Abstract
Early life adversity and stress in humans has been related to a number of psychological disorders including anxiety, depression, and addiction. The present study used isolation rearing, a well-characterized animal model of early life adversity, to examine its effects on social behavior and immediate early gene (IEG) expression produced by exposure to a novel social experience. Male and female rats were housed in same-sex groups or in isolation for 4 weeks beginning at weaning and were tested during late adolescence. The protein products of the IEGs c-fos and Arc, as well as the neurotrophic factor BDNF were assessed in medial prefrontal cortex (mPFC) subregions (anterior cingulate, prelimbic and infralimbic) using immunohistochemistry. Aggressive and non-aggressive behaviors during novel social exposure were also assessed. Exposure to a novel conspecific produced increases in Arc and c-fos activation in the mPFC of group reared animals in a sex- and subregion-dependent fashion compared to no social exposure controls, but this increase was blunted or absent in isolated animals. Isolates engaged in more social interactions and more aggressive behavior than group reared rats. Sex differences in some behaviors as well as in Arc and BDNF expression were observed. These results indicate that isolation rearing alters IEG activation in the mPFC produced by exposure to a novel conspecific, in addition to changing social behavior, and that these effects depend in part on sex.
Keywords: Isolation rearing, prefrontal cortex, Arc, c-fos, BDNF, social interaction, sex differences
1. Introduction
Early life adversity and stress in humans has been implicated as a factor in the etiology of a number of psychological disorders, including affective, anxiety, behavior, and substance use disorders [1–3]. Adding to the complexity of the effects of early life adversity, it is increasingly appreciated that males and females differ in the behavioral and physiological responses to stress [4, 5], prevalence rates of a number of psychological disorders such as depression and anxiety [6–8], and both vulnerability factors and the protective factors that can arise from early adversity [9].
Adolescent social isolation, or isolation rearing, is an animal model of early life adversity resulting in behavioral abnormalities termed “isolation syndrome” [10]. Social isolation during the critical period of adolescence produces anxiety-like behaviors and abnormalities in social behavior, brain morphology, and brain neurochemistry [11–13]. Social interaction is rewarding for adolescent rats [14] and social deprivation, especially during adolescence, is thought to be a stressful experience. This is indicated by isolation-induced changes in stress-related behaviors and HPA axis function [13, 15, 16] and can be dependent on the sex of the animal [17, 18]. Several groups have observed increases in social interaction and aggressive behavior after isolation rearing, especially in male rats [19, 20], although there is some disagreement in the literature [13, 21, 22].
Chronic stress in rats results in structural changes such including decreased dendritic branching [23] and spine density [24] in the mPFC. The effects of adverse experiences differs across the lifespan and adolescence appears to be a particularly important period in the development of stress reactivity [25]. Prefrontal cortical responses to adversity during adolescence are of particular interest since the PFC is not fully mature during adolescence [26–28]. Furthermore, appropriate social behavior may be mediated by the mPFC [29, 30]. Evidence that isolation rearing can produce abnormalities in PFC structure and function include reductions in PFC volume [31, 32], dendritic complexity [21], expression of immediate early genes [32, 33], and synaptic-associated proteins [22]. Of special importance during adolescence is the development of social play behaviors, and deprivation of play behavior in adolescent rats impairs subsequent social behavior and decreases the dendritic complexity of the mPFC [34].
The goal of the present study was to determine the effects of adolescent social isolation on functional changes within the mPFC of male and female rats in response to a brief exposure to a novel same-sex rat (conspecific). Rats were housed in isolation for 4 weeks and tested during late adolescence. To determine mPFC activation, immunohistochemistry was used to label the protein products of two different immediate early genes (IEGs), c-fos and activity-regulated cytoskeleton associated protein (Arc), as well as brain-derived neurotrophic factor (BDNF). C-fos is commonly used as a marker of neuronal activation and is transiently induced in response to various types of stimuli [35]. Arc is thought to play a role in changes in synaptic transmission through alterations at receptor sites in the post-synaptic density [36]. It has been implicated in multiple forms of synaptic plasticity, and is thought to be associated with reorganization of synapses as a result of stressful experiences [37]. Evidence exists for a role for BDNF in dendritic branching [38], and dendritic complexity within the mPFC has been shown to be reduced by isolation rearing [21]. Rapid increases in BDNF expression paralleling the time course of the present study have been shown to occur [39–41]. Unbiased stereology was used to assess protein product expression throughout the entire mPFC, and density counts were performed in subregions of the mPFC: the anterior cingulate cortex (AC), prelimbic cortex (PL) and infralimbic cortex (IL). We hypothesized that isolates would exhibit increases in aggressive behavior during exposure to a novel conspecific and reductions in mPFC activation produced by exposure to a novel conspecific compared to group reared rats.
2. Methods and Materials
2.1. Animals
Male (n = 40) and female (n = 40) Sprague-Dawley rats (Harlan; Indianapolis, IN) were purchased at postnatal day (P) 21 and were housed in standard Plexiglas cages either individually or in same-sex groups of 4 with food and water freely available in a 12:12 light:dark cycle. Isolated rats were exposed to the sight, sound, and smell of other rats in the colony room but were deprived of physical contact. Rats were weighed weekly but were not otherwise handled. Vaginal smears were not performed because regular handling during the isolation period is known to reduce the effects of isolation rearing [42, 43] and the stress of monitoring cycle status has been shown to increase adrenal weight and alter serotonin and HPA axis responses to subsequent stressor exposure [44]. Experimentation took place after 4 weeks of either isolation (ISO) or group (Group) rearing, between day P49 and P52, a period corresponding to late adolescence [26]. Rats were run in squads of 16 per day, counterbalanced by rearing condition, sex, and conspecific exposure. All experiments were conducted with protocols approved by the University of Colorado Animal Care and Use Committee.
2.2. Experimental procedures
2.2.1. Social Exposure Procedure
After 4 weeks of isolation or group rearing, rats from each group (n = 8 per group) were quasi-randomly chosen for social exposure, such that cages were randomly chosen for either social exposure or no exposure and all rats within a cage were exposed individually to a novel conspecific simultaneously. This was to ensure that rats within each cage were not deprived of social exposure or unduly disturbed by having cagemates removed. These rats were placed in a cage with a novel same-sex rat (conspecific) for 15 minutes. Slightly younger (P24 to P34), naïve, group housed rats were used as stimului in order to be less threatening to the experimental rats thus reducing aggressive behavior [45]. Exposure to novel conspecifics occurred in standard Plexiglas cages with a webcam above the cages recording each trial. Control rats were left in their home cages and all rats were sacrificed 90–100 min later after having been returned to their home cages. This time point was chosen as an optimal time point to measure the protein product of activity-dependent genes based on the time of peak protein expression following acute stimuli for the prototypical immediate-early gene, c-fos [46]. Behavior of the experimental rat was coded in a blinded fashion from the recordings using the following definitions:
Social Interaction
Overall time the experimental rat spends actively interacting (e.g. sniffing, following, grooming) with the novel conspecific [47].
Pinning
Standing over/holding down the novel conspecific while it is in a supine posture [48].
Chasing
Pursuit of the novel conspecific while it is running away from the experimental rat.
Aggressive Grooming
Vigorous grooming by the experimental rat of the novel conspecific when it is standing, crouching, supine, or trying to escape [48].
2.3. Tissue harvest
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with cold 0.9% saline followed by 4% paraformaldehyde in 0.01M PBS. Brains were postfixed for 4 hours in the same paraformaldehyde solution, cryoprotected in 30% sucrose for three days, then rapidly frozen in −30°C isopentane just prior to cryosectioning. Sections were taken (40 μm) through the prefrontal cortex using the atlas of Paxinos and Watson [49] from 3.2 mm to 2.2 mm anterior to bregma. Sections were stored at 4°C in cryoprotectant until immunohistochemistry was performed.
2.4. Immunohistochemistry
Immunohistochemistry was performed separately for Arc, c-fos, and BDNF labeled cells in the mPFC (Figure 1). Free-floating sections were first washed 3 times in 0.01 M PBS and between each subsequent step except as noted. Sections were incubated in 0.3% (Arc, BDNF) or 1% (c-fos) hydrogen peroxide, followed by 5% normal goat serum (NGS) and 0.25% Triton X in PBS. Sections were incubated overnight at RT (Arc, c-fos) or 48 hr at 4° C (BDNF) in primary antibodies directed against either Arc (rabbit anti-Arc, 1:3,000, Synaptic Systems), c-Fos (rabbit anti-Fos, 1:10,000, Santa Cruz Biotechnology), or BDNF (mouse anti-BDNF, 1:300, Calbiochem) in PBS with 5% NGS and 0.25% Triton X. Sections were then incubated in biotinylated goat anti-rabbit (Arc, c-fos) or anti-mouse (BDNF) secondary antibody (1:200, Jackson Labs) for 2 h, followed by incubation in avidin biotin complex (ABC kit, Vector Laboratories) for 2 h. Sections were washed 3 times in 0.1 M PB, then immunoreactivity was visualized with 3,3′-diaminobenzidine (DAB substrate kit, Vector Laboratories) and nickel ammonium sulphate as chromogens. Sections were mounted on slides using a 0.0015% gelatin solution, dehydrated using a series of ethanol solution, defatted using Histoclear (Sigma-Aldrich), and coverslipped with Permount (Sigma-Aldrich). Representative photomicrographs are shown in Fig. 1.
Figure 1.
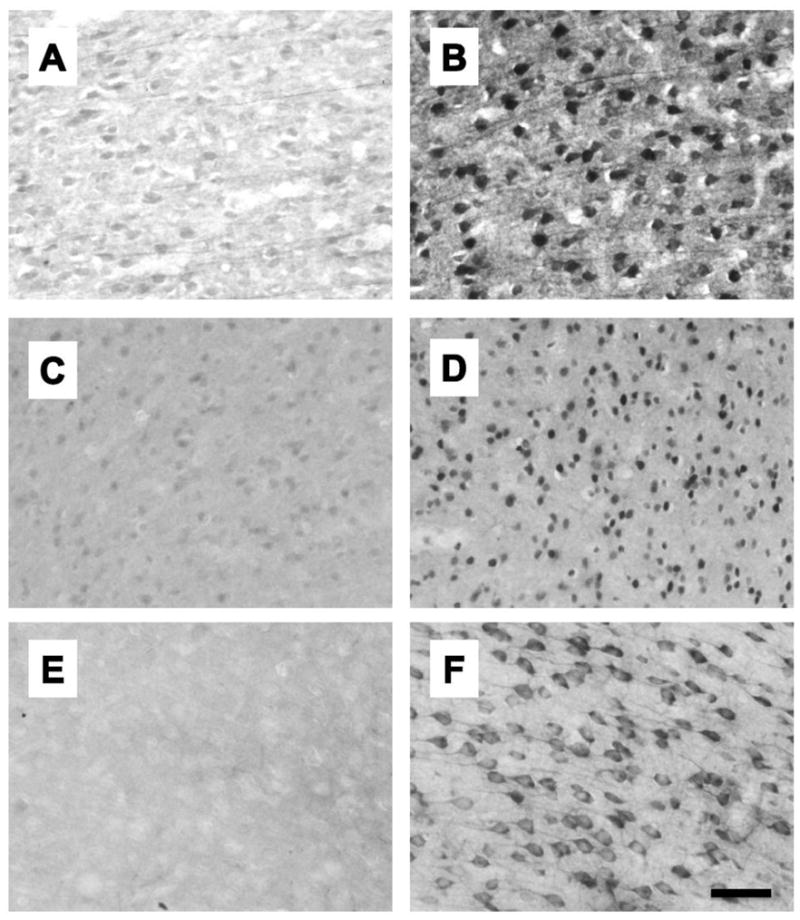
Representative photomicrographs showing immunohistochemistry for Arc (A, B), c-fos (C, D) and BDNF (E, F) from the AC of group reared males. A, C, and E are from rats that were not exposed to a novel conspecific and B, D, and F are from rats that were exposed to a novel conspecific.
2.5. Cell counts
Microscopy was performed using an Olympus BX51 microscope and VisioPharm software with the NewCAST module (VisioPharm, Hørsholm, Denmark). Unbiased stereology [50] was used to obtain estimates of total Arc, c-fos, and BDNF labeled cells in the mPFC. The boundaries of the mPFC and its subregions were determined using the rat brain atlas of Paxinos & Watson [51]. The mPFC was defined at 4x using the corpus callosum and the midline as shown in Fig. 2. The Cavalieri principal [50] was used to estimate mPFC volumes using a 92,886 μm2 point grid. Immunoreactive cells were counted using the optical dissector method, with a 1 μm guard zone. Random meander sampling was performed at 100x (oil) using sampling fractions of 1% and a 3,495 μm counting frame for Arc, c-fos, and BDNF. We chose to conduct density counts to determine cell numbers of immunoreactive cells within the subregions of the mPFC rather than stereology due to the lack of reliable landmarks to determine exact boundaries of the subregions with these antibodies. Immunolabeled cells in the anterior cingulate cortex (AC), the prelimbic cortex (PL), and the infralimbic cortex (IL) were counted by centering the field within each subregion at 10x using the corpus callosum as a guide, then shifting to a 40x objective; counts were performed within a 22,406 μm2 counting frame centered within the 40x field. This ensured that the counting frame was within the subregion of interest. Placement of the field of view for each subregion is shown in Fig 2. For each rat, 6–10 individual hemisphere measurements were assessed. For stereology, estimates of total numbers were calculated taking into account the sampling fraction, section thickness, and estimated surface volume, and was corrected for the number of hemispheres included. For density counts, mean values were calculated and expressed as mm2.
Figure 2.
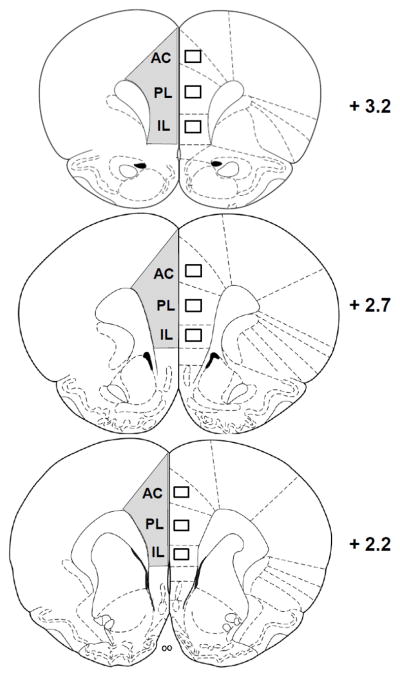
Regions within the mPFC assessed with stereology (gray area, left hemisphere), and density counts within the subregions AC, PL, and IL (right, boxes). Numbers indicate distance from bregma according to the atlas of Paxinos and Watson (1998).
2.6. Locomotor activity
In a separate experiment using different rats, we determined whether isolation rearing produced differences in locomotor activity in a familiar open field. The open field consisted of a conditioned place preference (CPP) apparatus each of which contains two separate rooms. Each room measures 30 cm × 30 cm × 30 cm and has black and white striped walls, either horizontally or vertically oriented. The apparatus is kept in a sound-proof room with dim lighting (20–25 lux) and masking noise produced by a small fan. Males and females were tested in parallel in separate apparatuses to keep odors separate, and each apparatus was washed with 70% alcohol between rats. Rats were tested in one side of the apparatus in a counterbalanced fashion. Rats were habituated to the apparatus for 10 min/day beginning on P49 for 5 days prior to testing. On test day rats were placed in the apparatus for 10 min. Distance traveled (meters) was recorded by Logitech Quickcam Pro 5000 webcams (Fremont, CA, USA) mounted 1.0 m above the center of the apparatus and interfaced with AnyMaze software (Stoelting Co., Wood Dale, IL, USA).
2.7. Statistics
Arc, c-fos, and BDNF were analyzed with 2 × 2 × 2 factorial ANOVAs with sex (female or male), rearing (group or ISO), and social cue (no social or social) as between-groups variables. Behavioral data were analyzed using 2 × 2 factorial ANOVAs with sex (female or male) and rearing (group or ISO) as between-groups variables. When significant interactions were obtained, Tukey’s HSD post-hoc tests were performed to determine differences between groups. Alpha was set at .05. In some cases we tested a priori hypotheses in the absence of significant interactions using Tukey’s HSD tests with alpha set at .01. Bivariate Pearson correlations were performed to determine relationships between IEG positive cell density within the mPFC subregions and behaviors, with alpha set at .05 (two-tailed). Statistical analyses were performed using SPSS (IBM SPSS Version 19, Chicago, IL).
3. Results
3.1. Stereology
3.1.1. mPFC volumes
There were no significant main effects of rearing, sex, or social cue on mPFC volumes, and there were no significant interactions. mPFC volumes are shown in Table 1.
Table 1.
Stereological estimates of mPFC volumes (mm3) of males and females after isolation or group rearing. Values are means and SEMs of 8 rats per group.
| Female
|
Male
|
|||
|---|---|---|---|---|
| Social | No social | Social | No social | |
|
|
||||
| Group reared | 3.387 ± 0.11 | 3.679 ± 0.12 | 3.494 ± 0.15 | 3.675 ± 0.08 |
| Isolation reared | 3.448 ± 0.22 | 3.462 ± 0.09 | 3.451 ± 0.11 | 3.539 ± 0.11 |
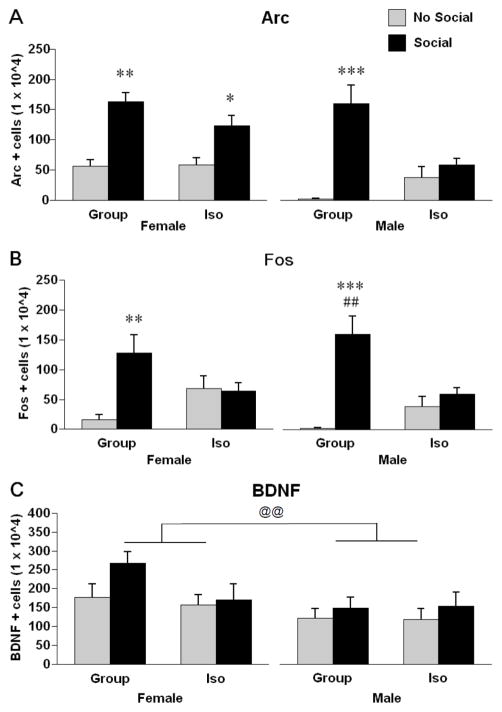
3.1.2. Arc
Exposure to a novel conspecific produced robust increases in the numbers of Arc positive cells in the mPFC, particularly in group-reared rats (Fig. 3A). There was a significant rearing X social cue interaction, F (1, 56) = 8.70, p < .01. Post hoc tests revealed that group-reared males and females, as well as isolation reared females (but not males), had increased numbers of Arc immunoreactive cells after exposure to a novel conspecific.
Figure 3.
Stereological estimates of cells positive for Arc (A), c-fos (B), and BDNF (C) immunoreactivity in the mPFC of male and female rats exposed to either group or isolation rearing. Rats then were subjected to either a 15 minute exposure to a novel same-sex conspecific (Social) or remained in their home cages (No Social). * p < .05, ** p < .01, *** p < .001, Significantly different from same sex, same rearing condition rats not exposed to a novel conspecific. ## p < .01, significantly different from same-sex, isolation reared rats exposed to a novel conspecific. @@ p < .01, significant difference between males and females. Values are means ± SEMs.
3.1.3. Fos
Exposure to a novel conspecific produced robust increases in the numbers of Fos positive cells in the mPFC, but only in group-reared rats (Fig. 3B). There was a significant rearing X social cue interaction, F (1, 56) = 22.07, p < .0001. Post hoc tests revealed that group-reared rats, but not isolation-reared rats, had increased numbers of c-fos positive cells after exposure to a novel conspecific.
3.1.4. BDNF
Exposure to a novel conspecific produced only modest increases in the numbers of BDNF positive cells in the mPFC. However, the numbers of BDNF positive cells depended on the sex of the animal (Fig. 3C). There was a significant main effect of sex on numbers of BDNF positive cells in the mPFC, F (1, 49) = 6.41, p = .01; females had greater numbers of BDNF positive cells. There was no significant main effect of social cue on numbers of BDNF positive cells in the mPFC but there was a trend, F (1, 49) = 3.24, p = .07; rats that had been exposed to a novel conspecific exhibited slightly increased numbers of BDNF positive cells. There were no significant interactions. The smaller numbers of animals in this experiment is due to damage to some of the tissue during processing.
3.2. Density counts in mPFC subregions
3.2.1. Arc
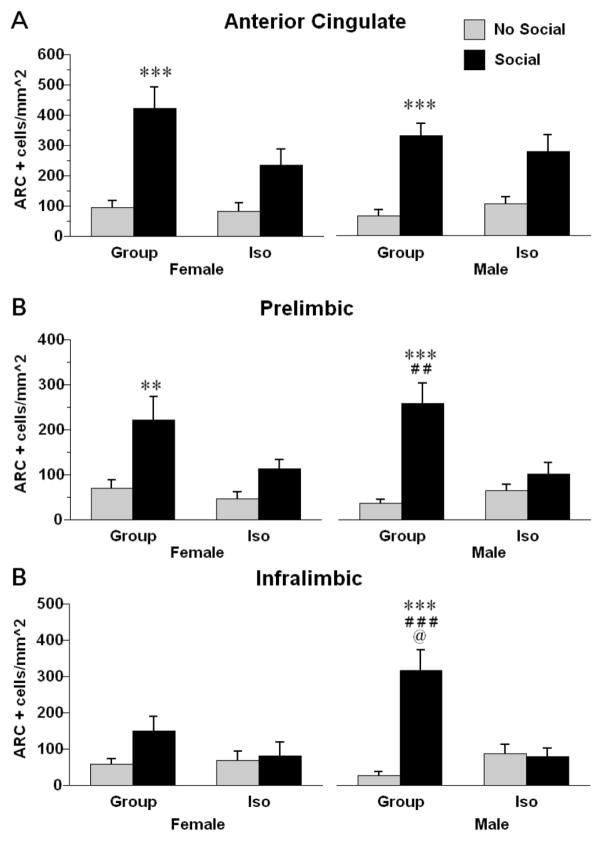
Exposure to a novel conspecific increased the density of Arc positive cells in the mPFC subregions in a rearing and sex-dependent manner. In the AC (Fig. 4A), there was a significant rearing X social cue interaction, F (1, 56) = 5.5.69, p < .05. Post hoc tests revealed that male and female group reared rats that had been exposed to a novel conspecific had greater levels of Arc protein expression than no-social controls. In the PL (Fig. 4B), there was a significant rearing X social cue interaction, F (1, 56) = 12.65, p < .001. Post hoc tests revealed that group reared rats had greater increases in Arc after exposure to a novel conspecific than isolation reared rats. In the IL (Fig. 4C), there was a significant rearing X social cue interaction, F (1, 56) = 18.78, p < .0001. Post hoc tests revealed that group reared rats had greater increases in Arc after exposure to a novel conspecific than isolation reared rats. In the IL there was a significant sex X rearing X social cue interaction, F (1, 56) = 6.018, p < .05. Post-hoc tests indicated that male group reared rats exposed to a novel conspecific had significantly greater levels of Arc expression than all other groups.
Figure 4.
Density counts of cells positive for Arc immunoreactivity in the AC (A), PL (B), or IL (C) subregions of the mPFC of male and female rats exposed to either group or isolation rearing (means . Rats then were subjected to either a 15 minute exposure to a novel same-sex conspecific (Social) or remained in their home cages (No Social). ** p < .01, *** p < .001, Significantly different from same sex, same rearing condition rats not exposed to a novel conspecific. ## p < .01, significantly different from same-sex, isolation reared rats exposed to a novel conspecific. @ p < .05, significantly different from female group-reared rats exposed to a novel conspecific. Values are means ± SEMs.
3.2.2. c-Fos
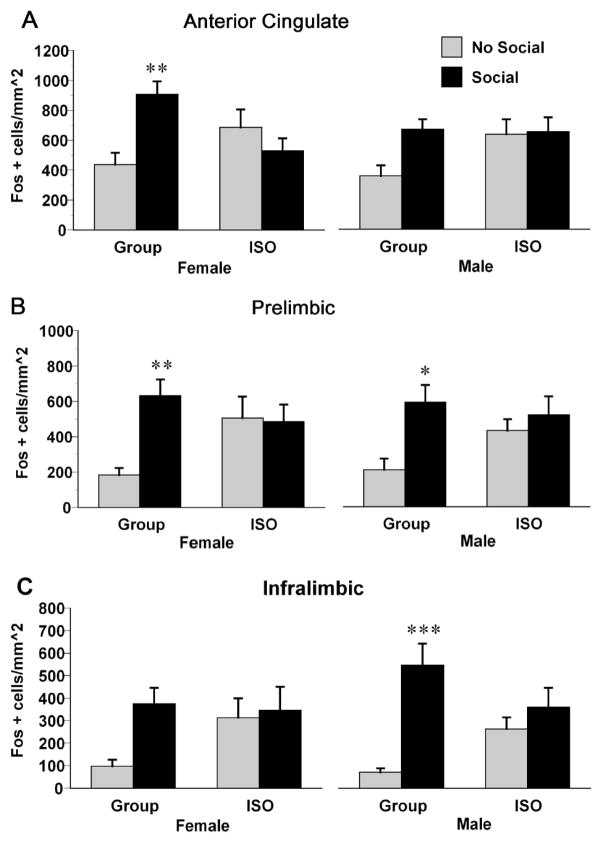
Exposure to a novel conspecific increased the density of c-fos positive cells in the mPFC subregions, but only in group-reared rats. In the AC (Fig. 5A), there was a significant rearing X social cue interaction, F (1, 55) = 15.83, p < .001; post-hoc tests revealed that group reared rats had greater increases in c-fos than isolation-reared rats but a priori tests confirmed that this reached significance only in females (p < .01) and not males (p = .19). In the PL (Fig. 5B) there was a significant rearing X social cue interaction, F (1, 55) = 12.27, p < .001; post-hoc tests revealed that group reared rats had greater increases in c-fos than isolation-reared rats. In the IL (Fig. 5C) there was a significant rearing X social cue interaction, F (1, 55) = 9.26, p < .01; post-hoc tests revealed that group reared rats had greater increases in c-fos in the IL than isolation-reared rats but a priori tests confirmed that this reached significance only in males (p < .10) but not females (p = .15). There were no significant differences between isolation-reared rats not exposed to a social cue relative to group-reared rats not exposed to a social cue.
Figure 5.
Density counts of cells positive for c-fos immunoreactivity in the AC (A), PL (B), or IL (C) subregions of the mPFC of male and female rats exposed to either group or isolation rearing. Rats then were subjected to either a 15 minute exposure to a novel conspecific (Social) or remained in their home cages (No Social). ** p < .01, *** p < .001, significantly different from same sex, same rearing condition rats not exposed to a novel conspecific. ## p < .01, significantly different from same-sex, isolation reared rats exposed to a novel conspecific. Values are means ± SEMs.
3.2.3. BDNF
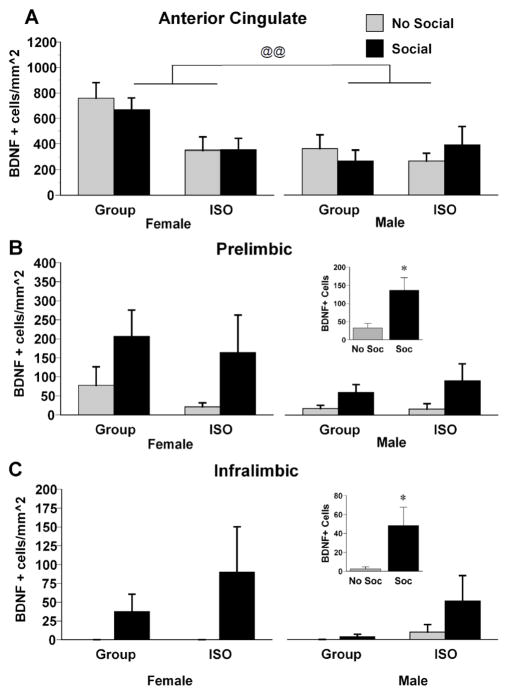
Exposure to a novel conspecific increased the density of BDNF positive cells only in the PL and IL, while greater densities of BDNF positive cells were observed in females than in males in the AC. In the AC (Fig. 6A), there was a significant main effect of Sex, F (1, 49) = 10.00, p < .01. Females had more BDNF positive cells in the AC than males. There was a trend for a significant sex X rearing interaction, F (1, 49) = 3.32, p = .07, reflecting a greater number of BDNF positive cells in group-reared females. In the PL (Fig. 6B), there was a significant main effect of social cue, F (1, 49) = 6.10, p < .05; there were more BDNF positive cells in the PL in rats that had been exposed to a novel conspecific. There was also a near-significant trend toward a main effect of sex in the PL, F (1, 49) = 3.69, p = .06, reflecting a greater number of BDNF positive cells in the PL of females. In the IL (Fig. 6C), there was a significant main effect of social cue, F (1, 49) = 5.03, p < .05. There were more BDNF positive cells in the IL in rats that had been exposed to a novel conspecific. Some groups (female and male group reared, and female ISO) that were not exposed to a social cue had no BDNF positive cells in the IL.
Figure 6.
Density counts of cells positive for BDNF immunoreactivity in the AC (A), PL (B), or IL (C) subregions of the mPFC of male and female rats exposed to either group or isolation rearing. Rats then were subjected to either a 15 minute exposure to a novel same-sex conspecific (Social) or remained in their home cages (No Social). * p < .05, significantly different from rats not exposed to a novel conspecific. @@ p < .01, significant difference between males and females. Values are means ± SEMs.
3.3. Social behavior
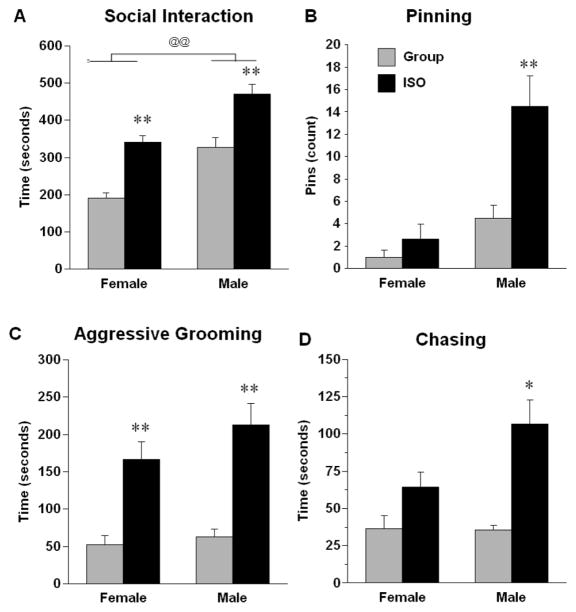
Adolescent social isolation produced an increase in time spent interacting with a novel conspecific and increased aggressive behaviors. For total time spent in social interaction (Fig. 7A), there was a main effect of sex, F (1, 28) = 36.25, p < .0001; males spent more time in social interactions than females. There was also a main effect of rearing on social interaction, F (1, 28) = 44.34, p < .0001; ISO rats spent more time in social interactions with a novel conspecific than group reared rats. For pinning (Fig. 7B), there was a significant main effect of rearing, F (1, 28) = 11.55, p < .01; ISO rats pinned more than group-reared rats. There was also a significant sex x rearing interaction, F (1, 28) = 6.99, p = .01; post hoc tests indicated that ISO males pinned significantly more than all other groups. For aggressive grooming (Fig. 7C), there was a main effect of rearing F (1, 28) = 42.43, p < .0001; ISO rats spent more time in aggressive grooming of a novel conspecific than group reared rats. For chasing (Fig. 7D), there was a main effect of rearing, F (1, 28) = 22.31, p < .0001; ISO rats spent more time chasing a novel conspecific than group reared rats. There were also strong trends for sex F (1, 28) = 3.92, p = .058 and for a sex x rearing interaction, F (1, 28) = 4.15, p = .051; post hoc tests indicated that ISO males spent more time chasing the novel conspecific than all other groups.
Figure 7.
Behaviors of male and female rats that had experienced either group or isolation rearing during 15 minutes of exposure to a novel same-sex conspecific. * p < .05, ** p < .01, significantly different from same sex, same rearing condition rats not exposed to a novel conspecific. @@ p < .01, significant difference between males and females. Values are means ± SEMs.
3.4. Correlations between behavior and expression of IEG protein products
We assessed relationships between density counts of Arc, c-fos, and BDNF in the AC, PL, and IL and the behaviors observed. Several significant negative correlations were observed that were dependent on sex (Table 2). Negative correlations for all IEGs in the AC and social interaction were significant for females, while no interactions with IEGs and social interaction were significant for males. Significant negative correlations between Arc and aggressive grooming, chasing, and pinning were observed only in males and were primarily in ventral subdivisions. These findings further support the dorsal/ventral sexual dimorphism observed for Arc immunoreactive cells in subregions of the mPFC described here, and suggest divergent roles for dorsal and ventral regions in these different behaviors for males and females.
Table 2.
Correlations between density counts of cells immunoreactive for IEG protein products within subregions of the mPFC and behaviors of male and female rats exposed to a novel conspecific.
| Subregion | Female
|
Male
|
|||||
|---|---|---|---|---|---|---|---|
| Arc | c-Fos | BDNF | Arc | c-Fos | BDNF | ||
|
|
|||||||
| Social Interaction | AC | −0.611a | −0.518a | −0.615a | −0.401 | 0.292 | 0.081 |
| PL | −0.529a | −0.171 | −0.258 | −0.39 | 0.096 | −0.153 | |
| IL | −0.13 | 0.078 | 0.107 | −0.351 | 0.028 | −0.075 | |
|
| |||||||
| Aggressive Grooming | AC | −0.405 | −0.423 | −0.317 | −0.46 | 0.266 | −0.021 |
| PL | −0.306 | −0.288 | −0.172 | −0.601a | 0.047 | −0.067 | |
| IL | 0.071 | −0.023 | 0.159 | −0.653a | −0.149 | 0.129 | |
|
| |||||||
| Chasing | AC | −0.229 | −0.521a | −0.325 | −0.497 | 0.108 | −0.009 |
| PL | −0.400 | −0.495 | −0.160 | −0.572a | 0.099 | −0.188 | |
| IL | −0.084 | −0.267 | 0.065 | −0.614a | −0.088 | −0.076 | |
|
| |||||||
| Pinning | AC | −0.579a | −0.013 | −0.154 | −0.565a | 0.127 | 0.148 |
| PL | −0.423 | 0.268 | −0.421 | −0.515a | 0.281 | −0.348 | |
| IL | 0.253 | 0.463 | −0.211 | −0.514a | 0.159 | −0.034 | |
p < .05
3.5. Locomotor activation
There was a significant main effect of sex on distance traveled in the familiar apparatus F(1,28) = 4.165, p = .05; females traveled a significantly greater distance than males (35.96 ± 1.81 and 31.58 ± 1.16 meters, respectively). There was no main effect of rearing condition in distance traveled (34.14 ± 1.81 and 33.40 ± 1.40 meters, respectively) and no rearing by sex interaction.
4. Discussion
The present results suggest that isolation rearing has profound effects on mPFC IEG expression and social behavior. Exposure to a novel conspecific produced robust increases in Arc and c-fos protein expression throughout the mPFC in group-reared male and female rats, but consistent with our hypothesis this increase was blunted after adolescent social isolation. We also observed a slight but significant increase in BDNF protein expression in response to a novel conspecific that did not depend on rearing condition. Subregion-specific sex differences in Arc and BDNF in group-reared rats were also observed. In contrast to reports of reduced mPFC volume after 7 or 8 weeks of isolation rearing [31, 32], no reductions in PFC volumes were observed. Isolation rearing produced increases in social interaction with a novel conspecific in both males and females, and this was accounted for by increases in aggressive behavior. Negative correlations were observed between some IEG protein products and behaviors, in a sex and subregion specific manner.
4.1. Effects of exposure to a novel conspecific on Arc in the mPFC
Robust effects of social interaction-induced Arc expression in the mPFC were dependent on rearing condition and sex in a subregion-dependent fashion. Stereological estimates revealed that exposure to a novel conspecific produced substantial increases in Arc protein expression in the mPFC in group-reared male and female rats. This increase was not observed in rats that had been exposed to adolescent social isolation. Further examination into the subregions of the mPFC revealed a sex difference in Arc activation in group-reared rats that was subregion specific and consisted of a dorsal-ventral gradient of activation that was opposite in males and females. Thus in group-reared females, social interaction-induced Arc activation was most robust in the AC, less so in the PL, and was not significantly increased in the IL. In males, this trend was reversed such that Arc activation was most robust in the IL, less so in the PL, and still less (though still significant) in the AC. Indeed, social interaction-induced Arc levels in the IL of male group-reared rats was significantly greater than those of group-reared females. The subregions of mPFC project differentially to a number of subcortical regions. For example, AC and PL project preferentially to the dorsolateral and dorsomedial striatum, respectively [52, 53]. Differences in projection of the ventral aspect of the mPFC include projections from PL to the basolateral amygdala, nucleus accumbens, ventral tegmental area and raphe nuclei [52–54], and IL to visceromotor regions such as the nucleus of the solitary tract and the parabrachial nucleus [52, 54]. IL also projects to the intercalated cells of the amygdala [55], a region responsible for inhibiting the basolateral nucleus of the amygdala. It may be that males and females differ in the extent that these regions interact during novel social interactions.
Social interaction-induced Arc activation was not significantly increased in any individual subregions in isolation-reared male or female rats. There was a significant increase in estimated Arc numbers using stereology in female isolates, but density counts did not reveal an increase in this group. This inconsistency may be due to the combined effect of slightly increased Arc density levels in all of the mPFC subregions in female isolates after exposure to a novel conspecific. It appears that Arc activation was greatest in the AC and it is likely that AC numbers drive the stereology counts as the density of immunoreactive cells is the greatest in that subregion. Another possibility is that different distributions of Arc positive cells in the middle layers (III–V) of the mPFC, where the density counts were performed, may be somewhat different than those in the deep and superficial layers, which were included in the stereology sampling. These results suggest that activation of Arc in the mPFC may be involved in the normal social interactions with a novel conspecific, at least during late adolescence. The expression of Arc mRNA has been shown to increase in the mPFC of adolescent and adult male rats after social defeat stress [56]. However, Arc mRNA was not increased in the PL of rats after social interaction with a cagemate after a 24 hr separation [57]. The present findings are the first to explore the impact of non-aggressive social interactions with novel conspecifics, which are presumably much less stressful. This is especially likely inasmuch as younger, smaller stimulus rats were used in the present study, in part to exclude this possibility. To the best of our knowledge this is the first demonstration of attenuation of experience-dependent Arc protein expression in the mPFC of isolation-reared rats, although it has previously been reported that constitutive levels of mRNA for Arc and other IEGs are reduced in the PFC of isolation-reared male rats [33]. This suppression of experience-dependent Arc expression may underlie the structural deficits in the mPFC of isolation-reared rats observed by others [21, 22, 31, 32].
4.2. Effects of rearing condition on social interaction-induced c-fos expression in the mPFC
The increases in social interaction-induced c-fos expression in the mPFC depend largely on rearing condition, in that the increases observed in group-reared rats were completely absent in isolation reared rats. Similar though slightly different patterns of activation were observed for c-fos as for Arc throughout the mPFC. Stereological estimates of total c-fos numbers in the mPFC showed that as with Arc, there was a robust social interaction-induced increase in both male and female group reared rats. In contrast to the modest increase in Arc in isolation-reared females, there was no increase in numbers of c-fos labeled cells in the mPFC of male or female isolates. Consistent with this there were no increases in the density of c-fos labeled cells in any subregion of the mPFC in isolates exposed to a novel conspecific. Similarly to Arc, a dorsal-ventral gradient in the response of male and female group-reared rats to a novel conspecific was observed. Thus, females had robust activation in the dorsal subregions (AC and PL) but non-significant activation of the IL. In contrast, males exhibited non-significant increases in the AC, somewhat greater increases in the PL, and robust increases in the IL. We have previously observed sex differences in c-fos activation in response to stress [58]. In that study, c-fos mRNA expression in females, as assessed with in situ hybridization, was less than that of males in all of the mPFC subregions immediately after a session of inescapable stress [58]. This discrepancy suggests differential dependence on the mPFC subregions depending on the stressfulness of the stimulus, but may also reflect differences in assessing protein versus mRNA expression. In accordance with the latter interpretation, Stack et al. [59] have observed that in female rats exposed to social interaction expression of the immediate early gene zif268 is less than that of males when assessed with in situ hybridization. It is important to note that in the study of Stack et al. [59], downregulation of zif268 in the mPFC of males abolished sex differences in social interaction [59]. However, other immediate early genes may contribute to the regulation of social behavior.
There was a notable (though not statistically significant) increase in the density of c-fos (and to a lesser extent, Arc) positive cells in mPFC subregions of isolation-reared rats that were not exposed to a novel conspecific, especially females. One possible reason for this is an increase in locomotor reactivity produced by isolation rearing which has been reported by others [18, 60]. However, it has been reported that this hyperlocomotion is strain-specific and does not occur in Sprague-Dawley rats [12, 61], those used in the present study. Indeed, here we did not observe differences in locomotor activity produced by differential rearing. Interestingly, we have observed that ISO rats appear more alert, though not more active, when we enter the vivarium during the light phase (Bland et al., unpublished observations). In line with this, isolation rearing has been shown to dysregulate the diurnal rhythmicity of a number of markers including circulating corticosterone [62], prolactin [62–64], and testosterone [64] in male rats; to the best of our knowledge the effect of isolation rearing on diurnal rhythms of female rats is unknown. In the mPFC and other regions, c-fos expression shows a diurnal pattern, such that expression is increased during the dark cycle [65], suggesting the possibility that altered circadian drive is responsible for the elevations in c-fos expression in ISO rats that were not exposed to a social cue. In a related vein, this elevation of c-fos expression may reflect the chronic stress of isolation rearing (which may in turn be responsible for the observed changes in diurnal rhythms [62]). Although beyond the scope of the present study, this important issue is worthy of further investigation.
4.3. Effects of rearing condition on social interaction-induced BDNF expression in the mPFC
The modest effects of social interaction on BDNF expression in the mPFC were primarily in the ventral subregions, with no impact of rearing condition, while a robust effect of sex on BDNF expression was largely dorsal. Exposure to a novel conspecific produced much smaller increases in BDNF than Arc or c-fos expression in the mPFC. Stereological estimates of the entire mPFC revealed a small increase, and results of the subregion assessment indicated that this reflected increases preferentially in the PL and IL, primarily in females. Consistent with this we have previously observed sex differences in constitutive expression of BDNF mRNA in the mPFC in the same direction [58]. The single time point was chosen to be optimal for expression of c-fos protein, and the induction of Arc follows a similar time course [66]. This may not be the optimal time point for BDNF protein expression; however, rapid induction of BDNF mRNA has been observed as early as 15 minutes [41], 20 minutes [40], and 30 minutes [39]. We did not observe an effect of rearing condition on constitutive BDNF expression, consistent with the results of Scaccianoce et al. [67], but in contrast to the findings of Meng et al. [68] in which increases in BDNF protein expression were observed in the mPFC of isolation-reared rats. An important difference is that in the study of Meng et al. [68], rats were isolation-reared for 4 weeks, then group housed for an additional 4 weeks, thus in that study BDNF may have increased as a result of rehousing.
4.4. Effects of isolation rearing on social behavior
Isolation rearing increased social interaction and aggressive grooming in both male and female rats. Females exhibited less overall social interaction, consistent with several other reports [69, 70], regardless of rearing condition. In concordance with this, females also engaged in a lesser amount of the specific behaviors examined. However, the finding of robust increases in social interaction in both male and female rats after isolation rearing is in contrast to the findings of Ferdman et al [21] in which males, but not females, engaged in increased social interaction after isolation rearing. Numerous methodological details might affect the impact of isolation rearing on social interaction, such as the stressfulness of the apparatus, the length of the isolation period, and the age of the animals when tested (here testing occurred in late adolescence), and these may account for the different results of the present study and that of Ferdman et al. [21] and Hermes et al. [22]. There were also large increases in aggressive grooming in both male and female isolation-reared rats. To the best of our knowledge, this is the first report of increased aggressive behavior in females after isolation rearing, and has been replicated in our laboratory in a separate experiment (data not shown). Aggressive grooming is associated with increased corticosterone and adrenal hypertrophy [48], suggesting that isolates were more stressed by the novel conspecific than were group reared rats. It will be important in future studies to assess social cue-induced corticosterone levels after isolation rearing to address this issue. Male, but not female, isolates engaged in more pinning than group-reared males. Pinning is an aggressive play behavior that peaks during mid-adolescence and is more common in males than females during this period [71]. One interpretation is that isolation rearing results in a developmental delay such that even at near-adulthood, rats in the present study were still exhibiting the behavioral traits of early adolescence.
4.5. Relationships between IEG expression in the mPFC and social behavior
Arc expression was most strongly related to social behavior of the IEGs investigated. The negative relationships observed reflected the greater activation of IEGs in group-reared rats, which tended to engage in lesser amounts of the behaviors assessed. Unique patterns of relationships were observed for males and females. In females, all IEGs in the AC were negatively related to social interaction, and this suggests preferential reliance on this subregion during social behavior, perhaps to suppress inappropriate behavior by exerting effects on projection regions such as the striatum. For males, the finding of negative relationships between Arc and c-fos in the ventral subregions of the mPFC during aggressive grooming and chasing may reflect a greater reliance on PFC-limbic region interactions in the suppression of inappropriate social behavior.
Isolation rearing is typically viewed as a stressor and it clearly represents a kind of early adversity. However, an alternative view is that isolation rearing deprives adolescent rats of the daily stresses of living in a complex social environment, experiences that might be considered examples of both distress and eustress. Adolescents learn to master their environment (and conversely, must learn to cope when they cannot master it) when engaging in social play and when determining dominance hierarchies. Thus, it is likely that during adolescent play behavior and other social experiences individuals are learning about control, which is known to require the mPFC [72]. If so, additional social experiences, even after a lengthy isolation period, might reverse the changes in mPFC function observed here. Alternatively, there may be a critical period early in adolescence during which these social experiences must take place.
In considering the increased social interaction and aggressive behavior produced by isolation rearing in the present study, it is important to note that ISO rats remained in isolation throughout the testing period, thus it is unknown whether these effects were due to chronic or acute social isolation. Several groups have investigated the reversibility of the effects of isolation rearing on a number of outcomes. One report demonstrated that shorter periods of isolation (4 to 7 days) increased social interaction in male rats, but aggressive behavior increased only when isolates were tested with other isolates and not with group housed rats [73]. Several studies have investigated the effects of resocialization on measures of anxiety, which is an important mediator of social interaction [47]. One effect of isolation rearing, increased latency to emerge into a novel open field, was reversed if the rats were resocialized after the adolescent social isolation [10]. Similarly, isolation rearing-induced increases in locomotion and pain sensitivity were also reversed by resocialization [74]. However, resocialization did not reverse isolation rearing-induced increases in anxiety on the elevated X maze [75]. Less is known of the effects of resocialization after isolation rearing on social behavior. Thus, this remains an important question and future studies will address this issue.
In the present experiment socially exposed rats were exposed to a new, clean cage (identical to their standard housing cages) in a different room in addition to a novel conspecific. Thus environmental novelty alone, rather than a novel social experience, may have contributed to the increased IEG expression observed in our group-reared rats. However, the primary finding of blunted activation in the mPFC of isolates is still of considerable interest, although it may generalize to hypofrontality in response to diverse novel experiences including novel social interaction. It is also important to note that estrous phase, which can affect mPFC function and behavior, was not assessed. However, it has been previously demonstrated that neither social interaction nor social interaction-induced zif268 in the mPFC are dependent on stage of estrous in the rat [69]. We have previously observed estrous cycle related variability in tailshock stress-induced c-fos mRNA in the mPFC such that estrus and proestrus, but not diestrus females had blunted stress-induced c-fos mRNA relative to males, however, females in the estrous stage groups did not differ significantly from each other for either c-fos or BDNF mRNA (Bland et al, 2005). It remains unknown what impact, if any, ovarian hormones may have had on the present results. In accordance with McCarthy et al. [76], this phase of our investigation merely sought to determine a sex difference, independent of estrous cycle. Future studies will address these possibilities.
In conclusion, novel social interaction induced large increases in Arc and c-fos protein expression in the mPFC of group reared rats, and isolation rearing produced marked alterations in IEG expression the mPFC as reflected by blunted social cue-induced Arc and c-fos activation. Arc in particular appears to be inversely related to social behaviors, and may have a sex- and subregion-dependent role in the suppression of inappropriate behavior. Because isolation rearing is a widely used model of early adversity and its potential for increasing the vulnerability to psychopathologies such as anxiety, depression, and psychosis, these results have implications for the important role of mPFC function in social deficits associated with these disorders.
Highlights.
Increased aggressive behaviors were observed in isolation-reared males and females.
Social interaction increased c-fos, Arc, and BDNF in the prefrontal cortex (PFC) in group-reared rats.
Isolation rearing eliminated the increases in c-fos and Arc protein in the PFC.
Sex differences in Arc and BDNF were observed in subregions of the PFC.
Negative correlations were observed between social behaviors and expression of PFC c-fos, Arc, and BDNF in a sex and subregion specific manner.
Acknowledgments
We would like to thank Natalie Foster and Imane Benjelloun for their excellent technical assistance. Supported by a NARSAD Young Investigator Award (STB) and NIH grant R03DA029673 (STB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLaughlin KA, et al. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) III: associations with functional impairment related to DSM-IV disorders. Psychol Med. 2010;40(5):847–59. doi: 10.1017/S0033291709991115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green JG, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner HA, Finkelhor D, Ormrod R. The effect of lifetime victimization on the mental health of children and adolescents. Soc Sci Med. 2006;62(1):13–27. doi: 10.1016/j.socscimed.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Gallucci WT, et al. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 1993;12(5):420–5. doi: 10.1037//0278-6133.12.5.420. [DOI] [PubMed] [Google Scholar]
- 5.Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–33. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 7.Bruce SE, et al. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 2005;162(6):1179–87. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean CP, et al. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45(8):1027–35. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veijola J, et al. Sex differences in the association between childhood experiences and adult depression. Psychol Med. 1998;28(1):21–7. doi: 10.1017/s0033291797006089. [DOI] [PubMed] [Google Scholar]
- 10.Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10(2):123–32. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- 11.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12(1–2):129–62. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 12.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(6):1087–102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Lukkes JL, et al. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 15.Lapiz MD, et al. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33(1):13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- 16.Serra M, et al. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress. 2005;8(4):259–64. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 18.Weiss IC, et al. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152(2):279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75(1–2):27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- 20.Vale AL, Montgomery AM. Social interaction: responses to chlordiazepoxide and the loss of isolation-reared effects with paired-housing. Psychopharmacology (Berl) 1997;133(2):127–32. doi: 10.1007/s002130050382. [DOI] [PubMed] [Google Scholar]
- 21.Ferdman N, et al. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180(2):174–82. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Hermes G, et al. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104(2):354–9. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Goldwater DS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164(2):798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 52(3):244–53. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- 26.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 28.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15(5–6):341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Avale ME, et al. Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 2011;25(7):2145–55. doi: 10.1096/fj.10-178558. [DOI] [PubMed] [Google Scholar]
- 30.Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30(5):944–57. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- 31.Schubert MI, et al. Effects of social isolation rearing on the limbic brain: a combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience. 2009;159(1):21–30. doi: 10.1016/j.neuroscience.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Day-Wilson KM, et al. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141(3):1113–21. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 33.Levine JB, et al. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145(1):42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 34.Bell HC, Pellis SM, Kolb B. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res. 2010;207(1):7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–85. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 36.Pinaud R. Experience-dependent immediate early gene expression in the adult central nervous system: evidence from enriched-environment studies. Int J Neurosci. 2004;114(3):321–33. doi: 10.1080/00207450490264142. [DOI] [PubMed] [Google Scholar]
- 37.Kozlovsky N, et al. The immediate early gene Arc is associated with behavioral resilience to stress exposure in an animal model of posttraumatic stress disorder. Eur Neuropsychopharmacol. 2008;18(2):107–16. doi: 10.1016/j.euroneuro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5(11):1177–84. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 39.Ernfors P, et al. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7(1):165–76. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 40.Gall CM. Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp Neurol. 1993;124(1):150–66. doi: 10.1006/exnr.1993.1186. [DOI] [PubMed] [Google Scholar]
- 41.Lauterborn JC, et al. Differential effects of protein synthesis inhibition on the activity-dependent expression of BDNF transcripts: evidence for immediate-early gene responses from specific promoters. J Neurosci. 1996;16(23):7428–36. doi: 10.1523/JNEUROSCI.16-23-07428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krebs-Thomson K, et al. Post-weaning handling attenuates isolation-rearing induced disruptions of prepulse inhibition in rats. Behav Brain Res. 2001;120(2):221–4. doi: 10.1016/s0166-4328(00)00374-0. [DOI] [PubMed] [Google Scholar]
- 43.Rosa ML, et al. Routine post-weaning handling of rats prevents isolation rearing-induced deficit in prepulse inhibition. Braz J Med Biol Res. 2005;38(11):1691–6. doi: 10.1590/s0100-879x2005001100018. [DOI] [PubMed] [Google Scholar]
- 44.Sfikakis A, et al. Stress through handling for vaginal screening, serotonin, and ACTH response to ether. Pharmacol Biochem Behav. 1996;53(4):965–70. doi: 10.1016/0091-3057(95)02089-6. [DOI] [PubMed] [Google Scholar]
- 45.Christianson JP, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193(1):87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20(6):665–72. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 47.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 48.Hurst JL, et al. Housing and welfare in laboratory rats: effects of cage stocking density and behavioural predictors of welfare. Anim Behav. 1999;58(3):563–586. doi: 10.1006/anbe.1999.1165. [DOI] [PubMed] [Google Scholar]
- 49.Paxinos G, Watson C. The rat Brain in stereotaxic coordinates. London: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 50.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. p. xxvi. [237] of plates. [Google Scholar]
- 52.Gabbott PL, Bacon SJ. Calcineurin immunoreactivity in prelimbic cortex (area 32) of the rat. Brain Res. 1997;747(2):352–6. doi: 10.1016/s0006-8993(96)01376-5. [DOI] [PubMed] [Google Scholar]
- 53.Sesack SR, et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 54.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 55.Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coppens CM, et al. Social Defeat during Adolescence and Adulthood Differentially Induce BDNF-Regulated Immediate Early Genes. Front Behav Neurosci. 2011;5:72. doi: 10.3389/fnbeh.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton DA, et al. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res. 2010;214(1):66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bland ST, et al. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051(1–2):90–9. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 59.Stack A, et al. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2009;35(2):570–80. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heidbreder CA, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100(4):749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 61.Lim AL, Taylor DA, Malone DT. A two-hit model: behavioural investigation of the effect of combined neonatal MK-801 administration and isolation rearing in the rat. J Psychopharmacol. doi: 10.1177/0269881111430751. [DOI] [PubMed] [Google Scholar]
- 62.Perello M, et al. Effect of social isolation on 24-h pattern of stress hormones and leptin in rats. Life Sci. 2006;78(16):1857–62. doi: 10.1016/j.lfs.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 63.Esquifino AI, et al. 24-hour pattern of circulating prolactin and growth hormone levels and submaxillary lymph node immune responses in growing male rats subjected to social isolation. Endocrine. 2004;25(1):41–8. doi: 10.1385/ENDO:25:1:41. [DOI] [PubMed] [Google Scholar]
- 64.Esquifino AI, et al. d24-hour changes in circulating prolactin, follicle-stimulating hormone, luteinizing hormone and testosterone in male rats subjected to social isolation. J Circadian Rhythms. 2004;2(1):1. doi: 10.1186/1740-3391-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144(1):344–55. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 66.Guzowski JF, et al. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21(14):5089–98. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scaccianoce S, et al. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res. 2006;168(2):323–5. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 68.Meng Q, et al. Effects of adolescent social isolation on the expression of brain-derived neurotrophic factors in the forebrain. Eur J Pharmacol. 2011;650(1):229–32. doi: 10.1016/j.ejphar.2010.09.061. [DOI] [PubMed] [Google Scholar]
- 69.Stack A, et al. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35(2):570–80. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49(2):245–50. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 71.Pellis SM, V, Pellis C. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23(3):215–31. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- 72.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8(3):365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 73.Niesink RJ, van Ree JM. Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol Behav. 1982;29(5):819–25. doi: 10.1016/0031-9384(82)90331-6. [DOI] [PubMed] [Google Scholar]
- 74.Gentsch C, et al. Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and reversed by resocialization. Physiol Behav. 1988;43(1):13–6. doi: 10.1016/0031-9384(88)90091-1. [DOI] [PubMed] [Google Scholar]
- 75.Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50(6):1129–32. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- 76.McCarthy MM, et al. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–7. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]