Abstract
Bcl-2 family proteins, known for their apoptosis functioning at the mitochondria, have been shown to localize to other cellular compartments to mediate calcium (Ca2+) signals. Since the proper supply of Ca2+ in cells serves as an important mechanism for cellular survival and bioenergetics, we propose an integrating role for Bcl-2 family proteins in modulating Ca2+ signaling. The endoplasmic reticulum (ER) is the main Ca2+ storage for the cell and Bcl-2 family proteins competitively regulate its Ca2+ concentration. Bcl-2 family proteins also regulate the flux of Ca2+ from the ER by physically interacting with inositol 1,4,5-trisphosphate receptors (IP3Rs) to mediate their opening. Type 1 IP3Rs reside at the bulk ER to coordinate cytosolic Ca2+ signals, while type 3 IP3Rs reside at mitochondria-associated ER membrane (MAM) to facilitate mitochondrial Ca2+ uptake. In healthy cells, mitochondrial Ca2+ drives pyruvate into the citric acid (TCA) cycle to facilitate ATP production, while a continuous accumulation of Ca2+ can trigger the release of cytochrome c, thus initiating apoptosis. Since multiple organelles and Bcl-2 family proteins are involved in Ca2+ signaling, we aim to clarify the role that Bcl-2 family proteins play in facilitating Ca2+ signaling and how mitochondrial Ca2+ is relevant in both bioenergetics and apoptosis. We also explore how these insights could be useful in controlling bioenergetics in apoptosis-resistant cell lines.
Keywords: Calcium signaling; Apoptosis; Bcl-2; Mitochondria-associated; ER membrane (MAM); Bioenergetics; ER; Mitochondria; Inositol; 1,4,5-trisphosphate receptor (IP3R)
Introduction
The growing Bcl-2 family (Fig. 1) consists of both anti- and pro-apoptotic members that share up to four conserved regions known as Bcl-2 homology domains (BH1-4). Anti-apoptotic family members typically contain all four BH domains and include Bcl-2 and Bcl-xL in which the BH4 domain is relevant for anti-apoptotic activity. Other anti-apoptotic members; however, such as Mcl-1, merely possess strong sequence homology in the BH1-3 domains. Additionally, HAX-1 has anti-apoptotic properties although it shares sequence homology within BH1 and BH2 only. Pro-apoptotic proteins are subdivided into multi-domain Bcl-2 effector molecules such as Bax and Bak, which contain BH1-3, and BH3-only proteins. BH3-only proteins are further characterized as activators like Bid, Bim, and Puma that directly activate Bax and Bak, or sensitizers including Bad, Bik, and Nix/BNIP3 that interact with anti-apoptotic proteins to induce the release of activated multi-domain pro-apoptotic proteins (Brunelle and Letai 2009; Gallenne et al. 2009). Interactions between family members mediated by BH domains are vital for many aspects of their functioning. One of the unique features of anti-apoptotic Bcl-2 family proteins is a hydrophobic pocket defined by the BH1-3 domains, which allows for heterodimerization with pro-apoptotic proteins through insertion of their BH3 domains (Burlacu 2003; Petch and Al-Rubeai 2004; Gélinas and White 2005).
Fig. 1.
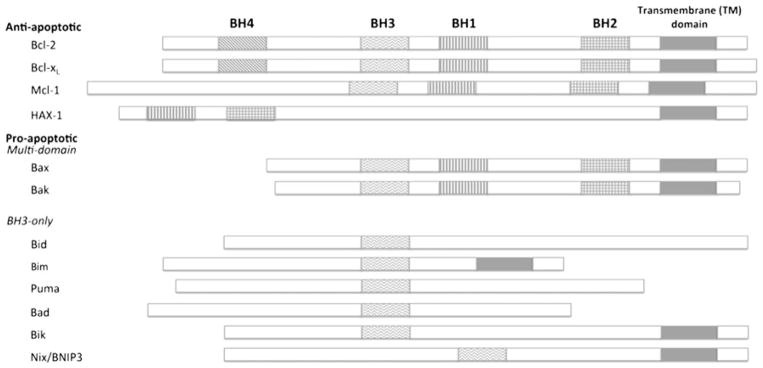
Schematic representation of key members of the Bcl-2 family of proteins. Anti-apoptotic members are typically comprised of all four BH domains and a transmembrane domain for membrane anchoring. Pro-apoptotic members contain multi-domain members that include BH1-3, or BH3-only members. Not all pro-apoptotic Bcl-2 family members; however, have a transmembrane domain
Bcl-2 family proteins are traditionally believed to reside or translocate to mitochondria and function as apoptotic regulators by controlling mitochondrial membrane permeability. Ultimately, the ratio between anti- and pro-apoptotic Bcl-2 proteins determines whether cells live or die (Hanson et al. 2008). As a result, Bcl-2 family proteins have been used to genetically engineer recombinant mammalian cells such as Chinese hamster ovary (CHO) and human embryonic kidney (HEK) 293 cell lines used to produce many biotherapeutics (Itoh et al. 1995; Chiang and Sisk 2005; Majors et al. 2007).
The Bcl-2 family; however, has additionally been detected at other subcellular locations in healthy cells including the endoplasmic reticulum (ER), nuclear membrane and within the cytosol and have also been linked to non-apoptotic functions including calcium (Ca2+) homeostasis, inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ signaling and mitochondrial bioenergetics (Vander Heiden and Thompson 1999; Pinton et al. 2002; Majors et al. 2007). One can speculate that localization of these proteins may in some way have an impact on their function inasmuch as apoptosis and bioenergetics are dependent on the proper supply of Ca2+ by organelle channeling. Indeed, that Ca2+ signaling regulation might serve as an integrating role in which the activity of Bcl-2 family members relates bioenergetics with apoptosis. A better understanding of how Bcl-2 family members promote bioenergetics would be useful to further advances in our understanding of cell physiology and metabolic engineering. Thus, we aim to clarify the multi-organelle localization and functions of Bcl-2 members in mediating Ca2+ signals and investigate the implications of Ca2+ as it relates to bioenergetics and apoptosis.
Intracellular Ca2+ dynamics
Cytosolic Ca2+ homeostasis
The plasma membrane consists of several transporters and channels to modulate intracellular Ca2+ concentration ([Ca2+]i) including store-operated Ca2+ channels (SOCs) and Ca2+-ATPases (PMCAs) to regulate Ca2+ entry and exit, respectively. Within the cytosol, Ca2+ chelators act as either buffers or sensors to regulate Ca2+ homeostasis, and include proteins from the annexin and EF-hand (a Ca2+-binding motif containing an E-helix-loop-F-helix) families. Calmodulin and proteins from the S100 family are typical examples of Ca2+ sensors that undergo conformational changes upon binding Ca2+ to interact with specific targets. Ca2+ buffers, on the other hand, maintain [Ca2+]i in the range of 20–100 nM under steady-state conditions. Cytosolic Ca2+ stores; however, are transient and serve to replenish ER and mitochondria Ca2+ stores and stimulate Ca2+ extrusion when cells are overloaded with Ca2+.
ER Ca2+ homeostasis
The ER functions as the predominant Ca2+ storage facility within cells. Housed within the ER are Ca2+ binding-proteins that can be categorized as buffers or chaperones that are responsible for mediating ER Ca2+ homeostasis. Buffers, such as calreticulin, have a large Ca2+-binding capacity and are responsible for maintaining ER Ca2+ concentration, [Ca2+]ER, within the physiological range of 0.2–1 mM. This allows the ER to generate Ca2+ signals directed into the cytosol or mitochondria that are central to a broad range of cellular and physiological functions. Additionally, Ca2+ can bind to the multiple Ca2+-binding sites found on chaperones such as binding immunoglobulin protein (BiP/GRP78), calnexin or ERp57 to regulate their activity (Görlach et al. 2006).
Ca2+ accumulation in the ER lumen is mediated by sarcoplasmic/endoplasmic reticulum Ca2+ ATPases (SERCAs; Fig. 2a). Local rises in cytosolic Ca2+ concentrations are able to stimulate increases in SERCA activity. However, SERCA-mediated ER Ca2+ uptake is almost exclusively regulated within the ER lumen. [Ca2+]ER plays an important role in controlling SERCA-dependent Ca2+ uptake, whereby high [Ca2+]ER inhibits SERCA activity and low [Ca2+]ER increases ER Ca2+ uptake by SERCA. Several studies have also shown that ER-resident proteins including, calnexin and calreticulin inhibit ER Ca2+ uptake by regulating SERCA activity (John et al. 1998; Roderick et al. 2000; Arnaudeau et al. 2002; Görlach et al. 2006).
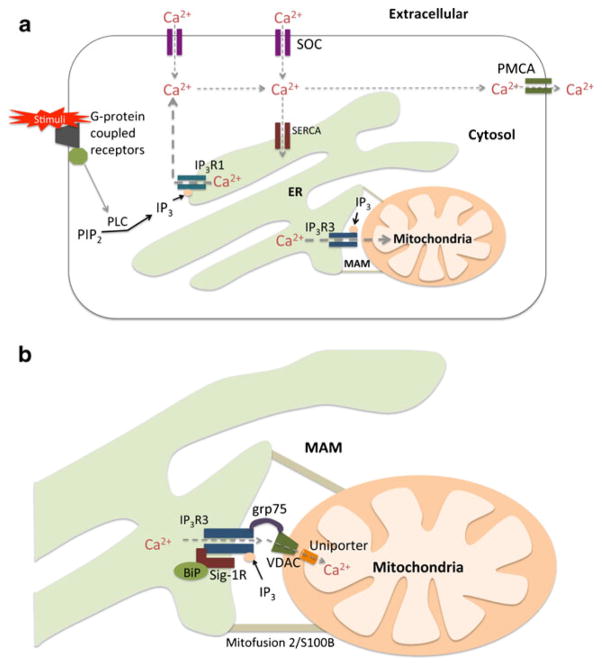
Fig. 2.
Schematic representation of intracellular Ca2+ dynamics. a Ca2+ entry and exit are regulated by SOC and PMCA channels, respectively, on the plasma membrane. Ca2+ is transported into the ER lumen by SERCA and released through IP3R. Type 1 IP3Rs (IP3R1) mediate cytosolic Ca2+ efflux and type 3 IP3Rs (IP3R3) mediate mitochondrial Ca2+ efflux. b IP3R3s enriched at MAM are found in a complex with Sig-1R and BiP, and are additionally linked to VDAC on the OMM by grp75. VDAC tightly controls Ca2+ permeation by IP3R3-mediated Ca2+ signals. Once Ca2+ transverses the OMM, Ca2+ uniporters transmit Ca2+ into the mitochondrial matrix
In the event of ER Ca2+ depletion, capacitative Ca2+ entry (CCE) is employed through SOCs on the plasma membrane. Stromal interaction molecule 1 (STIM1), an intraluminal ER Ca2+ sensor, plays an essential role in activation of CCE by communicating [Ca2+]ER to SOCs. Knockdown of STIM1 reduces store-operated currents in Jurkat T cells and store-operated Ca2+ entry in HEK293 cells and SH-SY5Y neuroblastoma cells (Putney 2005). Researchers later found that STIM1 aggregates in regions close to the plasma membrane to form a ternary complex with SOC subunits Orai1 and TRPC1 (canocial transient receptor potential 1) following ER Ca2+ depletion (Salido et al. 2009).
IP3R-mediated ER Ca2+ efflux
Stimulated release of Ca2+ from the ER occurs when ex-tracellular stimuli bind G-coupled protein receptors, activators of phospholipase C (PLC), to produce inositol 1,4,5- trisphosphate (IP3). The binding of IP3 provokes the opening of inositol 1,4,5-trisphosphate receptors (IP3Rs) and the release of Ca2+ (Fig. 2a). Three isoforms of IP3Rs (type 1, 2, and 3) have been identified with varying degrees of IP3 binding affinity and Ca2+ oscillations (Zhang et al. 2011). Knockdown studies of type 1 and type 3 IP3Rs in CHO cells showed that cytosolic Ca2+ was reduced when type 1 knockdown occurred and mitochondrial Ca2+ was reduced with type 3 knockdown. Indeed, Type 1 IP3Rs localize to the bulk ER to mediate Ca2+ efflux into the cytosol, whereas type 3 IP3Rs reside at the direct ER-mitochondrion contact termed MAM (mitochondria-associated ER membrane) and have been shown to preferentially facilitate the flux of Ca2+ into the mitochondria (Mendes et al. 2005).
MAM: direct ER to mitochondria Ca2+ signaling
Mitochondria-associated ER membrane (MAM) is a specialized area of the ER in direct contact with mitochondria that enables ER proteins to associate directly with proteins and lipids on the outer mitochondrial membrane (OMM). This physical interaction between the ER and mitochondria was determined based on co-sedimentation and electron microscopic observations (Shore and Tata 1977; Mannella et al. 1998) and it was later shown that up to 12 % of the ER is in direct contact with the mitochondria (Hayashi et al. 2009). Although the ER and mitochondria are both anchored to the cytoskeleton, the MAM is predominately held together by tethering proteins such as S100B and mitofusion 2, which maintain a minimum distance between the MAM and OMM at 10–25 nm ( Csordás et al. 2006; Hayashi et al. 2009).
The functional role of MAM in lipid metabolism has been well established in mammalian cells, where phosphatidylethanolamine (PE) synthesis depends on the transport of phosphatidylserine (PS) transport from the ER to mitochondria. Studies have shown that the enzymes involved in this process, including PS synthase and PE N-methyltransferase-2, are enriched at the MAM (Vance 2003). Likewise, key calcium signaling machinery has been observed to accumulate at the MAM; facilitating the efflux of Ca2+ directly from the ER into the mitochondria (Fig. 2b).
Furthermore, sigma-1 receptors (Sig-1Rs) in conjunction with the ER-resident chaperone, BiP, form a complex that stabilizes type 3 IP3Rs at the MAM. Upon IP3R activation by IP3, Sig-1Rs dissociate from BiP and directly bind type 3 IP3Rs to prevent degradation and prolong Ca2+ signaling (Hayashi and Su 2007). Ca2+ signaling at the MAM is additionally facilitated by the formation of “Ca2+ tunnels.” “Ca2+ tunnels” are multi-molecular complexes comprised of IP3Rs at the ER membrane linked to voltage-dependent anion channels (VDACs) on the OMM by glucose-regulated protein 75 (grp75). VDAC on the OMM functions to tightly control Ca2+ permeation during type 3 IP3R-mediated Ca2+ signaling. The IP3R3-VDAC complex shown in Fig. 2b ensures that Ca2+ released through IP3R3 generates high Ca2+ microdomains at ER-mitochondria contact sites (Szabadkai et al. 2006).
Ca2+ transport into mitochondria: VDAC and uniporter
Ca2+ permeation through the mitochondria is controlled by two major components: VDAC on the OMM and Ca2+ uniporters on the inner mitochondrial membrane (IMM; Fig. 2b). The OMM was thought to freely-transmit Ca2+; however, new evidence shows that VDAC may be tightly controlling Ca2+ permeation during short-lasting IP3R-mediated Ca2+ signaling (Spät et al. 2008). This was demonstrated using truncated Bid (tBid) to increase the permeability of the OMM, while keeping the IMM intact. It was observed that the exposure of tBid enhanced IP3R-mediated Ca2+ signaling, but failed to affect Ca2+ permeation into the mitochondria (Hajnóczky et al. 2002). Interestingly, overexpression of VDAC in HeLa cells stimulated with histamine, to generate IP3, caused an increase in mitochondrial Ca2+ uptake (Rapizzi et al. 2002). Cytosolic components such as NADH and metabolic enzymes; however, are also able to alter the gating properties of VDAC (Rizzuto et al. 2009). Once Ca2+ transverses the OMM, highly selective Ca2+ uniporters on the IMM are engaged to transmit Ca2+ into the mitochondrial matrix (Hajnóczky et al. 2002; Spät et al. 2008). A recently discovered gene, mitochondrial calcium uniporter (MCU), was determined to encode for the pore-forming subunit of the uniporter and has been determined to be critical for mitochondrial Ca2+ uptake. Indeed, when MCU is silenced mitochondrial Ca2+ uptake is diminished (Baughman et al. 2011; Chaudhuri et al. 2013). The Ca2+ concentration within the mitochondrial matrix plays a crucial role in mediating oxidative phosphorylation and ATP production through regulation of the TCA cycle (Hayashi and Su 2007; Rizzuto et al. 2009), while also controlling the opening of permeability transition pore (PTP), which plays a role in apoptosis (White et al. 2005; Walter and Hajnóczky 2005; Gunter and Sheu 2009).
Subcellular localization and redistribution of the Bcl-2 family
Distribution of anti-apoptotic Bcl-2 family proteins
Bcl-2 and Bcl-xL are tail-anchored proteins containing a C- terminal transmembrane (TM) domain consisting of a hydrophobic α-helix which functions as a membrane insertion device. The TM domain of Bcl-xL, in particular, possesses an X-TMB sequence that is flanked by two basic amino acids and specifically targets it to the outer mitochondrial membrane (OMM), which accounts for its predominant localization at the mitochondria. Bcl-2, on the other hand, contains an X/2-TMB sequence within its TM domain that is far less basic and has no sequence homology when compared with the X-TMB sequence of Bcl-xL (Kaufmann et al. 2003). As a result, Bcl-2 does not specifically target the mitochondria and can be found largely at the ER. In fact, Bcl-2 relies on the mitochondrial chaperone protein FKBP38, an atypical member of the FK506-binding immunophilin protein family, to shuttle it to the mitochondrial membrane (Portier and Taglialatela 2006).
The predominate ER localization of Bcl-2 can be attributed to its interaction with a reticulum (RTN) family protein, RTN- xs. Cell fractionation of D98/AH2 cells stably expressing Bcl-2 showed a shift in subcellular localization from the mitochondrial fraction to the microsomal fraction when transiently transfected with RTN-xs (Tagami et al. 2000). Interestingly, it has also been demonstrated that Bcl-2 is enriched at MAM (Meunier and Hayashi 2010). A small fraction of Bcl-xL has also been detected on the ER membrane due to interactions with RTN family members RTN-xs and NSP-C (Tagami et al. 2000).
Mcl-1 is found largely on the OMM, but curiously lacks a mitochondrial targeting sequence in its TM domain. Thus, two mechanisms have been proposed as to how Mcl-1 targets to the mitochondria. Firstly, Mcl-1 mitochondrial targeting has been linked to its association with translocase of the outer mitochondrial membrane 70 (Tomm70). The internal domain of Mcl-1 contains an EELD sequence that binds Tomm70 and works in conjunction with its TM domain to facilitate membrane insertion (Chou et al. 2006). Mitochondrial targeting is also achieved by the first 79 amino acids on the NH2-terminus of Mcl-1, which contains a PEST domain and several phosphorylation sites that promotes its association with the mitochondria. Deletion of the NH2-terminal domain of Mcl-1 diminishes mitochondrial targeting and the anti-apoptotic functioning of the protein (Germain and Duronio 2007).
The first 43 residues on the NH2-terminus of HAX-1 contain a predicated mitochondrial-targeting motif and acid box, which are essential for mitochondrial targeting (Chou et al. 2006), although a small fraction of HAX-1 has also been detected on the ER membrane. HAX-1 contains a binding region for the ER protein phospholamban (PLN) in its trans-membrane domain (aa 203–245), and when bound to PLN, HAX-1 distributes to the ER membrane. Indeed, when the transmembrane domain of HAX-1 is deleted, truncated HAX-1 (HAX-1 aa 1–181) only distributes to the mitochondria. Additionally, when compared to full length HAX-1 cotransfected with PLN, truncated HAX-1 cotransfected with PLN did not show distribution to the ER membrane (Kaufmann et al. 2003; Yap et al. 2010).
Although anti-apoptotic proteins reside mainly at the OMM and/or ER membranes, it is important to note that they have also been detected at other cellular locations. Bcl-2 and HAX-1, for instance, have been observed at outer nuclear membranes, while Mcl-1 and Bcl-xL exists partially within the cytosol. Cytosolic Bcl-xL, in particular, exists as a homodimer with its TM domain inserted into the hydrophobic pocket of the reciprocal dimer partner (Jeong et al. 2004).
Distribution of pro-apoptotic Bcl-2 family proteins
Bak mainly localizes to the OMM and integrates via its C- terminal TM domain. Similar to the TM domain of Bcl-xL, the TM domain of Bak is flanked by basic amino acids that specifically target the mitochondria (Lindsay et al. 2011). Its pro-apoptotic activity; however, is restrained by association with Bcl-xL, Mcl-1, or VDAC2 (Willis et al. 2005). In contrast, Bax in its inactive form is mainly found in the cytosol by hiding its C-terminal α helix containing a TM domain in its own binding pocket, while a fraction of Bax is also tethered to the OMM (George et al. 2010; Edlich et al. 2011). Subcellular fractionation studies have also shown that approximately 10–15 % of Bak and Bax reside at the ER (Zong et al. 2003).
Bik contains a C-terminal TM domain that exclusively targets it to the ER membrane (Mathai et al. 2002; Zhao et al. 2008). The hydrophobic C-terminal TM domain of Puma predominately targets it to the mitochondria, but is expressed at very low levels in cells until activation by p53 or alterations in intracellular Ca2+ (Nakano and Vousden 2001; Reimertz et al. 2003). Similarly, the C-terminal TM domain of Nix/BNIP3 also targets it to the mitochondria, although a small amount is also detected at the ER (Zhang et al. 2009; Chen et al. 2010). Most other inactive forms of BH3-only proteins including Bid, Bad, and Bim; however, are found within the cytosol. Bid remains cytosolic since it lacks a membrane targeting sequence. Bad is regulated by phosphor-ylation of three serine sites—S112, S136, and S155. The three serines are phosphorylated by a number of kinases including protein kinase A, Akt/protein kinase B, and p21-activated kinases. When S112 and S136 are phosphorylated, Bad is cytosolically retained through interaction with 14-3-3 scaffold proteins (Masters et al. 2001). The two major isoforms of Bim, BimEL and BimL, are sequestered to the microtubule- associated dynein motor complex by binding to the LC8 dynein light chain (Puthalakath et al. 1999).
Translocation of Bcl-2 family proteins
BH3-only pro-apoptotic proteins mainly found in the cytosol serve to detect apoptotic stimuli in cells and are characterized as activators or sensitizers. Activators, such as Bid, directly activate multi-domain pro-apoptotic proteins. Upon stimulation, Bid is proteolytically cleaved by caspase-8 and undergoes a posttranslational modification to form truncated Bid (tBid), which then targets the mitochondria and activates Bax and Bak. Activated Bax dimerizes and undergoes a conformational change that exposes its transmembrane domain followed by translocation to mitochondrial and ER membranes. Subsequently, tBid induces a conformational change in Bak that breaks its association with Bcl-xL, Mcl-1 or VDAC2 at the OMM (Korsmeyer et al. 2000). Alternatively, when activated, the BH3-only sensitizers like Bim and Bad translocate and then bind anti-apoptotic proteins to neutralize their activity. For example, apoptotic stimuli activate the release of Bim from its association with the dynein motor complex, and Bad undergoes dephosphorylation to dissociate from 14-3-3. The sensitizers are then able to bind Bcl-2 or Bcl-xL to disrupt their complexes with multi-domain pro-apoptotic proteins; thereby activating the apoptotic cascade (Puthalakath et al. 1999; Masters et al. 2001). Bad has also been shown to hinder the activity of Bcl-xL by inhibiting dimer formation. Interestingly; however, this also triggers the translocation of cytosolic Bcl-xL to mitochondrial membranes by displacing homodimers of Bcl-xL to expose Bcl-xL TM domains (Jeong et al. 2004).
Although the mechanism is unknown, cytosolic Mcl-1 is also known to translocate to the OMM upon apoptotic stimulation. Interestingly, Mcl-1 degradation within the cytosol and at the OMM also facilitates cytosolic Bcl-xL and Bax translocation to the mitochondria (Nijhawan et al. 2003). Once in the OMM, Bcl-xL heterodimerizes with multi-domain and pro-apoptotic proteins to deter apoptosis (Cheng et al. 2001; Yi et al. 2003; Ruffolo and Shore 2003; Shore and Nguyen 2008). In particular, Bcl-xL is able to retrotranslocate Bax from the mitochondria to the cytosol depending on its concentration at the OMM (Edlich et al. 2011).
Multi-organelle coordination of Ca2+ signaling by the Bcl-2 family
Bcl-2 family at the ER
Bcl-2 mediates ER Ca2+ stores
There is conflicting evidence as to the roles anti-apoptotic Bcl-2 family proteins play in ER Ca2+ homeostasis. Some studies show that ER Ca2+ is preserved when anti-apoptotic Bcl-2 proteins are overexpressed, while others report that Bcl-2 overexpression results in diminished Ca2+ stores. These different findings demonstrate the complexity of Ca2+ signaling and may be the result of unique differences between cell types or even clonal isolates. The data supporting the preservation of ER Ca2+ will be considered first. Under extracellular Ca2+-free conditions, it was observed that Bcl-2 overexpression preserves ER luminal Ca2+ during apoptosis induced by inter-leukin (IL-3) withdrawal in IL-3-dependent hematopoetic cells. Indeed, direct measurement of ER luminal Ca2+ by Fura-2FF AM showed no difference between Bcl-2 positive and negative clones (Baffy et al. 1993). It was further demonstrated for W.Hb12 cells stably overexpressing Bcl-2 that ER Ca2+ was preserved due to enhanced ER Ca2+ uptake (He et al. 1997). A similar finding was observed in MCF10A cells stably expressing Bcl-2. It was additionally determined that Bcl-2 overexpressing MCF10A cells have upregulated SERCA2 mRNA and protein levels. Therefore, increased ER Ca2+ uptake was attributed to increased SERCA activity, and it was speculated that Bcl-2 might interact with SERCA pumps at the ER (Chen et al. 2004).
Evidence supporting reduced ER luminal Ca2+ in cells overexpressing anti-apoptotic proteins has been presented by a number of researchers using ER-targeted sensors including cameleons, Mag-fura 2-AM, and AEQ chimeras. There are three proposed mechanisms to explain how reduced ER luminal Ca2+ may occur. Some researchers believe that Bcl-2 overexpression decreases SERCA activity, thereby reducing the amount of Ca2+ that can enter the ER. This was demonstrated in a study showing that Bcl-2 overexpression decreases SERCA2b and calreticulin expression in LNCaP cells (Vanden Abeele et al. 2002). Furthermore, it was reported that anti-apoptotic HAX-1, although residing predominately at the mitochondria, can translocate to the ER to bind with and significantly downregulate SERCA2 thereby causing reduced ER Ca2+ loading (Vafiadaki et al. 2009). Another proposed mechanism for reduced ER luminal Ca2+ is that anti-apoptotic Bcl-2 family members increase Ca2+ leakage across the ER in order to reduce steady-state Ca2+ levels. Researchers observed a decrease in ER luminal Ca2+ in DT40 cells expressing Bcl-2 or Mcl-1; however, in the presence of IP3R inhibitor heparin that blocks the exit of Ca2+ from the ER, ER luminal Ca2+ was preserved. Furthermore, Bcl-2 and Mcl-1 expression had no effect on ER Ca2+ stores in DT40 cells with all three IP3R isoforms knocked-out (Eckenrode et al. 2010). An alternative scenario under consideration is that Bcl-2 indirectly decreases capacitative Ca2+ entry by downregulating SOC activity at the plasma membrane to limit the amount of Ca2+ stored in the ER (Li et al. 2002).
Pro-apoptotic Bcl-2 members regulate ER Ca2+ stores
Pro-apoptotic Bcl-2 family proteins have also be shown to regulate luminal ER Ca2+. Experimentation with Bax−/Bak−double-knockout (DKO) cells (mouse embryo fibroblast cells deficient in Bax and Bak) showed a decrease in ER Ca2+ stores, which resulted in reduced flux of Ca2+ from the ER into the cytosol and mitochondria compared to wild-type cells under thapsigargin (Tg) stimulation. Expression of recombinant Bax in DKO cells restored ER Ca2+ to nearly wild-type levels; however, expression of mitochondria-targeted Bax in DKO cells had no effect on ER Ca2+ stores. Thus, the expression of ER-targeted Bax/Bak may function to increase the ER luminal Ca2+ concentration (Scorrano et al. 2003; Oakes et al. 2005).
BH3-only pro-apoptotic proteins Bik and Nix/BNIP3 have been observed to increase ER Ca2+ leakage. In Hep3B cells with Bik overexpression, there was an observed increase in cytosolic Ca2+ over a 72 h time frame after depletion of ER Ca2+ (Zhao et al. 2008). Another study using kidney epithelial cells derived from wild-type or Bax−/Bak− DKO mice demonstrated that Bik overexpression results in the release of ER Ca2+ in wild-type cells, although Ca2+ release was not observed in DKO cells. Hence, Bik appears to function through a Bax/Bak-dependent mechanism to release ER Ca2+ stores (Mathai et al. 2005). Researchers have also observed greater increases in cytosolic Ca2+ in Nix-overexpressing mice compared to Nix-knockout mice and controls following caffeine stimulation (Diwan et al. 2009). Transiently overexpressing BNIP3 in Mes 23.5 cells was additionally determined to increase ER Ca2+ leakage, resulting in increased mitochondrial Ca2+ accumulation (Zhang et al. 2009).
Puma regulates ER Ca2+ depletion-induced apoptosis
Following ER Ca2+ depletion stimulated by Tg, researchers observed transcriptional upregulation of Puma, a BH3-only pro-apoptotic protein, in HCT116 human colon cancer cells. Increased Puma mRNA and protein levels resulted in activation of caspase-3, 8, and 9 and Bid as well as the release of cytochrome c into the cytosol (Luo et al. 2005). Likewise, increases in Puma mRNA and protein levels were detected in SH-SY5Y neuroblastoma cells after 4 and 12 h treatment, respectively, with tunicamycin (an ER stress inducer that increases intracellular Ca2+ levels). Corresponding increases in activated caspase-3 and 9 as well as cytochrome c release was observed. Morphological changes characteristic of apoptosis and substantial increases in cell death were also observed in GFP-positive SH-SY5Y cells transiently co-transfected with hemagglutinin-tagged Puma compared to control cells (Reimertz et al. 2003).
Researchers have found that Bax-KO cells provided some resistance to Tg-induced apoptosis. Increased Puma upregulation was also observed in these cells compared to controls, indicating an attempt by Puma to compensate for Bax deficiency. Thus, Puma appears to facilitate ER-stress-induced apoptosis through a Bax-dependent pathway. Additional evidence shows that Puma is vital for initiation of ER stress-induced apoptosis. BiP, an unfolded protein response target gene that aids in restoring normal ER functioning, was upregulated in response to prolonged Tg treatment in HCT116 control and Puma-knockout (KO) cells. Consistent with previous results Tg also triggered the upregulation of Puma in control cells. However, Puma-KO cells had substantially less apoptosis after exposure to Tg compared to controls (Reimertz et al. 2003; Luo et al. 2005).
Bcl-2, Bcl-xL, and Mcl-1 bind IP3Rs
Several studies confirm that Bcl-2, Bcl-xL, and Mcl-1 bind to all three isoforms of IP3Rs (Eckenrode et al. 2010). Researchers also observed enhanced Bcl-2 and type 1 IP3R binding in Bax−/Bak− double-knockout (DKO) MEF cells by immunoprecipation of Bcl-2 from ER fractions when compared to wild-type cells (Oakes et al. 2005). Furthermore, an increase in Bcl-2 and type 3 IP3R interactions was shown by immunoprecipation of type 3 IP3Rs or Bcl-2 in T47D breast cancer cells after apoptosis induction using gefitinib, a tyro-sine kinase inhibitor (Zannetti et al. 2008). Most studies; however, focus on determining the binding sites for Bcl-2 family protein interacting with type 1 IP3Rs.
Pull-down studies using glutathione-S-transferase (GST) fusion constructs followed by immunoblotting showed that Bcl-2 binds to domain 3 (amino acids, aa, 293–1581) in the regulatory and coupling domain of type 1 IP3Rs, and less significantly with domain 6 (aa 2590–2749) at the C-terminus. By dividing the binding region into smaller fragments and it was determined that Bcl-2 binds most strongly with amino acids 1347–1426 of domain 3 (Rong et al. 2009). In a similar study, Bcl-xL was shown to interact with domain 6 at the C-terminus of type 1 IP3R; interaction with domain 3 was not investigated (White et al. 2005). Furthermore, Flag-tagged Bcl-2, Bcl-xL, and Mcl-1 were pulled-down by domain 6 (GST-IP3R-TM6 + C; aa 2570–2749) of all three types of IP3Rs (Eckenrode et al. 2010). Researchers have also been able to determine that the BH4 domain of Bcl-2 is necessary for binding with type 1 IP3Rs. This was demonstrated using GST-IP3R-domain 3 to pull down full-length Bcl-2, whereas ΔBH4Bcl-2 was not pulled down by either GST-IP3R-domain 3 or 6 (Rong et al. 2009).
Bcl-2, Bcl-xL, and Mcl-1 regulate IP3R-mediated Ca2+ signaling
By binding IP3Rs, Bcl-2 family proteins function to modulate ER Ca2+ efflux. Using a single type 1 IP3R channel incorporated into an artificial planar lipid bilayer, Bcl-2 overexpression was shown to markedly reduce IP3R channel opening (Rong et al. 2009) and decrease the binding affinity of IP3Rs to IP3. During single cell imaging with Fura- 2-loaded DT40 cells the initial amplitude of released Ca2+ was reduced in Bcl-2 overexpressing cells. However, Bcl-2 and Mcl-1 overexpression contributed to more sustained and extended Ca2+ oscillations. Separately, experimentation with siRNA against Bcl-2 showed an increase in the number of cells that responded to ATP stimulation as well as an increase in the amplitude of Ca2+ signals when compared to control cells (Chen et al. 2004).
Experiments with a single type 1 IP3R channel showed that Bcl-xL increased channel activity by increasing the binding affinity of IP3Rs to IP3 (White et al. 2005; Li et al. 2007). Interestingly, however; Bcl-xL was also shown to diminish IP3R expression. In fact, total RNA purified from FL5.12 cells stably transfected with Bcl-xL, showed a marked decrease in the expression of type 1 IP3R genes. Western blotting data showed lower levels of type 1 and 3 IP3R expression in Bcl-xL overexpressing FL5.12 and 2B4.11 cells when compared to control cells. Thus, Bcl-xL overexpression resulted in an over-all decrease in the percentage of ER Ca2+ release as observed by researchers in which they showed that Bcl-xL overexpressing vesicles release less than 25 % of total Ca2+, compared to control vesicles that released approximately 40 % of total Ca2+ (Li et al. 2002).
Bcl-2 family at the mitochondria
Bcl-2 family proteins mediate VDAC opening on the OMM
VDAC is a highly abundant protein on the OMM and functions to facilitate the entry and exit of ATP, Ca2+, cytochrome c, and other metabolites between the mitochondria and other cellular compartments. As previously discussed, VDAC is also known to form “Ca2+ tunnels” with IP3R3 at the MAM via linkage with grp75 to tightly control ER Ca2+ signals into the mitochondria. There is some controversy; however, as to whether the open or closed conformation of VDAC assists in bioenergetics or induces cell death.
One theory is that VDAC closure sensitizes cells to apoptotic signals, while VDAC opening facilitates cellular metabolism (Vander Heiden et al. 2000; Tan and Colombini 2007). In its closed conformation, VDAC was observed to be highly permeable to Ca2+, but has reduced permeability to metabolites and ATP. Alternatively, the open conformation of VDAC allows for metabolite flux, has a low permeability to Ca2+ and prevents cytochrome c release. In fact, recombinant Bcl-xL was shown to increase the conductance of VDAC (Vander Heiden et al. 2001), while tBid induces VDAC channel closure (Rostovtseva et al. 2004).
Other researchers hypothesized that, in healthy cells, VDAC is continuously opening and closing to promote cellular metabolism. However, when pro-apoptotic proteins bind VDAC it causes the channel to remain in its open conformation, which is permeable to cytochrome c and leads to apoptosis. Anti-apoptotic proteins are able to counteract the effects of pro-apoptotic proteins by closing VDAC channels, but have no effect of VDAC conformation in healthy cells (Tsujimoto and Shimizu 2000). This was demonstrated by electrophysiological studies in which Bax caused a 4-fold increase in VDAC conductance, while Bcl-xL almost completely closed the channel (Shimizu et al. 2000a). Of note, it was demonstrated that the BH4 domains of Bcl-2 and Bcl- xL are required to inhibit VDAC opening, while the BH1 domain of Bcl-xL is also required (Shimizu et al. 2000b).
Mcl-1 and BNIP3 regulate mitochondrial Ca2+ uptake
Although the mechanism is unknown, Mcl-1 has been shown to modulate mitochondrial Ca2+ uptake. Rhod-2 measurements of mitochondrial Ca2+ using confocal microscopy were used to compare Mz-ChA-1 cells transfected with Mcl-1, siRNA against Mcl-1, or were not transfected. Those cells transfected with siRNA against Mcl-1 or non-transfected cells showed an increase in mitochondrial Ca2+ when stimulated with ATP, while cells transfected with Mcl-1 showed lower mitochondrial Ca2+ increases. Researchers also observed an increase in mitochondrial Ca2+ in Mcl-1 knockdown cells, while mitochondrial Ca2+ remained constant in Mcl-1 overexpressing cells when stimulated with staurosporine to induce apoptosis (Minagawa et al. 2005).
Conversely, Mes 23.5 cells transiently overexpressing BNIP3 were observed to stimulate mitochondrial Ca2+ uptake. Cells were loaded with Fluo-4 and Rhod-2 Ca2+ dyes to monitor cytosolic and mitochondrial Ca2+, respectively, after Tg-induced ER Ca2+ depletion. When compared to control, BNIP3 increased the ratio of mitochondrial-to-cytosolic Ca2+ over an 8 h period. This increase in mitochondrial Ca2+ was hindered when cells were first incubated with Ru360, a mito-chondrial uniporter inhibitor. Furthermore, transiently transfecting Bcl-2 in addition to BNIP3 also reduced mitochondrial Ca2+ uptake (Zhang et al. 2009).
Bcl-2 family at other subcellular localizations
Cytosolic Ca2+ increases modulate apoptosis
Increases in intracellular Ca2+ have been found to change the phosphorylation state of Bad through calcineurin, a serine-threonine phosphatase that is stimulated by Ca2+. Immunoprecipitation studies have determined that phosphorylated Bad reside in the cytosol in complex with 14-3-3 scaffold proteins and calcineurin. After cytosolic Ca2+ concentration is increased by stimulating cells with Tg, ionomycin, A23187, or glutamate, Bad becomes dephosphorylated and dissociates from calcineurin and 14-3-3. Dephosporylated Bad then trans- locates to the mitochondria to associate with Bcl-xL and results in increases in the activated form of caspase-3 (Wang 1999; Springer et al. 2000).
Ca2+ has also been found to activate a family of cytosolic cysteine proteinases called calpains (Khorchid and Ikura 2002). Active calpains cleave a number of target proteins in order to regulate apoptosis including members of the Bcl-2 family and caspases. Indeed, calpains are able to cleave Bid into its truncated form (tBid), resulting in cytochrome c release. Calpains can also activate Bax by cleaving its N-terminal region, which is responsible for heterodimerization with Bcl-2 and Bcl-xL. Bcl-2 and Bcl-xL can also be direct targets of calpains. Researchers determined that calpain- truncated Bcl-2/Bcl-xL undergo conformational changes that make them unable to homo- or heterodimerize. Of note, calpain-truncated Bcl-2, but not calpain-truncated Bcl-xL, was able to induce cytochrome c release. Taken together, the above data support the notion that calpain cleavage of Bcl-2 family proteins facilitates apoptosis. However, it is largely tissue-dependent whether calpain-cleaved caspases promotes death or cell survival (Gil-Parrado et al. 2002; Łopatniuk and Witkowski 2011).
Nuclear envelope-associated Bcl-2 is pro-apoptotic
The nuclear envelope acts as a barrier responsible for housing the genetic material of the cell within the nucleus. Continuous with the ER membrane, the cisternal space of the nuclear envelope also acts as a Ca2+ store mediated by SERCA pumps and IP3Rs. The nuclear pore complex (NPC) is a large protein channel that spans both lipid layers of the nuclear envelope and function to exclusively regulate the transport of macro-molecular molecules into and out of the nucleus. The NPC is composed of over 100 proteins, including 16–25 copies of glycoprotein 210 that contains several Ca2+-binding domains that extend into the cisternae of the nuclear envelope. Thus, cisternae Ca2+ stores were determined to regulate NPC structure (Gerace et al. 1982; Greber and Gerace 1995). Particularly, cisternal Ca2+ depletion causes the NPC to undergo conformational changes that increase NPC diameter, alter transport, and impede selectivity; all of which are detrimental to the cell (Erickson et al. 2006; Strasser et al. 2012).
Since NPC structure is mediated by cisternae Ca2+ concentration, it was hypothesized that nuclear envelope-associated Bcl-2 may function to mediate nuclear envelope permeability. In fact, it was determined that Bcl-2 overexpression lowers steady-state Ca2+ concentrations in the nucleus and increases the size-exclusion limit of the NPC when compared to control cells. Furthermore, only ER- and nuclear envelope-target Bcl-2, and not mitochondrial localized Bcl-2, increased nuclear envelope permeability. Since increased nuclear envelope permeability can lead to apoptosis, Bcl-2 has a pro-apoptotic role when localized at the nuclear envelope (Portier and Taglialatela 2006; Strasser et al. 2012) and further supports the notion that subcellular localization is important to Bcl-2 family functioning.
Bcl-2 mediates Ca2+ entry at the plasma membrane
Bcl-2 has been shown to modulate Ca2+ entry into the cell, although conflicting evidence may be the result of unique differences between cell types. Some studies observed a decrease in Ca2+ entry in Bcl-2 expressing cells. For example, measurement of cytosolic Ca2+ concentration ([Ca2+]c) using AEQ chimeras in HeLa cells transiently overexpressing Bcl-2 resulted in a lower increase in [Ca2+]c compared to controls after CCE was activated (Pinton 2000). Similar results were also seen in LNCaP cells stably overexpressing Bcl-2. These cells were treated with fura-2 to measure [Ca2+]c, and researchers observed a longer latency period and slower rate of Ca2+ influx through SOCs. Further experimentation suggested that Bcl-2 expression decreases the number of functional SOCs, resulting in the observed downregulation of capacitative Ca2+ entry (Vanden Abeele et al. 2002). Conversely, experiments with HL60 and PW cell lines resulted in enhanced CCE in Bcl-2 overexpressing cell lines (Williams et al. 2000).
The role of Ca2+ in bioenergetics and apoptosis
The principle input for cellular carbon metabolism in mammalian cells is glucose. During glycosis, one glucose molecule is converted into two pyruvate molecules, and produces energy in the form of ATP. Pyruvate can then be transported into the mitochondria to enter the tricarboxylic acid (TCA) cycle for the production of NADH and FADH2, or remains in the cytosol where it is converted into lactate. NADH and FADH2 are then used in the electron transport chain to drive ATP production by means of oxidative phosphorylation. Ca2+ in the mitochondria assist in the regulation of cellular bioenerget-ics by stimulating Ca2+-dependent enzymes in the TCA cycle (pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase), as well as ATP synthase, adenosine nucleotide translocase (ANT; the ATP translocator), and sites on the electron transport chain (Fig. 3).
Fig. 3.
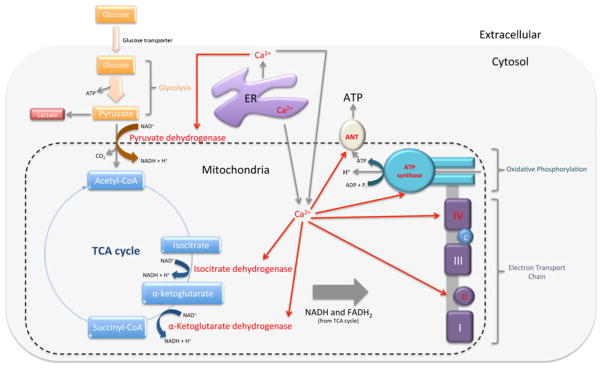
Ca2+-dependent enzymes in bioenergetics are facilitated by Ca2+ signals from the ER into the cytosol and mitochondria. Pyruvate, produced during glycolysis, can remain in the cytosol where it is converted into lactate, or it is transported into the mitochondria by Ca2+-dependent pyruvate dehydrogenase where it enters the TCA cycle. The TCA cycle consists of two other Ca2+-dependent enzymes—isocitrate, and α-ketoglutarate dehydrogenases—and produces NADH and FADH2. NADH and FADH2 are used by the electron transport chain to drive oxidative phosphorylation and ATP production. Complex II and IVof the electron transport chain are Ca2+-dependent as well as ATP synthase and ANT
The electron transport chain is also responsible for maintaining the inner mitochondrial transmembrane potential (Δψm) through regulation of the permeability transition pore (PTP). For many years the PTP was thought to consist of ANT found on the IMM and VDAC found on the OMM. However, PTP opening has been observed in the absence of these molecules (Kokoszka et al. 2004; Baines et al. 2007). In fact, new evidence indicates that the PTP is composed of ATP synthase dimers that bind cyclophilin D, a known regulator of the PTP. Although the mechanism is unknown, it is speculated that Ca2+ accumulated in the mitochondrial matrix binds ATP synthase causing conformational changes that induce PTP formation, with cyclophilin D increasing the Ca2+ binding affinity to the PTP (Giorgio et al. 2013).
Two open conformations of the PTP have been observed; a low-conductance state and a high-conductance state. At a low-conductive state, the PTP is permeable to small ions such as Ca2+ and K+ and contributes to mitochondrial Ca2+ homeo-stasis by controlling mitochondrial Ca2+-induced Ca2+ release (CICR). During CICR, Ca2+ is released quickly during transient PTP opening without causing pathological increases in Δψ m. The high-conductive conformation of the PTP; however, is permeable to pro-apoptotic molecules like cytochrome c and irreversibly dissipates Δψm leading to apoptosis (Jouaville et al. 1998; Huang et al. 2000). Once in the cytosol, cytochrome c interacts with apoptotic-protease-activating factor-1 (Apaf-1) to form the apoptosome. The apoptosome will then activate initiator caspases to trigger a caspase cascade, thus resulting in apoptosis. Cytochrome c can also bind IP3Rs to boost the influx of Ca2+ into the mitochondria and enhance apoptotic signaling (Boehning et al. 2003; Jeong and Seol 2008). The switch from a metabolic to apoptotic functioning of the PTP is dependent on matrix pH, which is dictated by the rate of mitochondrial Ca2+ influx and not the absolute amount (Jouaville et al. 1998). Thus, rapid or transient increases in mitochondrial Ca2+ will result in low-conductive opening of the PTP, while prolonged Ca2+ signals or mitochondrial Ca2+ overload causes high-conductive PTP opening.
There is some controversy as to whether the PTP regulates necrosis or apoptosis (Fig. 4). Bcl-2 overexpression was determined to diminish Ca2+ activation of the PTP, and block the dissipation of Δψm by ER-targeted BNIP3 (Murphy et al. 2001; Zhang et al. 2009); thus supporting an apoptotic role of the PTP. However, a study using cyclophilin D-deficient mice indicates that PTP opening is vital for necrosis (Nakagawa et al. 2005). This is further supported by the fact that ER localization of Nix also plays a role in PTP-dependent necrosis (Chen et al. 2010). There is additional evidence supporting the release of cytochrome c through the mitochondrial apoptosis-induced channel (MAC) formed on the OMM during early apoptosis. Although there is not a clear link between Ca2+ signaling and MAC, this channel is highly regulated by Bcl-2 family proteins (Guo et al. 2004; Martinez-Caballero et al. 2004, 2005; Dejean et al. 2005, 2006). There is also speculation that the PTP and MAC work together to facilitate cytochrome c release during mitochondrial mediated apoptosis. For instance, Ca2+ overload in the mitochondria matrix would cause highly-conductive PTP opening and dissipation of Δψm resulting in elevated cytosolic Ca2+ concentration. Increased cytosolic Ca2+ in return would lead to activation of pro-apoptotic proteins such as tBid and Bad, and induce MAC formation; therefore leading to OMM permeability and cyto-chrome c release (Wang 1999; Springer et al. 2000; Chen et al. 2002; Hajnóczky et al. 2009).
Fig. 4.
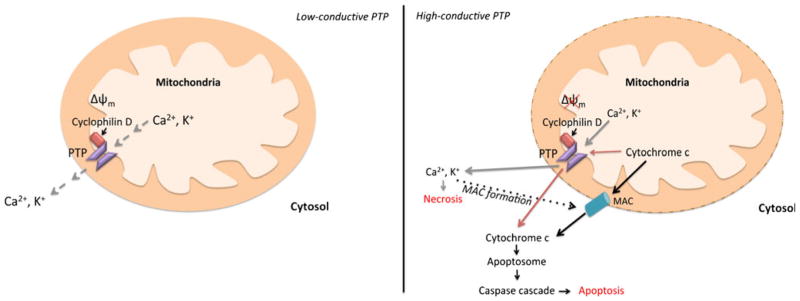
PTP opening is controlled by the rate of mitochondrial Ca2+ influx. Rapid or transient increases in mitochondrial Ca2+ influx results in low-conductive PTP opening permeable to small ions and controls transient Ca2+ release. Prolonged Ca2+ signals or mitochondrial Ca2+ overload causes high-conductive PTP opening, which increases cyctosolic Ca2+. The highly-conductive PTP was observed to directly facilitate necrosis (gray lines) and apoptosis (red lines). There is additional evidence that the PTP works in conjunction with MAC during apoptosis initiation (black lines). High-conductive opening of the PTP increases cytosolic Ca2+ concentration, which results in activation of pro-apoptotic proteins, inhibition of anti-apoptotic proteins, and MAC assembly. MAC then functions to permeabilize the OMM and release cyto-chrome c, which leads to apoptosis
The Bcl-2 family: linking apoptosis and bioenergetics through Ca2+
The relative amounts of anti- and pro-apoptotic proteins determine whether a cell remains viable or enters into apoptosis. In healthy cells, anti-apoptotic Bcl-2 family proteins dominate and function at the ER, mitochondria, nuclear envelope, and plasma membrane to mediate Ca2+ homeostasis, IP3R-mediate Ca2+ signaling and mitochondrial Ca2+ uptake in order to maintain concentrations within physiological levels (Table 1). Sustained and complete release of Ca2+ into the mitochondria, alterations in the spatiotemporal pattern, or changes at the level of effectors activated by Ca2+ can cause Ca2+ signals to switch from physiological functioning to apoptosis initiation (Pinton et al. 2002), leading Bcl-2 family proteins to translocate to the mitochondrial membrane in order to regulate Δψm (Fig. 4). If death signals prevail, the PTP switches from a low-conductive state to a high-conductive state which dissipates Δψm (Jouaville et al. 1998).
Table 1.
Overview of Bcl-2 family functioning in intracellular Ca2+ homeostasis and IP3R-mediated Ca2+ signaling at various subcellular localizations
| Bcl-2 family protein | Localization | Functioning |
|---|---|---|
| Anti-apoptotic | ||
| Bcl-2 | ER |
|
| Mitochondria |
|
|
| Plasma membrane |
|
|
| Nuclear envelope |
|
|
| Bcl-xL | ER |
|
| Mitochondria |
|
|
| Cytosol |
|
|
| Mcl-1 | ER |
|
| Mitochondria |
|
|
| Cytosol |
|
|
| HAX-1 | ER |
|
| Mitochondria |
|
|
| Nuclear envelope |
|
|
| Pro-apoptotic | ||
| Bax/Bak | ER |
|
| Mitochondria |
|
|
| Bid/tBid | Mitochondria |
|
| Puma | Mitochondria |
|
| Cytosol |
|
|
| Bad | Mitochondria |
|
| Cytosol |
|
|
| Bik | ER |
|
| BNIP3 | ER |
|
| Mitochondria |
|
|
| Nix | ER |
|
| Mitochondria |
|
|
Genetic engineering of mammalian cells has evolved to combat apoptosis through overexpression of anti-apoptotic proteins and the knockdown or silencing of pro-apoptotic proteins. A number of studies have centered on overexpression of Bcl-2, in which these cell lines had higher viabilities and improved productivity compared to control cells when exposed to apoptotic triggers (Mastrangelo et al. 1999, 2000). Overexpression of other anti-apoptotic Bcl-2 proteins, such as Bcl-xL and Mcl-1, or combinations of anti-apoptotic proteins have also resulted in increases in viability and titer (Chiang and Sisk 2005; Nivitchanyong et al. 2007; Majors et al. 2009; Dorai et al. 2009). Likewise, Bax−/Bak− double-knockouts in DHFR knockout CHO suspension cells resulted in increased levels of recombinant protein production when compared to wild-type cells (Cost et al. 2010).
Since Bcl-2 family proteins also regulate bioenergetics, changes in cellular metabolism were observed in some apoptosis-resistant cell lines. For example, Bcl-2 expressing Myc-transformed Rat1 cells were shown to have an altered glucose metabolism with a lower molar ratio of lactate production to glucose consumption, indicating a more efficient shuttling of pyruvate into the TCA cycle (Papas et al. 1999). Apoptosis-resistant CHO cells were also shown to consume lactate at a higher rate when compared to control cells (Dorai et al. 2009), and lactate consuming cells were found to be more energy efficient based on metabolic flux analysis (Martínez et al. 2013). Thus, it was hypothesized that apoptosis-resistant cell lines could thrive in high glucose conditions without producing toxic levels of lactate. Indeed, anti-apoptotic cell lines could be cultured effectively in a high glucose medium, where viability, peak viable cell density, and antibody titer all significantly increased compared to controls (Dorai et al. 2009).
It has become clear that subcellular localization of Bcl-2 family proteins also plays an important role in mediating Ca2+ signals important in both bioenergetics and apoptosis. Thus, in the future, it may be useful to target Bcl-2 family proteins to specific subcellular locations to control bioenergetics, along with apoptosis. For example, ER-targeted Bcl-2 was shown to protect cells from apoptosis by Bax overexpression, while wild-type and mitochondrial-targeted Bcl-2 did not (Wang et al. 2001). Additionally co-expression of Bcl-2 and FKBP38, a mitochondrial chaperone protein that shuttles Bcl-2 to the mitochondrial membrane, reduced pro-apoptotic nuclear envelope-associated Bcl-2 and increased cell survival (Portier and Taglialatela 2006). More studies are needed; however, to determine if subcellular targeting of Bcl-2 family proteins can be effective in controlling bioenergetics or other aspects of cellular physiology beyond the cell death cascade.
Acknowledgments
This work is supported in part by the Intramural Research Program of the National Institute on Drug Abuse, the National Institutes of Health and the Department of Health and Human Services.
Contributor Information
Abasha Lewis, Email: anlewis@jhu.edu, Department of Chemical and Biomolecular Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, USA, Cellular Pathobiology Section, IRP, NIDA, NIH, DHHS, 333 Cassell Drive, Baltimore, MD, USA.
Teruo Hayashi, Seiwakai Nishikawa Hospital, 293-2 Minato-Machi, Hamada, Shimane, Japan.
Tsung-Ping Su, Cellular Pathobiology Section, IRP, NIDA, NIH, DHHS, 333 Cassell Drive, Baltimore, MD, USA.
Michael J. Betenbaugh, Department of Chemical and Biomolecular Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, USA
References
- Arnaudeau S, Frieden M, Nakamura K, et al. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem. 2002;277:46696–46705. doi: 10.1074/jbc.M202395200. [DOI] [PubMed] [Google Scholar]
- Baffy G, Miyashita T, Williamson JR, Reed JC. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, et al. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D, Sancak Y, Mootha VK, Clapham DE. MCU encodes the pore conducting mitochondrial calcium currents. Elife. 2013;2:e00704. doi: 10.7554/eLife.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Won D-J, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- Chen R, Valencia I, Zhong F, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lewis W, Diwan A, et al. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Sisk WP. Bcl-x(L) mediates increased production of humanized monoclonal antibodies in Chinese hamster ovary cells. Biotechnol Bioeng. 2005;91:779–792. doi: 10.1002/bit.20551. [DOI] [PubMed] [Google Scholar]
- Chou C, Lee R, Yang-Yen H-F. An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway. Mol Biol Cell. 2006;17:3952–3963. doi: 10.1091/mbc.E06-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost GJ, Freyvert Y, Vafiadis A, et al. BAK and BAX deletion using zinc-finger nucleases yields apoptosis-resistant CHO cells. Biotechnol Bioeng. 2010;105:330–340. doi: 10.1002/bit.22541. [DOI] [PubMed] [Google Scholar]
- Csordás G, Renken C, Várnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta. 2006;1762:191–201. doi: 10.1016/j.bbadis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Diwan A, Matkovich SJ, Yuan Q, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai H, Kyung YS, Ellis D, et al. Expression of anti-apoptosis genes alters lactate metabolism of Chinese Hamster Ovary cells in culture. Biotechnol Bioeng. 2009;103:592–608. doi: 10.1002/bit.22269. [DOI] [PubMed] [Google Scholar]
- Eckenrode EF, Yang J, Velmurugan GV, et al. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson ES, Mooren OL, Moore D, et al. The role of nuclear envelope calcium in modifying nuclear pore complex structure. Can J Physiol Pharm. 2006;84:309–318. doi: 10.1139/y05-109. [DOI] [PubMed] [Google Scholar]
- Gallenne T, Gautier F, Oliver L, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas C, White E. BH3-only proteins in control: specificity regulates MCL-1 and BAK-mediated apoptosis. Gene Dev. 2005;19:1263–1268. doi: 10.1101/gad.1326205. [DOI] [PubMed] [Google Scholar]
- George NM, Targy N, Evans JJD, et al. Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process. J Biol Chem. 2010;285:1384–1392. doi: 10.1074/jbc.M109.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L, Ottaviano Y, Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982;95:826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Duronio V. The N terminus of the anti-apoptotic BCL-2 homologue MCL-1 regulates its localization and function. J Biol Chem. 2007;282:32233–32242. doi: 10.1074/jbc.M706408200. [DOI] [PubMed] [Google Scholar]
- Gil-Parrado S, Fernández-Montalván A, Assfalg-Machleidt I, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem. 2002;277:27217–27226. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Sign. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Greber UF, Gerace L. Depletion of calcium from the lumen of endoplasmic reticulum reversibly inhibits passive diffusion and signal-mediated transport into the nucleus. J Cell Biol. 1995;128:5–14. doi: 10.1083/jcb.128.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Sheu S-S. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Pietkiewicz D, Pavlov EV, et al. Effects of cytochrome c on the mitochondrial apoptosis-induced channel MAC. Am J Physiol Cell Physiol. 2004;286:C1109–C1117. doi: 10.1152/ajpcell.00183.2003. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Csordás G, Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32:363–377. doi: 10.1016/s0143416002001872. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Roy SS, Madesh M, et al. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson CJ, Bootman MD, Distelhorst CW, et al. The cellular concentration of Bcl-2 determines its pro- or anti-apoptotic effect. Cell Calcium. 2008;44:243–258. doi: 10.1016/j.ceca.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T-P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T-P, Rizzuto R, Hajnoczky G. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, McCormick TS, Lam M, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol. 1997;138:1219–1228. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhai D, Huang Y. Study on the relationship between calcium-induced calcium release from mitochondria and PTP opening. Mol Cell Biochem. 2000;213:29–35. doi: 10.1023/a:1007138818124. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ueda H, Suzuki E. Overexpression of bcl-2, apoptosis suppressing gene: Prolonged viable culture period of hybridoma and enhanced antibody production. Biotechnol Bioeng. 1995;48:118–122. doi: 10.1002/bit.260480205. [DOI] [PubMed] [Google Scholar]
- Jeong S-Y, Seol D-W. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Jeong S-Y, Gaume B, Lee Y-J, et al. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 2004;23:2146–2155. doi: 10.1038/sj.emboj.7600225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P. Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol. 1998;142:963–973. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Mazat JP. Modulation of cell calcium signals by mitochondria. Mol Cell Biochem. 1998;184:371–376. [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, et al. Characterization of the signal that directs Bcl-xL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorchid A, Ikura M. How calpain is activated by calcium. Nat Struct Biol. 2002;9:239–241. doi: 10.1038/nsb0402-239. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Li C, Fox CJ, Master SR, et al. Bcl-X(L) affects Ca(2+) homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. Proc Natl Acad Sci U S A. 2002;99:9830–9835. doi: 10.1073/pnas.152571899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang X, Vais H, et al. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria–specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Łopatniuk P, Witkowski JM. Conventional calpains and programmed cell death. Acta Biochim Pol. 2011;58:287–296. [PubMed] [Google Scholar]
- Luo X, He Q, Huang Y, Sheikh MS. Transcriptional upregulation of PUMA modulates endoplasmic reticulum calcium pool depletion-induced apoptosis via Bax activation. Cell Death Differ. 2005;12:1310–1318. doi: 10.1038/sj.cdd.4401659. [DOI] [PubMed] [Google Scholar]
- Majors BS, Betenbaugh MJ, Chiang GG. Links between metabolism and apoptosis in mammalian cells: applications for anti-apoptosis engineering. Metab Eng. 2007;9:317–326. doi: 10.1016/j.ymben.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Majors BS, Betenbaugh MJ, Pederson NE, Chiang GG. Mcl-1 overexpression leads to higher viabilities and increased production of humanized monoclonal antibody in Chinese hamster ovary cells. Biotechnol Progr. 2009;25:1161–1168. doi: 10.1002/btpr.192. [DOI] [PubMed] [Google Scholar]
- Mannella CA, Buttle K, Rath BK, Marko M. Electron microscopic tomography of rat-liver mitochondria and their interactions with the endoplasmic reticulum. BioFactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- Martínez VS, Dietmair S, Quek L-E, et al. Flux balance analysis of CHO cells before and after a metabolic switch from lactate production to consumption. Biotechnol Bioeng. 2013;110:660–666. doi: 10.1002/bit.24728. [DOI] [PubMed] [Google Scholar]
- Martinez-Caballero S, Dejean LM, Kinnally KW. Some amphiphilic cations block the mitochondrial apoptosis-induced channel, MAC. FEBS Lett. 2004;568:35–38. doi: 10.1016/j.febslet.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Martinez-Caballero S, Dejean LM, Jonas EA, Kinnally KW. The role of the mitochondrial apoptosis induced channel MAC in cytochrome c release. J Bioenerg Biomembr. 2005;37:155–164. doi: 10.1007/s10863-005-6570-z. [DOI] [PubMed] [Google Scholar]
- Masters SC, Yang H, Datta SR, et al. 14-3-3 inhibits Bad-induced cell death through interaction with serine-136. Mol Pharmacol. 2001;60:1325–1331. doi: 10.1124/mol.60.6.1325. [DOI] [PubMed] [Google Scholar]
- Mastrangelo AJ, Zou S, Hardwick JM, Betenbaugh MJ. Antiapoptosis chemicals prolong productive lifetimes of mammalian cells upon Sindbis virus vector infection. Biotechnol Bioeng. 1999;65:298–305. [PubMed] [Google Scholar]
- Mastrangelo AJ, Hardwick JM, Bex F, Betenbaugh MJ. Part I. Bcl-2 and Bcl-x(L) limit apoptosis upon infection with alphavirus vectors. Biotechnol Bioeng. 2000;67:544–554. [PubMed] [Google Scholar]
- Mathai JP, Germain M, Marcellus RC, Shore GC. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 2002;21:2534–2544. doi: 10.1038/sj.onc.1205340. [DOI] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Shore GC. BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- Mendes CCP, Gomes DA, Thompson M, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Meunier J, Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J Pharmacol Exp Ther. 2010;332:388–397. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa N, Kruglov EA, Dranoff JA, et al. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Schneider E, Kinnally KW. Overexpression of Bcl-2 suppresses the calcium activation of a mitochondrial megachannel. FEBS Lett. 2001;497:73–76. doi: 10.1016/s0014-5793(01)02440-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nijhawan D, Fang M, Traer E, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Gene Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivitchanyong T, Martinez A, Ishaque A, et al. Anti-apoptotic genes Aven and E1B-19K enhance performance of BHK cells engineered to express recombinant factor VIII in batch and low perfusion cell culture. Biotechnol Bioeng. 2007;98:825–841. doi: 10.1002/bit.21479. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas KK, Sun L, Roos ES, et al. Change in lactate production in Myc-transformed cells precedes apoptosis and can be inhibited by Bcl-2 overexpression. FEBS Lett. 1999;446:338–342. doi: 10.1016/s0014-5793(99)00240-9. [DOI] [PubMed] [Google Scholar]
- Petch A, Al-Rubeai M. The Bcl-2 family. In: Al-Rubeai M, Fussenegger M, editors. Cell engineering. Kluwer Academic Publishers; Netherlands: 2004. pp. 25–47. [Google Scholar]
- Pinton P. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, et al. A role for calcium in Bcl-2 action? Biochimie. 2002;84:195–201. doi: 10.1016/s0300-9084(02)01373-1. [DOI] [PubMed] [Google Scholar]
- Portier BP, Taglialatela G. Bcl-2 localized at the nuclear compartment induces apoptosis after transient overexpression. J Biol Chem. 2006;281:40493–40502. doi: 10.1074/jbc.M606181200. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O’Reilly LA, et al. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimertz C, Kögel D, Rami A, et al. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Marchi S, et al. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick HL, Lechleiter JD, Camacho P. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y-P, De Smedt H, Bultynck G, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci U S A. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva TK, Antonsson B, Suzuki M, et al. Bid, but not Bax, regulates VDAC channels. J Biol Chem. 2004;279:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- Ruffolo SC, Shore GC. BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J Biol Chem. 2003;278:25039–25045. doi: 10.1074/jbc.M302930200. [DOI] [PubMed] [Google Scholar]
- Salido GM, Sage SO, Rosado JA. Biochemical and functional properties of the store-operated Ca2+ channels. Cell Signal. 2009;21:457–461. doi: 10.1016/j.cellsig.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem. 2000a;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A. 2000b;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore GC, Nguyen M. Bcl-2 proteins and apoptosis: choose your partner. Cell. 2008;135:1004–1006. doi: 10.1016/j.cell.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Shore GC, Tata JR. Two fractions of rough endoplasmic reticulum from rat liver. II. Cytoplasmic messenger RNA’s which code for albumin and mitochondrial proteins are distributed differently between the two fractions. J Cell Biol. 1977;72:726–743. doi: 10.1083/jcb.72.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spät A, Szanda G, Csordás G, Hajnóczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Nottingham SA, Kennedy SE. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser C, Grote P, Schäuble K, et al. Regulation of nuclear envelope permeability in cell death and survival. Nucleus. 2012;3:540–551. doi: 10.4161/nucl.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Várnai P, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami S, Eguchi Y, Kinoshita M, et al. A novel protein, RTN-XS, interacts with both Bcl-XL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity. Oncogene. 2000;19:5736–5746. doi: 10.1038/sj.onc.1203948. [DOI] [PubMed] [Google Scholar]
- Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- Vafiadaki E, Arvanitis DA, Pagakis SN, et al. The Anti-apoptotic Protein HAX-1 Interacts with SERCA2 and Regulates Its Protein Levels to Promote Cell Survival. Mol Biol Cell. 2009;20:306–318. doi: 10.1091/mbc.E08-06-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog Nucleic Acid Re. 2003;75:69–111. doi: 10.1016/s0079-6603(03)75003-x. [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F, Skryma R, Shuba Y, et al. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Li XX, et al. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci U S A. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, et al. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Walter L, Hajnóczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- Wang H. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SS, French JN, Gilbert M, et al. Bcl-2 overexpression results in enhanced capacitative calcium entry and resistance to SKF-96395-induced apoptosis. Cancer Res. 2000;60:4358–4361. [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Gene Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap SV, Vafiadaki E, Strong J, Kontrogianni-Konstantopoulos A. HAX-1: a multifaceted antiapoptotic protein localizing in the mitochondria and the sarcoplasmic reticulum of striated muscle cells. J Mol Cell Cardiol. 2010;48:1266–1279. doi: 10.1016/j.yjmcc.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Yi X, Yin X-M, Dong Z. Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem. 2003;278:16992–16999. doi: 10.1074/jbc.M300039200. [DOI] [PubMed] [Google Scholar]
- Zannetti A, Iommelli F, Fonti R, et al. Gefitinib induction of in vivo detectable signals by Bcl-2/Bcl-xL modulation of inositol trisphosphate receptor type 3. Clin Cancer Res. 2008;14:5209–5219. doi: 10.1158/1078-0432.CCR-08-0374. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Liu H, et al. BNIP3 mediates cell death by different pathways following localization to endoplasmic reticulum and mitochondrion. FASEB J. 2009;23:3405–3414. doi: 10.1096/fj.08-124354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Fritz N, Ibarra C, Uhlén P. Inositol 1,4,5-trisphosphate receptor subtype-specific regulation of calcium oscillations. Neurochem Res. 2011;36:1175–1185. doi: 10.1007/s11064-011-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang L, Sun Y, et al. The endoplasmic reticulum (ER)-target protein Bik induces Hep3B cells apoptosis by the depletion of the ER Ca2+ stores. Mol Cell Biochem. 2008;312:33–38. doi: 10.1007/s11010-008-9718-4. [DOI] [PubMed] [Google Scholar]
- Zong W-X, Li C, Hatzivassiliou G, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]