Abstract
Dietary chromium supplementation for the treatment of diabetes remains controversial. The prevailing view that chromium supplementation for glucose regulation is unjustified has been based upon prior studies showing mixed, modest-sized effects in patients with type 2 diabetes (T2DM). Based on chromium's potential to improve insulin, dopamine, and serotonin function, we hypothesize that chromium has a greater glucoregulatory effect in individuals who have concurrent disturbances in dopamine and serotonin function – that is, complex patients with comorbid diabetes, depression, and binge eating. We propose, as suggested by the collective data to date, the need to go beyond the “one size fits all” approach to chromium supplementation and put forth a series of experiments designed to link physiological and neurobehavioral processes in the chromium response phenotype.
Diabetes, Depression, and Binge Eating Comorbidity
Worldwide, over 370 million people have diabetes, and diabetes-related healthcare costs are approaching 500 billion U.S. dollars [1]. Lifestyle interventions are effective but difficult to sustain over time [2], and existing drug therapies are limited by side effects and durability. Therefore, significant future progress in treating and preventing diabetes hinges on developing tailored strategies that focus on individuals at greatest risk and that are easily assimilated and sustained.
Diabetes patients that struggle with depression and disordered eating comprise an important high-risk group because they are less compliant with treatment recommendations and suffer worse diabetes-related complications and poorer outcomes [3-8]. An estimated 17.6 % [9] to 31 % [10] of diabetic patients have a depressive disorder, and as high as 40 % of T2DM patients [11] and 58 % of type 1 patients [12] have disordered eating, with binge eating being the predominant pathology [13]. Binge eating significantly increases the risk of new-onset T2DM [14] and is associated with higher glycated hemoglobin and body mass index [11] and less weight loss [15] in persons with T2DM. An estimated 1.4 % [16] to 10 % [13] of patients with T2DM meet full diagnostic criteria for binge eating disorder [17].
We hypothesize that the “complex” diabetes patient who suffers with comorbid depression and disordered eating may have a good clinical response to treatment with dietary chromium because of its ability to regulate key neurobiological substrates that are dysregulated in these conditions.
Neurobiological basis for the hypothesis
T2DM and binge eating are linked through shared cortical and subcortical neural circuitry and signaling pathways involved in food reward, particularly the dopaminergic system, which is highly sensitive to the actions of insulin. The mesolimbic dopamine (DA) pathway has been specifically implicated in the incentive, reinforcing and motivational aspects of food intake [18, 19]. The dopaminergic neurons of the midbrain nucleus, the ventral tegmental area (VTA), play a key role in motivated eating and in processing cues related to the rewarding properties of palatable food [20]. Insulin receptors are expressed on these VTA neurons, and insulin in the VTA can suppress feeding [21]. Importantly, insulin directly inhibits VTA DA neurons [22], which primarily project to the ventral striatum, a key site for regulation of motivated feeding behavior [23]. Insulin-mediated decrease of DA in the VTA is thought to normally suppress the salience of food upon reaching satiety. A plausible consequence of the hyperinsulinemia of T2DM is insulin-resistance of VTA neurons, akin to that seen in other systems of the body. To our knowledge, the possibility of insulin-resistance of VTA DA neurons has not been investigated, but if it occurs, it is proposed that by reducing insulin-induced suppression of VTA DA neuron firing, it would blunt the normally associated waning of food-cue salience with increasing satiety. In addition to the mesolimbic DA projection, VTA DA neurons can project to frontal regions, where DA supports the coupling of attention-related networks [24] that may underlie attentional biases to food cues and reward sensitivity observed in persons who binge eat [25-27].
T2DM, binge eating, and depression are further linked through shared circuits and signaling pathways within the serotonergic system. Like the dopaminergic system, the serotonergic system is highly sensitive to the actions of insulin. In the hypothalamus, insulin administration increases hypothalamic serotonin (5-HT) release [28, 29]. Reciprocally, 5-HT modulates insulin action in the brain [30, 31] having a direct effect on hypothalamic glucose homeostasis [32], which appears impaired in T2DM [33]. 5-HT is also involved in the regulation of bias toward selection of immediate over delayed rewards in dynamic delay discounting tasks [34], control of meal size [35], and sweet preference, as indexed by increased sweet calorie intake following depletion of the serotonin precursor tryptophan [36]. Finally, a large and converging body of evidence from animal and human studies links 5-HT deficiency with depression [37] and demonstrates that lowering 5-HT levels through acute tryptophan depletion precipitates depression symptoms in vulnerable individuals [38, 39].
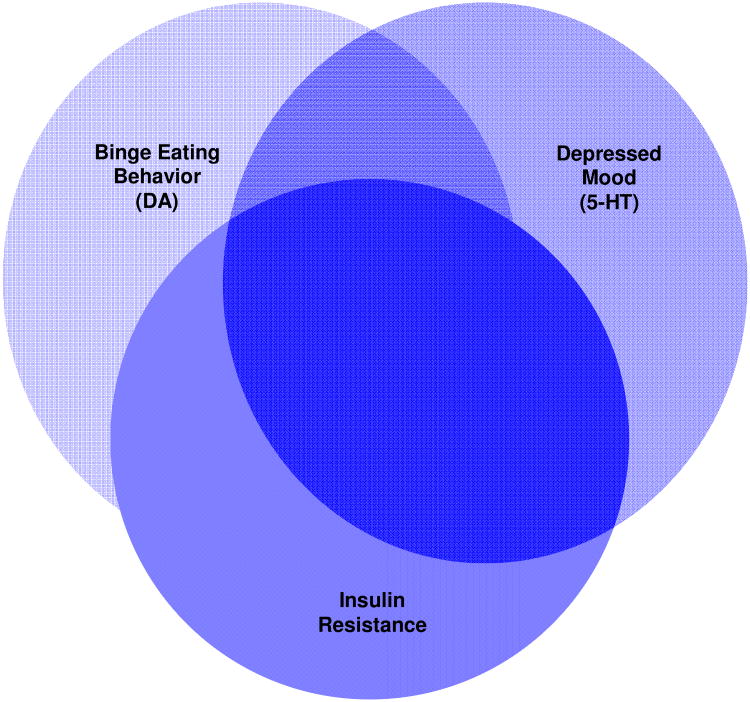
The micronutrient, chromium, has been known to be an essential element in carbohydrate metabolism [40-43]. Given this role in insulin action, chromium may be an ideal alternative therapy for complex T2DM patients with comorbid depression and binge eating because of its “triple-threat” capacity to affect insulin, DA, and 5-HT neurotransmission and thus target the behavioral and psychological correlates of poor glycemic control (see heuristic model in Figure 1). Chromium enhances cellular insulin activity [44-50] which, in turn, promotes increased entry of tryptophan into the brain thereby increasing brain serotonin synthesis [44]. Chromium treatment significantly lowers the cortisol response to 5-hydroxytryptophan suggesting, in particular, enhanced sensitivity of central serotonin2A (5-HT2A) receptors [45, 46]. Because of reciprocal interaction of serotonin and DA through direct synaptic connections [51, 52] and through physical 5-HT2A–DA2 receptor heterocomplexes [53, 54], the possibility exists for chromium to also influence DA function indirectly via the 5-HT system. That these neural connections occur in the VTA in neurons projecting to the nucleus accumbens provides a plausible neurobiological basis for chromium's capacity to influence eating behavior and mood.
Figure 1. Heuristic Model of the High Chromium Response Phenotype.
Supportive evidence for the hypothesis from human studies
Hexavalent chromium (chromium VI) is highly toxic and carcinogenic. However, according to the Council for Responsible Nutrition, dietary supplementation with trivalent chromium (chromium III) is generally considered safe for adults at levels up to 1,000 mcg/day [55]; side-effects are typically mild and include unexplained bruising, nosebleed, rash, decreased urination, lethargy, loss of appetite, nausea or vomiting, sleep disturbances, headache, and dizziness. Except at supra-physiologic doses over extended treatment periods, trivalent chromium has essentially no known genotoxic or carcinogenic risk [56]; and, since 2005, the FDA has recognized trivalent chromium in the form of chromium picolinate as a safe nutritional supplement that may reduce the risk of insulin resistance and possibly T2DM. The combination of chromium picolinate combined with biotin, which is thought to synergize the effects of chromium [57, 58], is also well tolerated and associated with minimal adverse effects [59, 60]. In addition to chromium picolinate, other trivalent chromium complexes include chromium niacin, chromium D-phenylalanine, and chromium salicylate. Like chromium picolinate, these complexes, as well as the novel chromium chelate, chromium histidinate, exert glucoregulatory and immunoregulatory effects in diabetes [61, 62].
In placebo-controlled trials, dietary supplementation with chromium with and without the addition of biotin has been reported to improve glucose regulation, depression, and binge eating in patient populations [59, 63, 64]. Studies in diabetes have nearly exclusively focused on patients with T2DM but there are case report data suggesting benefit in type 1 diabetes, as well [65]. Recent meta-analyses indicated that chromium supplementation lowers blood glucose in T2DM patients [66-68] but not all patients derive this benefit [60, 69-71] or show improvements in glycated hemoglobin [72]; pre-treatment insulin sensitivity explains ∼40 % of the clinical response to chromium [71, 73], with individuals who are more insulin resistant trending toward greater improvements in glucose regulation post-treatment. In contrast, most, but not all, studies focused on dietary chromium supplementation in samples of non-diabetic overweight or obese individuals have failed to find a significant impact of chromium picolinate on glucose regulation [74]; the reasons for this lack of an effect in obesity, per se, are not precisely known. However, given that hyperglycemia, by definition, is present in diabetic compared to the non-diabetic state, this metabolic parameter could be postulated as a major contributing reason to explain this observation. Chromium supplementation has also been shown to reduce depression symptoms and carbohydrate cravings in antidepressant-refractory patients with dysthymic disorder [75-77] and to reduce food intake and hunger levels in non-depressed overweight women with carbohydrate cravings [78]. In addition, our group recently reported reduced binge eating in overweight individuals with binge eating disorder following a 6-month chromium supplementation trial [79], possibly due to improvements in cognitive processes (i.e., inhibitory control) that underlie healthy eating behavior.
In adults with mild cognitive impairment, chromium supplementation increased cerebral activation and enhanced performance during various learning, recall, and recognition memory tasks, independent of any changes in peripheral glucose or insulin concentration [80]. Cognitive deficits that contribute to the development and maintenance of obesity and eating disorders are especially pronounced among obese persons with co-morbid binge eating disorder [81, 82]. Binge eating, like other binge-type substance use, is linked to excess allocation of attention to binge-associated stimuli [83, 84]. This attentional bias may reflect profound Pavlovian learning, as demonstrated in models of alcohol abuse and smoking [85-87]. Through such control of attention, food cues may thus facilitate compulsive food intake. The neural basis of attentional bias is not well understood, but may involve dysfunction in executive control signals from the prefrontal cortex regulating mesolimbic DA projections.
The Hypothesis
Identifying other explanatory factors is the key to unlocking chromium's full potential as a complementary approach to T2DM. As illustrated in Figure 1, we hypothesize that patients with T2DM along with comorbid depression and binge eating will have a greater response to chromium than patients without these comorbidities. Treatment trials that examine the effects of chromium on neurocircuitry underlying the cognitive, emotional, and behavioral processes that contribute to poor glucose control are needed. Studies that systematically examine food-cue attentional bias and fronto-mesolimbic connectivity during DA and 5-HT precursor depletion studies in patients with T2DM would be especially instrumental in advancing our understanding of the chromium response phenotype. From such studies we would anticipate showing that (1) chromium supplementation improves DA and 5-HT functioning, as evidenced by post-treatment reductions in sensitivity to depletion of DA and 5-HT precursors and improved functional connectivity between the prefrontal cortex and ventral tegmental area, and that (2) these effects are more pronounced in patients with versus without comorbid symptoms of depression and binge eating. By demonstrating how chromium treatment alters neural circuits underlying depressed mood and conditioned responses to food cues, these studies may reveal novel therapeutic targets and increase understanding of how to optimize chromium use in diabetes treatment.
Acknowledgments
WTC is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health (NIH), which funds the Louisiana Clinical and Translational Science Center. LY was supported, in part, by the National Center for Complementary and AlternativeMedicine of the NIH under award number 5K23AT004946-06. CB is supported in part by P60AA011605 from the NIH. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors disclose no direct sources of grant support for the preparation of this manuscript.
Footnotes
Conflict of interest statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas Update 2012. 2012 Available at: http://www.idf.org/diabetesatlas/5e/Update2012. [PubMed]
- 2.Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int. 2009;4(24):416–427. doi: 10.1093/heapro/dap031. [DOI] [PubMed] [Google Scholar]
- 3.Davison KM. Eating disorders and diabetes: Current perspectives. Can J Diabetes. 2003;1(27):62–73. [Google Scholar]
- 4.Rotella F, Cresci B, Monami M, et al. Are psychopathological features relevant predictors of glucose control in patients with type 2 diabetes? A prospective study. Acta Diabetol. 2012;49(Suppl 1):179–184. doi: 10.1007/s00592-012-0403-4. [DOI] [PubMed] [Google Scholar]
- 5.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;4(63):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon C, Aime A, Belanger C, Markowitz JT. Comorbid diabetes and eating disorders in adult patients: assessment and considerations for treatment. Diabetes Educ. 2012;4(38):537–542. doi: 10.1177/0145721712446203. [DOI] [PubMed] [Google Scholar]
- 7.Katon W, Russo J, Lin EH, et al. Diabetes and poor disease control: is comorbid depression associated with poor medication adherence or lack of treatment intensification? Psychosom Med. 2009;9(71):965–972. doi: 10.1097/PSY.0b013e3181bd8f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;2(33):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;11(23):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;6(24):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 11.Meneghini LF, Spadola J, Florez H. Prevalence and associations of binge eating disorderin a multiethnic population with type 2 diabetes. Diabetes Care. 2006;12(29):2760. doi: 10.2337/dc06-1364. [DOI] [PubMed] [Google Scholar]
- 12.Fairburn CB, Peveler RC, Davies B, Mann JI, Mayou RA. Eating disorders in young adults with insulin dependent diabetes mellitus: a controlled study. Br Med J. 1991;303:17–20. doi: 10.1136/bmj.303.6793.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papelbaum M, Appolinario JC, Moreira Rde O, Ellinger VC, Kupfer R, Coutinho WF. Prevalence of eating disorders and psychiatric comorbidity in a clinical sample of type 2 diabetes mellitus patients. Rev Bras Psiquiatr. 2005;2(27):135–138. doi: 10.1590/s1516-44462005000200012. [DOI] [PubMed] [Google Scholar]
- 14.Hudson JI, Lalonde JK, Coit CE, et al. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am J Clin Nutr. 2010;6(91):1568–1573. doi: 10.3945/ajcn.2010.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorin AA, Niemeier HM, Hogan P, et al. Binge eating and weight loss outcomes in overweight and obese individuals with type 2 diabetes: results from the Look AHEAD trial. Arch Gen Psychiatry. 2008;12(65):1447–1455. doi: 10.1001/archpsyc.65.12.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allison KC, Crow SJ, Reeves RR, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obesity. 2007;5(15):1287–1293. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. DSM-5 Proposed Diagnostic Criteria for Binge Eating Disorder. [Accessed October 23, 2012];2010 http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=372.
- 18.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity Rev. 2013;1(14):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;11(12):638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 20.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;8(30):375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol. 2008;2(295):R388–394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mebel DM, Wong JC, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci. 2012;3(36):2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;5(86):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 24.Dang LC, O'Neil JP, Jagust WJ. Dopamine supports coupling of attention-related networks. J Neurosci. 2012;28(32):9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;8(65):654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Svaldi J, Tuschen-Caffier B, Peyk P, Blechert J. Information processing of food pictures in binge eating disorder. Appetite. 2010;3(55):685–694. doi: 10.1016/j.appet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M. Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev. 2013;9 Pt A(37):1985–1998. doi: 10.1016/j.neubiorev.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orosco M, Rouch C, Gerozissis K. Activation of hypothalamic insulin by serotonin is the primary event of the insulin-serotonin interaction involved in the control of feeding. Brain Res. 2000;1-2(872):64–70. doi: 10.1016/s0006-8993(00)02449-5. [DOI] [PubMed] [Google Scholar]
- 29.Orosco M, Gerozissis K. Macronutrient-induced cascade of events leading to parallel changes in hypothalamic serotonin and insulin. Neurosci Biobehav Rev. 2001;2(25):167–174. doi: 10.1016/s0149-7634(01)00004-5. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Berglund ED, Sohn JW, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nature neuroscience. 2010;12(13):1457–1459. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;5(6):398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade JM, Juneja P, MacKay AW, et al. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology. 2008;3(149):955–961. doi: 10.1210/en.2007-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papazoglou I, Berthou F, Vicaire N, et al. Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model. Mol Cell Endocrinol. 2012;1(350):136–144. doi: 10.1016/j.mce.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Schweighofer N, Bertin M, Shishida K, et al. Low-serotonin levels increase delayed reward discounting in humans. J Neurosci. 2008;17(28):4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leibowitz S, Shor-Posner G. Brain serotonin and eating behavior. Appetite. 1986;(7):1–14. doi: 10.1016/s0195-6663(86)80049-6. [DOI] [PubMed] [Google Scholar]
- 36.Pagoto SL, Spring B, McChargue D, et al. Acute tryptophan depletion and sweet food consumption by overweight adults. Eat Behav. 2009;1(10):36–41. doi: 10.1016/j.eatbeh.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;1601(367):2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altman SE, Shankman SA, Spring B. Effect of acute tryptophan depletion on emotions in individuals with personal and family history of depression following a mood induction. Neuropsychobiology. 2010;3(62):171–176. doi: 10.1159/000319358. [DOI] [PubMed] [Google Scholar]
- 39.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;4(12):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 40.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr. 1977;4(30):531–538. doi: 10.1093/ajcn/30.4.531. [DOI] [PubMed] [Google Scholar]
- 41.Anderson RA. Chromium, Glucose Intolerance and Diabetes. J Am Coll Nutr. 1998;6(17):548–555. doi: 10.1080/07315724.1998.10718802. [DOI] [PubMed] [Google Scholar]
- 42.Anderson RA, Polansky MM, Bryden NA, Bhathena SJ, Canary JJ. Effects of supplemental chromium on patients with symptoms of reactive hypoglycemia. Metabolism. 1987;4(36):351–355. doi: 10.1016/0026-0495(87)90206-x. [DOI] [PubMed] [Google Scholar]
- 43.Jeejeebhoy KN. The role of chromium in nutrition and therapeutics and as a potential toxin. Nutr Rev. 1999;11(57):329–335. doi: 10.1111/j.1753-4887.1999.tb06909.x. [DOI] [PubMed] [Google Scholar]
- 44.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;992(173):149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- 45.Franklin M, Odontiadis J. Effects of treatment with chromium picolinate on peripheral amino acid availability and brain monoamine function in the rat. Pharmacopsychiatry. 2003;5(36):176–180. doi: 10.1055/s-2003-43046. [DOI] [PubMed] [Google Scholar]
- 46.Attenburrow MJ, Odontiadis J, Murray BJ, Cowen PJ, Franklin M. Chromium treatment decreases the sensitivity of 5-HT2A receptors. Psychopharmacology (Berl) 2002;4(159):432–436. doi: 10.1007/s00213-001-0960-7. [DOI] [PubMed] [Google Scholar]
- 47.McCarty MF. Enhancing central and peripheral insulin activity as a strategy for the treatment of endogenous depression--an adjuvant role for chromium picolinate? Med Hypotheses. 1994;4(43):247–252. doi: 10.1016/0306-9877(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 48.Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry. 1997;15(36):4382–4385. doi: 10.1021/bi963154t. [DOI] [PubMed] [Google Scholar]
- 49.Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;4(23):313–319. doi: 10.1016/j.jnutbio.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;2(136):415–420. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 51.Di Giovanni G, Di Matteo V, Pierucci M, Esposito E. Serotonin-dopamine interaction: electrophysiological evidence. Prog Brain Res. 2008;172:45–71. doi: 10.1016/S0079-6123(08)00903-5. [DOI] [PubMed] [Google Scholar]
- 52.Van Bockstaele EJ, Cestari DM, Pickel VM. Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: potential sites for modulation of mesolimbic dopamine neurons. Brain Res. 1994;2(647):307–322. doi: 10.1016/0006-8993(94)91330-7. [DOI] [PubMed] [Google Scholar]
- 53.de Bartolomeis A, Buonaguro EF, Iasevoli F. Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology (Berl) 2013;1(225):1–19. doi: 10.1007/s00213-012-2921-8. [DOI] [PubMed] [Google Scholar]
- 54.Albizu L, Holloway T, Gonzalez-Maeso J, Sealfon SC. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology. 2011;4(61):770–777. doi: 10.1016/j.neuropharm.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hathcock JN. Chromium. In: MacKay D, Wong A, Nguyen H, editors. Vitamin and mineral safety, 3rd edition. 3rd. Council for Responsible Nutrition; Washington, D.C: 2014. pp. 128–134. [Google Scholar]
- 56.National Toxicology Program. NTP toxicology and carcinogenesis studies of chromium picolinate monohydrate (CAS No 27882-76-4) in F344/N rats and B6C3F1 mice (feed studies) 2010:1–194. [PubMed] [Google Scholar]
- 57.McCarty MF. High-dose biotin, an inducer of glucokinase expression, may synergize with chromium picolinate to enable a definitive nutritional therapy for type II diabetes. Med Hypotheses. 1999;5(52):401–406. doi: 10.1054/mehy.1997.0682. [DOI] [PubMed] [Google Scholar]
- 58.Sahin K, Tuzcu M, Orhan C, et al. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512004850. [DOI] [PubMed] [Google Scholar]
- 59.Albarracin C, Fuqua B, Geohas J, Juturu V, Finch MR, Komorowski JR. Combination of chromium and biotin improves coronary risk factors in hypercholesterolemic type 2 diabetes mellitus: a placebo-controlled, double-blind randomized clinical trial. J Cardiometab Syndr. 2007;2(2):91–97. doi: 10.1111/j.1559-4564.2007.06366.x. [DOI] [PubMed] [Google Scholar]
- 60.Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;1(24):41–51. doi: 10.1002/dmrr.755. [DOI] [PubMed] [Google Scholar]
- 61.Peng M, Yang X. Controlling diabetes by chromium complexes: The role of the ligands. J Inorg Biochem. 2015 doi: 10.1016/j.jinorgbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Selcuk MY, Aygen B, Dogukan A, et al. Chromium picolinate and chromium histidinate protects against renal dysfunction by modulation of NF-kappaB pathway in high-fat diet fed and Streptozotocin-induced diabetic rats. Nutr Metab (Lond) 2012;9:30. doi: 10.1186/1743-7075-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singer GM, Geohas J. The effect of chromium picolinate and biotin supplementation on glycemic control in poorly controlled patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized trial. Diabetes Technol Ther. 2006;6(8):636–643. doi: 10.1089/dia.2006.8.636. [DOI] [PubMed] [Google Scholar]
- 64.Geohas J, Daly A, Juturu V, Finch M, Komorowski JR. Chromium picolinate and biotin combination reduces atherogenic index of plasma in patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized clinical trial. Am J Med Sci. 2007;3(333):145–153. doi: 10.1097/MAJ.0b013e318031b3c9. [DOI] [PubMed] [Google Scholar]
- 65.Fox GN, Sabovic Z. Chromium picolinate supplementation for diabetes mellitus. J Fam Pract. 1998;1(46):83–86. [PubMed] [Google Scholar]
- 66.Broadhurst CL, Domenico P. Clinical studies on chromium picolinate supplementation in diabetes mellitus--a review. Diabetes Technol Ther. 2006;6(8):677–687. doi: 10.1089/dia.2006.8.677. [DOI] [PubMed] [Google Scholar]
- 67.Abdollahi M, Farshchi A, Nikfar S, Seyedifar M. Effect of chromium on glucose and lipid profiles in patients with type 2 diabetes; a meta-analysis review of randomized trials. J Pharm Pharm Sci. 2013;1(16):99–114. doi: 10.18433/j3g022. [DOI] [PubMed] [Google Scholar]
- 68.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;8(30):2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 69.Sharma S, Agrawal RP, Choudhary M, Jain S, Goyal S, Agarwal V. Beneficial effect of chromium supplementation on glucose, HbA(1) C and lipid variables in individuals with newly onset type-2 diabetes. J Trace Elem Med Biol. 2011 doi: 10.1016/j.jtemb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Ali A, Ma Y, Reynolds J, Wise JP, Sr, Inzucchi SE, Katz DL. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr Pract. 2011;1(17):16–25. doi: 10.4158/EP10131.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cefalu WT, Rood J, Pinsonat P, et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism. 2010;5(59):755–762. doi: 10.1016/j.metabol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Landman GW, Bilo HJ, Houweling ST, Kleefstra N. Chromium does not belong in the diabetes treatment arsenal: Current evidence and future perspectives. World J Diabetes. 2014;2(5):160–164. doi: 10.4239/wjd.v5.i2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang ZQ, Qin J, Martin J, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;12(56):1652–1655. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian H, Guo X, Wang X, et al. Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst Rev. 2013;11:CD010063. doi: 10.1002/14651858.CD010063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLeod MN, Gaynes BN, Golden RN. Chromium potentiation of antidepressant pharmacotherapy for dysthymic disorder in 5 patients. J Clin Psychiatry. 1999;4(60):237–240. doi: 10.4088/jcp.v60n0406. [DOI] [PubMed] [Google Scholar]
- 76.Docherty JP, Sack DA, Roffman M, Finch M, Komorowski JR. A double-blind, placebo-controlled, exploratory trial of chromium picolinate in atypical depression: Effect on carbohydrate craving. J Psychiatr Pract. 2005;11:302–314. doi: 10.1097/00131746-200509000-00004. [DOI] [PubMed] [Google Scholar]
- 77.McLeod MN, Golden RN. Chromium treatment of depression. Int J Neuropsychopharmacology. 2000;4(3):311–314. doi: 10.1017/S146114570000208X. [DOI] [PubMed] [Google Scholar]
- 78.Anton SD, Morrison CD, Cefalu WT, et al. Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther. 2008;5(10):405–412. doi: 10.1089/dia.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brownley KA, Von Holle A, Hamer RM, La Via M, Bulik CM. A double-blind, randomized pilot trial of chromium picolinate for binge eating disorder: results of the Binge Eating and Chromium (BEACh) study. J Psychosom Res. 2013;1(75):36–42. doi: 10.1016/j.jpsychores.2013.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD. Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr Neurosci. 2010;3(13):116–122. doi: 10.1179/147683010X12611460764084. [DOI] [PubMed] [Google Scholar]
- 81.Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;1(57):263–271. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 82.Duchesne M, Mattos P, Appolinario JC, et al. Assessment of executive functions in obese individuals with binge eating disorder. Rev Bras Psiquiatr. 2010;4(32):381–383. doi: 10.1590/s1516-44462010000400011. [DOI] [PubMed] [Google Scholar]
- 83.Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD, Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. 2012;5(45):657–663. doi: 10.1002/eat.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gearhardt AN, White MA, Potenza MN. Binge eating disorder and food addiction. Curr Drug Abuse Rev. 2011;3(4):201–207. doi: 10.2174/1874473711104030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hitsman B, MacKillop J, Lingford-Hughes A, et al. Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology (Berl) 2008;4(196):611–621. doi: 10.1007/s00213-007-0995-5. [DOI] [PubMed] [Google Scholar]
- 87.Robinson TE, Berridge KC. Addiction. Ann Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]