat, peanut, shellfish, fish; (Mean+/−SD)
IgE≥0.1 kU/l
Atopic sensitization to outdoor and indoor allergens
As required by the eligibility criteria, all children had at least one atopic sensitization on skin or serum sIgE test. Based on combined sIgE and SPT results, less children were sensitized to pollen (tree, grass and ragweed) than for indoor allergens (33–60% vs. 68–78%, p=0.02). The tree pollen results were omitted from the subsequent comparative analyses because different, only partially cross-reacting tree pollen mixes were used for skin and serum testing.
Discordance between allergen specific skin prick and serum sIgE test
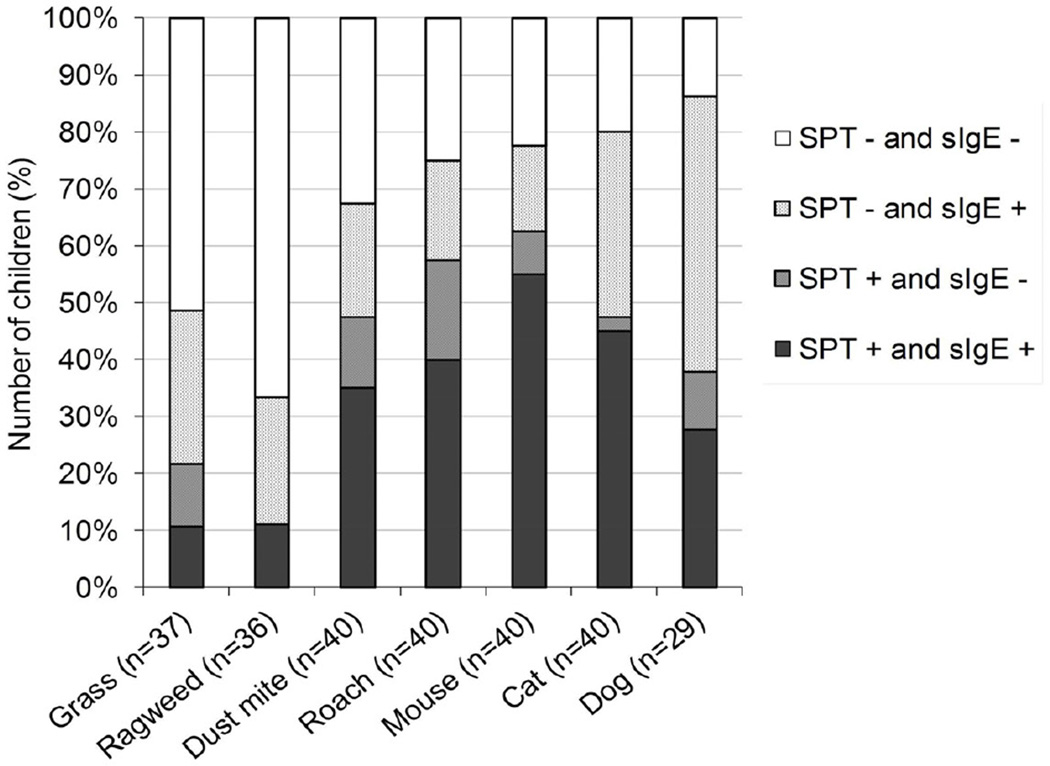
The proportion of tests with discordant (SPT positive/sIgE negative, i.e. <0.1 kU/l or SPT negative /sIgE positive, i.e. ≥0.1 kU/l) or concordant results (SPT positive/sIgE positive or SPT negative /sIgE negative) are shown in figure 1. There were discordant results between SPT and sIgE results for all of the allergens tested. The strength of agreement in detecting allergic sensitization by the two methods (i.e. either having negative SPT with sIgE < 0.1 kU/l or positive SPT with sIgE ≥ 0.1 kU/l) was assessed by using the kappa statistics. Agreement was poor (Kappa <0.20) or fair (Kappa 0.21–0.40) for nearly all allergens tested, only mouse testing had moderate agreement with kappa=0.50 (table 3). In average 42% (min. 19%, max. 67%) of all sensitizations diagnosed by combined serum IgE and SPT tests would have been missed if only skin testing had been done. In contrast, only 13% (min. 0%, max. 23%) of all sensitizations would have been missed if serum IgE testing alone had been done. We also calculated mismatches using the traditional cut-off level of ≥ 0.35 kU/l for sIgE. Between 2.5% – 19.4% of sIgE levels fell between ≥0.1 kU/l and < 0.35 kU/l with the amounts varying between allergens (roach 2.5%, dust mite 7.5%, mouse 10%, grass 10.8%, cat 17.5%, dog 16.2% and ragweed 19.4%). Accordingly, using the traditional cut-off for sIgE ≥ 0.35 kU/l, 30% (min. 14%, max. 48%) of all atopic sensitization would have been with skin testing alone, and 19% (min. 14%, max. 29%) had been missed if serum IgE testing had been done alone (data not shown).
Figure 1.
Concordances and discordances between SPT and sIgE for each aeroallergen tested.
1 For grass and ragweed some children had missing laboratory values for sIgE.
2 Eleven children were not skin tested for dog (because dog was tested on a second applicator that carried otherwise only food antigens that was not used in all children)
Table 3.
Strength of agreement between SPT and serum IgE testing. Agreement is defined as either having skin prick test negative with corresponding allergen specific sIgE negative (i.e. <0.1 kU/l) or skin prick test positive with corresponding allergen specific sIgE positive (i.e. ≥ 0.1 kU/l). Kappa < 0.20 indicates poor agreement, kappa 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement.
| Kappa (95% CI) | |
|---|---|
| Dog | −0.04 (−0.14, 0.06) |
| Grass | 0.12 (0.05, 0.19) |
| Roach | 0.28 (0.21, 0.36) |
| Cat | 0.32 (0.24, 0.39) |
| Dust mite | 0.35 (0.28, 0.43) |
| Ragweed | 0.40 (0.35, 0.45) |
| Mouse | 0.50 (0.44, 0.56) |
Number of children in whom at least one specific sensitization would have been missed with skin testing or serum allergen specific IgE testing alone
In 32 of 40 children (80%), one or more aeroallergen sensitizations would have been missed if only SPT had been performed. On the other hand, if only sIgE testing had been performed one or more aeroallergen sensitization would have been missed in 15 of 40 children (38%) of children. Only 3 (7.5%) of children had a perfect match between skin and sIgE testing.
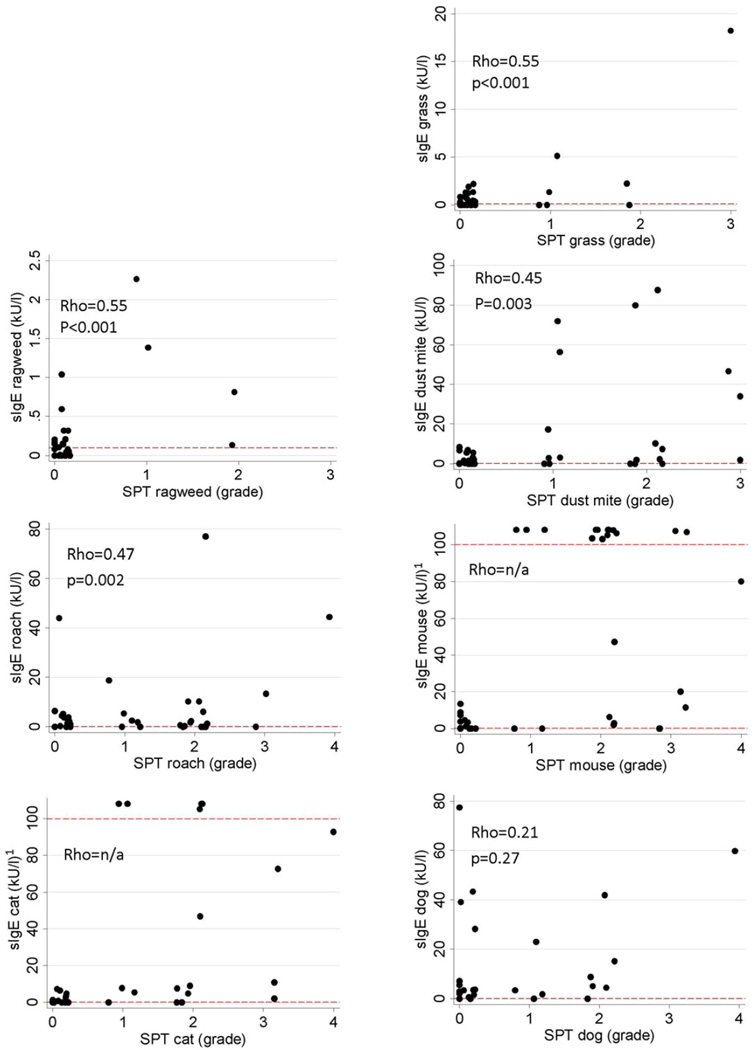
Correlation between skin test (grade) and level of specific sIgE testing by allergen
The association between level of aeroallergen specific sIgE and SPT grade showed fair correlation for most allergens (Spearman rank correlation, rho=0.45–0.55, p<0.005, figure 2). There was no correlation between SPT grade and sIgE level for dog (rho 0.27, p=0.27). Correlation could not be computed for cat and mouse because some children had cat or mouse specific IgE levels above 100 kU/l, the higher cut-off level for exact allergen specific sIgE measurement of the Immulite system. Therefore exact levels were not available in these children.
Figure 2.
Association between allergen specific sIgE levels (kU/l) and grade of SPT result. Each data point represents the results of one patient. 1 Values above sIgE 100 kU/l (the upper cut-off of the Immulite assay detection range) indicate test results reported as “>100 kU/l” and do not reflect precise values.
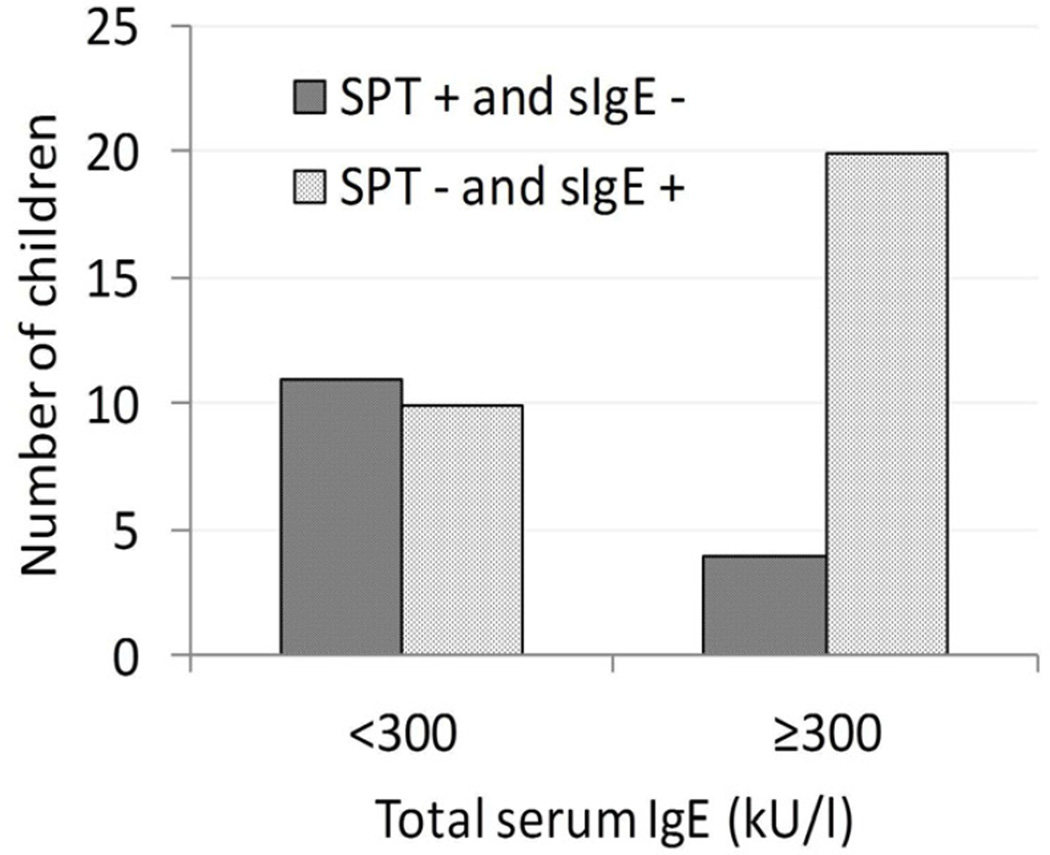
Children with high total serum IgE are more likely to have SPT negative/sIgE positive mismatches
Total serum IgE was measured in 38 of 40 children (2 missing values due to blood drawing or lab errors). Children with a high total serum IgE level (≥300 kU/l) were more likely to have SPT negative/sIgE positive (i.e. ≥0.1kU/l) mismatches and less likely to have SPT+/sIgE negative (i.e. <0.1 kU/l) mismatches than children with IgE levels < 300 kU/l (Fisher’s exact test p=0.025, figure 3). There was a large discrepancy between SPT positive/sIgE negative and SPT negative/sIgE positive mismatches among the 22 children with serum IgE ≥300 kU/l. Almost all of these children (20 children) had a total of 42 SPT negative/sIgE positive mismatches, while only 4 children had a total of 10 SPT positive/sIgE negative mismatches (Fisher’s exact p=0.003, figure 3). In contrast, among the 16 children whose total serum IgE was <300 kU/l, 11 had a total of 15 allergen specific SPT positive/sIgE negative (i.e. <0.1 kU/l) mismatches while about the same number of children (10), had a total of 21 SPT negative/sIgE positive mismatches (i.e. ≥0.1 kU/l) (Fisher’s exact p=0.154; note that some children had mismatches in both directions).
Figure 3.
Percent of children with discordant test results grouped by level of total serum IgE
Having a high total IgE (≥300 kU/l) remained a strong predictor for being less likely to have SPT+/IgE− mismatches after adjusting for age, race, weight and height (OR=0.06, 95%CI 0.01–0.40, p=0.004). There was no statistical significant difference in the number of SPT/sIgE mismatches in children with active eczema compared to children whose eczema was either inactive or who never had eczema (p>0.5). Age, BMI and ethnic background did not predict mismatch in either direction (p>0.1).
Even though it might have been expected that in children with SPT negative/sIgE positive (i.e. ≥0.1 kU/l) mismatches, the allergen specific serum IgE levels (with corresponding negative SPT) would have been low, this was not always the case. Serum IgE levels ranged between marginally detectable to as high as 19.2 kU/l for mouse, 40.7 kU/l for roach and 77.4 kU/l for dog, in children in whom skin tests to the corresponding allergen were negative. Specifically, median sIgE levels in children with SPT negative/sIgE positive mismatches were: grass 0. 6 kU/l (min 0.11, max 1.37 kU/l), ragweed 0.29 kU/l (min 0.17, max 0.97 kU/l), dust mite 0.62 kU/l (min 0.14, max 4.86 kU/l), cat 0.35 kU/l (min 0.12, max 7.64 kU/l), mouse 2.4 kU/l, (min 0.12, max 19.2 kU/l), roach 0.66 kU/l (min 0.32, max 40.7 kU/l) and dog 3.12 kU/l (min 0.22, max 77.4 kU/l).
Discussion
In our analysis of concordance between skin prick test results (Greer Laboratories, ComforTen™) with serum IgE testing (Immulite 2000™, detection cut-off 0.1 kU/l) to 7 common aeroallergens, we found significant discrepancies between skin and serum test results in a population of highly atopic, inner-city children <4 years of age. In average, 13% of all sensitizations would have been missed with serum IgE testing alone and 42% of sensitizations would have been missed if only skin testing had been done. Correlation between allergen specific IgE levels and grade of wheal diameter on skin testing was fair for most allergens. Similarly, agreement between positive or negative test results of the two methods was poor or fair for most allergens. Children with high total serum IgE levels (≥300 U/l) were more likely to exhibit sensitization by serum testing than skin testing, while children with total serum IgE levels of <300 kU/l were about as likely to have positive skin results with negative corresponding serum IgE results as vice versa.
Similar discrepancies between skin and serum testing were found in a study of 250 adults with allergic rhinitis who were tested for 53 different aeroallergens by means of serum (ImmunoCap) and skin testing (Quintip) [5]. The overall discrepancy between skin and serum test was nearly 20%. However, in contrast to our study in which we found predominantly skin test negative/serum positive mismatches, the authors reported an larger proportion of skin test positive/serum negative results overall (11.7%) as opposed to skin test negative/serum positive results (7.7%). However, in that study, 28% of participants also had a diagnosis of asthma, and 9.2% had eczema, which differs significantly from our study population. Accordingly, their overall study population had significantly lower total IgE levels compared to our cohort. Consistent with our findings, individuals with high total IgE (>200kU/l) were more likely to have skin test negative/serum positive results [5]. An alternative explanation could be variability between the ImmunoCap and Immulite system in detecting allergen specific IgE. While sIgE results of both systems appear to highly correlate, a recent analysis showed that the Immulite system appears to give higher values than the ImmunoCap system at least for food allergens [9]. A poor correlation between skin test grade and serum IgE levels similar to what we observed was also previously described in older children with allergic rhinitis (median age 10 years)[10].
In our study, there are several possible explanations for the observed discordance between skin testing and sIgE testing. First, differences in testing antigens (e.g. composition, concentration) present in skin and serum testing reagents may result in discrepancies between the two test results, especially grass pollen, cat, dog and mouse where antigenic substrates differed between both methods (table 1). Testing for separate pollen and mold antigens (i.e. separate grass pollen or mold) was omitted from serum and skin testing, due to the limited blood volume we were allowed to obtain and to minimize discomfort due to extensive skin testing in this young age group. Of note, only 3/40 children were positive to the mold mix extract using skin prick testing (serum testing not performed, data not shown). On the other hand, the northern grass pollen used for skin testing contain antigens that have been shown to be largely present in timothy grass [11, 12] which speaks against the argument that antigenic differences solely account for the observed discrepancies. Likewise, Dermatophagoides farinae (Der f1) and Dermatophagoides pteronyssinus (Der p1) have high sequence analogy, in agreement with their high cross reactivity [13]. Nevertheless, minor antigens may still be represented in different quantities among the two dust mite species and differences in the crystal or sequential antigen structures of major or minor allergens may contribute to the discrepancies observed in our study [13, 14]. For ragweed, high cross reactivity can be expected among giant and short ragweed [15]. Nevertheless in our cohort we found 8 children that had low-positive ragweed sIgE (short ragweed, all <1 kU/l but above detection level of 0.1 kU/l) but negative skin test results (giant and short ragweed) and none of the children had a mismatch in the opposite direction (positive skin test with negative sIgE), suggesting that reasons other than antigenic differences may be responsible for the mismatch between serum and skin test results. Similarly, one would expect similar representation of antigens in cockroach extracts used for skin testing and serum testing. However, in our study we found a high mismatch for cockroach testing in both directions (18%), when either skin test or sIgE testing alone was done. Similar to our results, a Brazilian study observed a similar discrepancy for cockroach allergy, although they report that skin testing appeared to be more sensitive in detecting cockroach allergy [16]. For dog, we used non-standardized conventional dog extract. The more potent, acetone-precipitated dog extract would have probably yielded a higher number of skin test positive results and less SPT negative/sIgE positive mismatches [17, 18].
Apart from antigenic differences between the two testing methods, technical factors may also contribute to the discrepancies between SPT and sIgE testing. We used the ComforTen system, which has recently been described as being less sensitive than Multitest II, at least in older populations [19]. The reason for choosing the ComforTen system was that, in our experience, the Multitest II or Greer Pick systems produce more dermographic responses in very young children, probably because the Multitest applicators have multiple spikes per test allergen, while the ComforTen system has only one spike per allergen. Because of the general smaller wheal sizes the ComforTen system yields for this reason, we a priori defined a positive skin test response as a wheal diameter at least 2 mm above the glycerin control (instead of the conventional 3mm above glycerin control). Of note, almost none of the children had any wheal response to the glycerin control (data not shown). In addition, skin testing can often be technically difficult in toddlers who frequently wiggle around during the skin testing procedure, possibly contributing to the variability in wheal size responses.
Another possible explanation for positive serum IgE results with corresponding negative skin test results would be if IgE non-specifically bound to the test matrix of the Immulite 2000 3g Allergy system. However, in this case, one may expect positive results for all tested allergens in these children rather than only a few.
Another hypothetical explanation for the observed discrepancies between skin and serum test results is a relative immaturity of the immune system in young age. For example, mast cells may not have trafficked to the epidermis in sufficient amounts to elicit a typical wheal and flare response, or young children’s mast cells may not be as reactive as those of adults.
It is important to understand that the goal of this analysis was not to investigate the correlation between clinical allergic response to allergens and allergic sensitization. This is because allergen challenge in the age group of our study population would be difficult to perform because objective, standardized procedures to measure allergic responses in the upper or lower airways are not available for this age group. Similarly it can be difficult to judge the clinical relevance of allergic sensitization based on the clinical exposure history because of the perennial presence of some antigens (e.g. cockroach and mouse) in most households, buildings or even public places in low-income city areas. Similarly, it is unknown to date if low levels allergic sensitization in early childhood may implicate a risk for developing clinical allergy later in life. Nevertheless, while the clinical relevance of low level allergic sensitization in young children remains to be elucidated, our data indicates that, both skin and serum testing are contributory in comprehensively diagnosing allergic sensitizations in inner-city, highly atopic children.
In conclusion, aeroallergen skin testing and serum IgE testing showed significant discrepancies in our cohort of young, highly atopic inner-city children. High total serum IgE (≥300 kU/l) was associated with having allergen specific serum IgE but often negative corresponding skin test. Antigenic differences in the testing materials as well as variability between testing instruments may be partially responsible for these findings and it is possible that these discrepancies are more common in younger populations as opposed to older individuals. However, since in most clinical or research settings neither of these variables can be controlled, it may be necessary to perform specific serum IgE testing in addition to skin testing, when accurate diagnosis of aeroallergen sensitization is required to define an appropriate intervention such as environmental remediation or even offer immunotherapy.
Acknowledgements
We owe great thanks to Margaret Chin, N.P. for help with the skin testing procedures and help in all practical aspects of the study. In addition, without the generous donation to our clinical trial by Mr. & Mrs. Avinadav & Pazia Siev in memory of her father, Shlomo Kritzman, this research would not have been possible. Furthermore, Xin Zheng, PhD and Jose Adames from the Research Information Core at the Einstein Institute for Clinical and Translational Research have contributed by providing the digital database. We also thank Shankar Viswanathan, PhD, for his statistical advices.
Funding: This publication was supported in part by the CTSA Grant UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. In addition, Mr. & Mrs. Avinadav & Pazia Siev provided generous financial support through a private donation in memory of her father, Shlomo Kritzman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution of each author
Gabriele de Vos, M.D. designed the study, supervised data collection and management, data analysis, drafted the initial manuscript and gave final approval of the version to be published.
Ramin Nazari, M.D. had the research idea, helped with data managment, data collection, skin testing and article revision and gave final approval of the version to be published.
Denisa Ferastraoaru, M.D. contributed through data analysis and review of the article and gave final approval of the version to be published.
Purvi Parikh, M.D. contributed through data analysis, literature review and revision of the article and gave final approval of the version to be published.
Rebecca Geliebter, (Medical student) contributed through data cleaning, analysis and revision of the article and gave final approval of the version to be published.
Yikania Pichardo, M.D., contributed through performing skin tests, study coordination and data entry as well as revision of the article and gave final approval of the version to be published.
Andrew Wiznia, M.D. contributed through interpretation, critical revisions of the article and general supervision and gave final approval of the version to be published.
David Rosenstreich, M.D. contributed through interpretation and critical revisions of the article and gave final approval of the version to be published.
Conflict of interest statement:
None of the contributing authors has any financial or other material conflict of interest in this research project or its results.
Clinical trial registration:
clinicaltrial.gov identifier NCT01028560
Contributor Information
Gabriele de Vos, Email: gabriele.de-vos@einstein.yu.edu.
Ramin Nazari, Email: raminnazari@yahoo.com.
Denisa Ferastraoaru, Email: denisa.ferastraoaru@nbhn.net.
Purvi Parikh, Email: purvi.parikh@nbhn.net.
Rebecca Geliebter, Email: Rebecca.geliebter@med.einstein.yu.edu.
Yikania Pichardo, Email: yikania.pichardo@nbhn.net.
Andrew Wiznia, Email: Andrew.wiznia@einstein.yu.edu.
David Rosenstreich, Email: david.rosenstreich@einstein.yu.edu.
References
- 1.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 2.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, Gern JE, Gerritsen J, Hamelmann E, Helms PJ, Lemanske RF, Martinez F, Pedersen S, Renz H, Sampson H, von Mutius E, Wahn U, Holt PG. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, Gern JE, Lemanske RF., Jr Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185(3):281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabria CW, Dietrich J, Hagan L. Comparison of serum-specific IgE (ImmunoCAP) and skin-prick test results for 53 inhalant allergens in patients with chronic rhinitis. Allergy Asthma Proc. 2009;30(4):386–396. doi: 10.2500/aap.2009.30.3258. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, Wood RA. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129(1):193–197. doi: 10.1542/peds.2011-2382. [DOI] [PubMed] [Google Scholar]
- 7.Control of Environmental Factors and Comorbid Conditions That Affect Asthma; 2007. 3 EP. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. http://wwwnhlbinihgov/guidelines/asthma/asthgdlnhtm. Section 3, Component 3. [Google Scholar]
- 8.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RG, Mudd K, White MA, Wood RA. Extension of food allergen specific IgE ranges from the ImmunoCAP to the IMMULITE systems. Ann Allergy Asthma Immunol. 2011;107(2):139–144. doi: 10.1016/j.anai.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Ciprandi G, De Amici M, Marseglia G. Comparison of serum specific IgE and skin prick test in polysensitized children. Clin Lab. 2011;57(1–2):83–85. [PubMed] [Google Scholar]
- 11.Schenk S, Breiteneder H, Susani M, Najafian N, Laffer S, Duchene M, Valenta R, Fischer G, Scheiner O, Kraft D, Ebner C. T cell epitopes of Phl p 1, major pollen allergen of timothy grass (Phleum pratense). Crossreactivity with group I allergens of different grasses. Adv Exp Med Biol. 1996;409:141–146. doi: 10.1007/978-1-4615-5855-2_19. [DOI] [PubMed] [Google Scholar]
- 12.Hiller KM, Esch RE, Klapper DG. Mapping of an allergenically important determinant of grass group I allergens. J Allergy Clin Immunol. 1997;100(3):335–340. doi: 10.1016/s0091-6749(97)70246-x. [DOI] [PubMed] [Google Scholar]
- 13.Chruszcz M, Chapman MD, Vailes LD, Stura EA, Saint-Remy JM, Minor W, Pomes A. Crystal structures of mite allergens Der f 1 and Der p 1 reveal differences in surface-exposed residues that may influence antibody binding. J Mol Biol. 2009;386(2):520–530. doi: 10.1016/j.jmb.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas WR, Smith W. House-dust-mite allergens. Allergy. 1998;53(9):821–832. doi: 10.1111/j.1398-9995.1998.tb03987.x. [DOI] [PubMed] [Google Scholar]
- 15.Leiferman KM, Gleich GJ, Jones RT. The cross-reactivity of IgE antibodies with pollen allergens. II. Analyses of various species of ragweed and other fall weed pollens. J Allergy Clin Immunol. 1976;58(1 PT. 2):140–148. doi: 10.1016/0091-6749(76)90149-4. [DOI] [PubMed] [Google Scholar]
- 16.Lopes MI, Miranda PJ, Sarinho E. Use of the skin prick test and specific immunoglobulin E for the diagnosis of cockroach allergy. J Pediatr (Rio J) 2006;82(3):204–209. doi: 10.2223/JPED.1487. [DOI] [PubMed] [Google Scholar]
- 17.Lent AM, Harbeck R, Strand M, Sills M, Schmidt K, Efaw B, Lebo T, Nelson HS. Immunologic response to administration of standardized dog allergen extract at differing doses. J Allergy Clin Immunol. 2006;118(6):1249–1256. doi: 10.1016/j.jaci.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Meiser JB, Nelson HS. Comparing conventional and acetone-precipitated dog allergen extract skin testing. J Allergy Clin Immunol. 2001;107(4):744–745. doi: 10.1067/mai.2001.114340. [DOI] [PubMed] [Google Scholar]
- 19.Dykewicz MS, Dooms KT, Chassaing DL. Comparison of the Multi-Test II and ComforTen allergy skin test devices. Allergy Asthma Proc. 2011;32(3):198–202. doi: 10.2500/aap.2011.32.3441. [DOI] [PubMed] [Google Scholar]