Abstract
It has been shown that genetic inhibition of p53 leads to enhanced proliferation of hematopoietic stem cells (HSCs). This could, in theory, contribute to the increased frequency of tumor development observed in p53-defcient mice and humans. In our previous work, we identified chemical p53 inhibitors (PFTs) that suppress the transactivation function of p53 and protect cultured cells and mice from death induced by gamma irradiation (IR). Here we found that when applied to bone marrow cells in vitro or injected into mice, PFTβ impeded IR-induced reduction of hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) population sizes. In addition, we showed that PFTβ stimulated HSC and HPC proliferation in the absence of IR in vitro and in vivo and mobilized HSCs to the peripheral blood. Importantly, however, PFTβ treatment did not affect the timing or frequency of tumor development in irradiated p53 heterozygous mice used as a model for determination of carcinogenicity. Thus, although PFTβ administration led to increased numbers of HSCs and HPCs, it was not carcinogenic in mice. These findings suggest that chemical p53 inhibitors may be clinically useful as safe and effective stimulators of hematopoiesis.
Keywords: p53 inhibitor, hematopoietic stem cells, cancer, lymphoma, sarcoma, radiation
Introduction
p53 is a multifunctional tumor suppressor protein. 100% of p53-knockout mice develop tumors (mostly lymphomas and sarcomas) early in life.1,2 Mice with decreased p53 gene dose or patients with Li-Fraumeni syndrome (mutation in one p53 allele) are also highly prone to early tumor development.3 One possible function for basal (as compared to stress-responsive) p53 activity is in the control of stem cell renewal.4,5 Upon activation by stresses such as DNA damage, p53 can, depending on the particular type of stress and type of cell, trigger programmed cell death (apoptosis), activate cell cycle checkpoints that prevent damaged cells from proliferating, or promote senescence (permanent cell cycle arrest). Consistent with these functions, inactivation of p53 facilitates expansion of aberrant cells and leads to genomic instability. Loss of p53 also promotes cellular immortalization—a state of long-term self-renewal that is one of the first steps towards cancer.6 Moreover, recent work has demonstrated that disruption of the p53 network enhances production of induced pluripotent stem (iPS) cells, converting, for example, differentiated normal fibroblasts into iPS cells that are (like “natural” stem cells) capable of self-renewal and of giving rise to multiple different types of differentiated cells.7–11 Thus, the role of p53 provides a remarkable link between the processes of stem cell reprogramming and oncogenic transformation. Even in the absence of any obvious stress, p53 can limit the self-renewal capacity of adult neural stem cells12 and regulate quiescence in hematopoietic stem cells (HSCs).4 These functions of p53 appear to be independent of its role as a regulator of stress responses.
Although p53-deficient mice have an increased HSC pool size, the proportion of the cells that exhibits quiescence is decreased.4 Also, it was found that p53 can regulate self-renewal of early hematopoietic progenitor cells (HPCs) by promoting their acquisition of stem-cell-like properties.13 The expansion of long-lived cells presents a possible mechanism by which p53 might contribute to oncogenesis, particularly, to development of lymphomas. Remarkably, transplantation of HSCs from p53 null mice into lethally irradiated recipients resulted in reduced engraftment as compared to HSCs from p53 wild type donors.14 Also, recipients that received p53 null HSCs did not display any increase in development of lymphomas or other tumors. In contrast, transplantation of whole BM from p53 null mice into lethally irradiated recipients led to enhanced engraftment as compared to transplantion of p53 wild type BM, but the recipients of p53 null BM developed lymphomas.14–16 Thus, transduction of only p53-deficient HSCs is not sufficient for development of lymphomas in mice. Additional cells and/or factors originating from whole BM appear to be necessary for lymphoma development.
Inhibition of p53 has been suggested as a therapeutic strategy to protect normal tissues from p53-mediated injury since p53-dependent apoptosis contributes to the hematopoietic (HP) component of acute radiation syndrome, the side effects of anticancer radio- and chemotherapy, and other pathologies associated with stress-mediated activation of p53.17,18 We have identified chemical p53 inhibitors (named pifithrins, PFTs) that are able to reversibly block p53-dependent transcriptional activation. We found that PFTs (namely, PFTα and its cyclic form PFTβ) not only suppressed radiation-induced activation of p53-responsive genes, but also protected cultured cells from subsequent p53-dependent apoptosis and mice from radiation-induced HP syndrome.19 The ability of PFTs to protect normal cells in the face of various p53-inducing stresses has been demonstrated in a number of systems, including neuro-, renal and cardio-protection (reviewed in ref 18).
It has been shown that exposure to IR causes both acute bone marrow suppression through induction of p53-dependent apoptosis in rapidly proliferating HPCs, as well as long-term residual HP injury involving senescence of HSCs which limits their capacity for self-renewal.20 Thus, the mechanism(s) by which PFTβ protects mice against HP acute radiation syndrome may include, in addition to protection of differentiated HP cells from acute apoptosis, protection of HSCs and HPCs through stimulation of their proliferation. In the current study, we tested whether (as shown for genetic p53 inhibition) chemical inhibition of p53 by PFTβ affects proliferation of HSCs and HPCs. We found that PFTβ stimulated proliferation of HSCs and HPCs both in vitro and in vivo and that the induced HSCs were fully functional in BM transplantation experiments and mobilized to the blood stream. While this, in addition to the demonstrated radioprotective efficacy of PFTs, suggests promising clinical uses for p53 inhibitors, it also presents the theoretical risk that enhanced proliferation of stem cells due to p53 inhibition could lead to appearance of transformed cells and thereby promote development of tumors (particularly lymphomas). However, we demonstrated that temporary inhibition of p53 by PFTβ did not change the frequency, timing or spectrum of radiation-induced tumors in cancer-prone p53+/−mice.21,22 Our finding that PFTs are non-carcinogenic strengthens the possibility of using pharmacological suppression of p53 as a safe and effective means to prevent and treat acute p53-dependent pathologies and to stimulate stem cell production.
Results
PFTβ protects against IR-induced loss of hematopoietic stem and progenitor cells
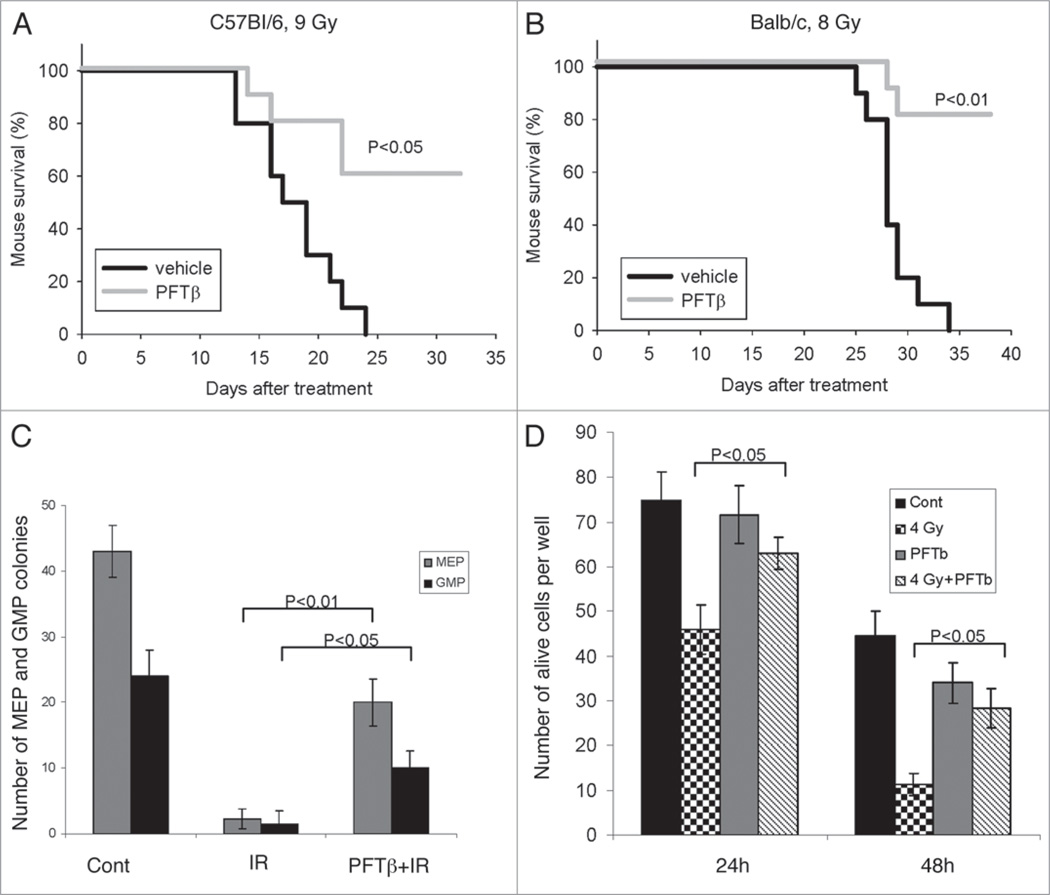
The hematopoietic (HP) component of acute radiation syndrome results from induction of massive p53-dependent apoptosis of HP cells, including hematopoietic stem and early progenitor cells in the bone marrow. We have shown that the p53 inhibitory small molecule PFTα protects against this type of tissue injury, thereby improving survival of irradiated mice.19,23 Here we tested the circularized form of PFTα, PFTβ, as a radioprotectant in two strains of wild type mice (C57Bl/6 and Balb/c). Mice were given a single intraperitoneal (i.p.) injection of PFTβ (25 mg/kg) or vehicle immediately before exposure to lethal doses of total body gamma irradiation (TBI, 9 Gy for C57Bl/6 and 8 Gy for Balb/c). 60% of C57Bl/6 mice and 80% of Balb/c mice that were injected with PFTβ survived beyond the time at which 100% of vehicle-injected animals were dead (day 24 and day 34 for C57Bl/6 and Balb/c, respectively) (Fig. 1A and B). The doses of TBI used in this experiment are known to cause death primarily through damage to the HP system, as compared to higher doses that invoke gastrointestinal and cerebrovascular damage as well as HP damage. Therefore, the effect of PFTβ on survival of irradiated mice in this experiment indicates that the compound acts as a radioprotectant of the HP system.
Figure 1.
PFTβ protects mice and hematopoietic cells from ionizing radiation (IR). (A and B) Survival curves for mice given a single i.p. injection of PFTβ (25 mg kg−1 body weight) or vehicle (“control”) just prior to lethal total body irradiation of 9 Gy in C57Bl/6J mice (A) and 8 Gy in Balb/c mice (B). The results shown are from a representative experiment. Three independent experiments were performed, each with 10 mice per group. (C) C57Bl/6J mice were injected (i.p.) with PFTβ (25 mg/kg) or vehicle 30 min before TBI (4 Gy). 24 h later, BM-MNCs were isolated from the mice and plated for CFC assays. Megakaryocyte-erythroid (MEP) and myeloid (GMP) colonies were counted 7 days after plating. Untreated and unirradiated mice were included as a control (“Cont”). The Y-axis shows the mean number of MEP and GMP colonies per plate. (D) BM-MNCs from C57Bl/6 mice (150 cells plated per well) were treated in vitro with PFTβ (15 µM) or vehicle just prior to irradiation (4 Gy) or without irradiation. Cell viability was determined by counting of trypan blue-excluding cells 24 h and 48 h after IR. The data in (C and D) are presented as mean +/− SE (n = 5).
Notably, the mice that survived irradiation due to PFTβ injection remained viable and healthy for a long period of time post-irradiation (>1 year). This suggested that PFTβ (and PFTα19,23) protected hematopoietic stem cells (HSCs) and early progenitor cells (HPCs) capable of fully repopulating the HP system for long-term function. To test this possibility, we assessed the effect of PFTβ on different populations of bone marrow cells (BMCs) in vivo (in mice) and in vitro. It was previously shown that gamma irradiation of bone marrow mononuclear cells (BM-MNCs) inhibits the clonogenic activity of HPCs in colony-forming cell (CFC) assays capable of distinguishing between two distinct types of colonies: those originating from megakaryocyte-erythrocyte progenitors (MEP) and those originating from granulocyte-macrophage progenitors (GMP).20 Therefore, we used this assay to determine the effect of PFTβ on the viability of HPCs in irradiated mice. We injected (i.p.) mice with PFTβ (25 mg/kg) or vehicle 30 min before TBI (4 Gy). 24 h later, we isolated BM-MNCs from the mice and plated them in culture dishes (4 × 104 cells/ml in 35 mm plates). MEP and GMP colonies were counted 7 days after plating. We found that PFTβ treatment significantly increased the number of both MEP and GMP colonies produced by BM HPCs of irradiated mice in this assay (Fig. 1C). This PFTβ-dependent improvement in the clonogenic activity of BM-MNCs isolated from irradiated mice suggests that the compound has a direct protective effect on HPCs within the BM. This was confirmed by in vitro experiments in which PFTβ (15 µM) was applied directly to BM-MNCs that consisted mostly of differentiated HP cells (in short-term culture held in non-proliferative conditions by exclusion of growth factors) prior to irradiation of the culture (4 Gy). PFTβ treatment resulted in a significant increase in the number of viable cells (based on trypan blue exclusion) remaining in the culture 24 h and 48 h after IR (Fig. 1D). Thus, PFTβ had a direct radioprotective effect on BM-MNCs.
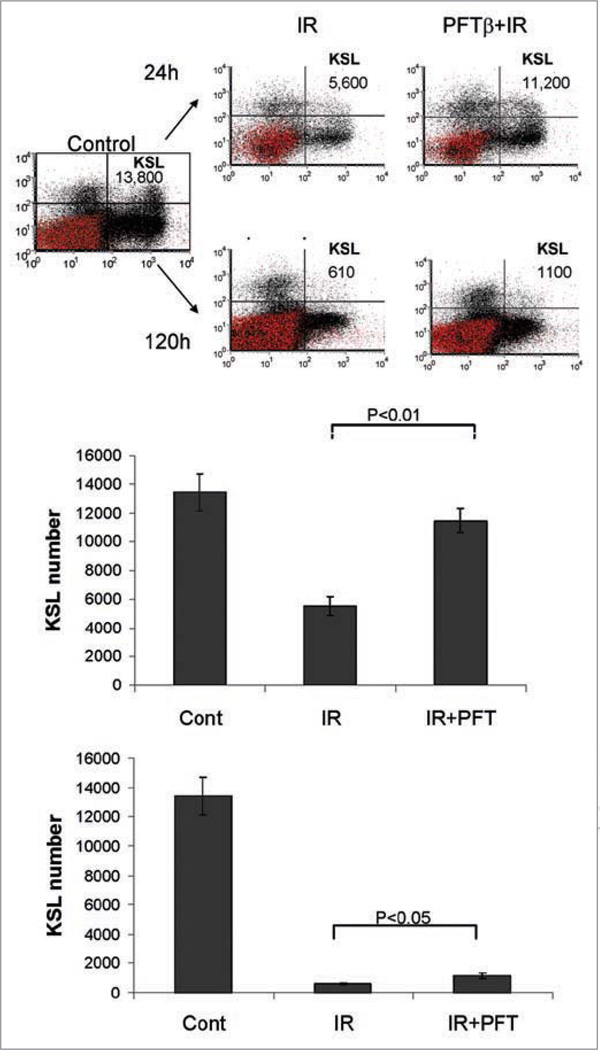
In addition to its effect on BM-MNCs and HPCs, IR also affects HSCs. It is well-established that HSCs lack expression of cell surface molecules characteristic of lineage-specific mature cells (Lin-)16,24,25 and usually express stem cell antigen 1 (Sca1+) and c-Kit (Kit+, CD117), a transmembrane tyrosine kinase that serves as a receptor for stem cell factor.26,27 Therefore, FACS analysis of Lin-Sca1+c-Kit+ (KSL) cells provides a means to specifically detect HSCs. It was previously found that HSC injury occurring in mice exposed to TBI was due to defective self-renewal of HSCs.20 TBI of mice resulted in a reduction in the frequency of HSCs (KSL) in the bone marrow.20 We have confirmed these data. In our hands, unirradiated control mice were determined to have, on average, 14,000 KSL cells within BM-MNCs isolated from 4 hind limb bones (tibia and femurs) (Fig. 2). Exposure of mice to TBI (4 Gy) led to a reduction in the number of KSL cells in each mouse that was time-dependent (increasing with increasing time-post-TBI) (left in Fig. 2 and data not shown). For example, 24 and 120 h post-irradiation, the average number of KSL cells per mouse was reduced from 14,000 to 5,600 and 610, respectively (left in Fig. 2).
Figure 2.
PFTβ decreases IR-induced reduction of HSCs. Flow cytometric analysis of BM-MNCs stained for detection of HSCs (Lin−, c-Kit+, Sca1+ “KSL” cells, upper right quadrant of scatter plots) from a representative control untreated mouse (left) or representative mice treated with a single injection of PFTβ (25 mg/kg) or vehicle just before exposure to TBI (4 Gy). Analysis was performed on BM-MNCs isolated from mice 24 h (top) or 120 h (bottom) post-IR. The total number of KSL cells per mouse (4 bones) is indicated. The bar graphs show the mean number of KSL cells per mouse +/− SE (n = 5) as determined by flow cytometry 24 h (top) and 120 h (bottom) after IR.
In order to determine whether PFTβ protects against this radiation-induced loss of HSCs, we injected mice with PFTβ (25 mg/kg) or vehicle just before exposure to TBI (4 Gy) and isolated BM-MNCs for FACS analysis at 24 h, 48 h, 120 h and 240 h post-irradiation. As shown in Figure 2 (representative results from the 24 h and 120 h timepoints), pretreatment with PFTβ resulted in a significant increase in the number of KSL cells in irradiated mice. PFTβ treatment of irradiated mice resulted in an approximately two-fold increase in KSL cells as compared to vehicle treated irradiated mice, such that the average number of HSCs per mouse approached that of control unirradiated mice (right in Fig. 2). These results demonstrate that, as for HPCs (see above), PFTβ impedes IR-induced reduction of HSCs in mice.
In the absence of IR, PFTβ increases the number of hematopoietic stem and progenitor cells
Radiation damage to the HP system involves at least two mechanisms that lead to acute and prolong myelosuppression: induction of apoptosis in rapidly proliferating HPCs and induction of senescence in HSCs.20 It was recently found that various hematopoietic growth factors including GM-CSF can significantly improve acute myelosuppression and promote recovery of BM hematopoietic function after IR and that this primarily involves stimulation of the proliferation and differentiation of HPCs and HSCs.28 Thus, the mechanism by which PFTβ protects mice against HP acute radiation syndrome may include, in addition to protection of differentiated HP cells from apoptosis, protection of HSCs and HPCs by stimulation of their proliferation and differentiation.
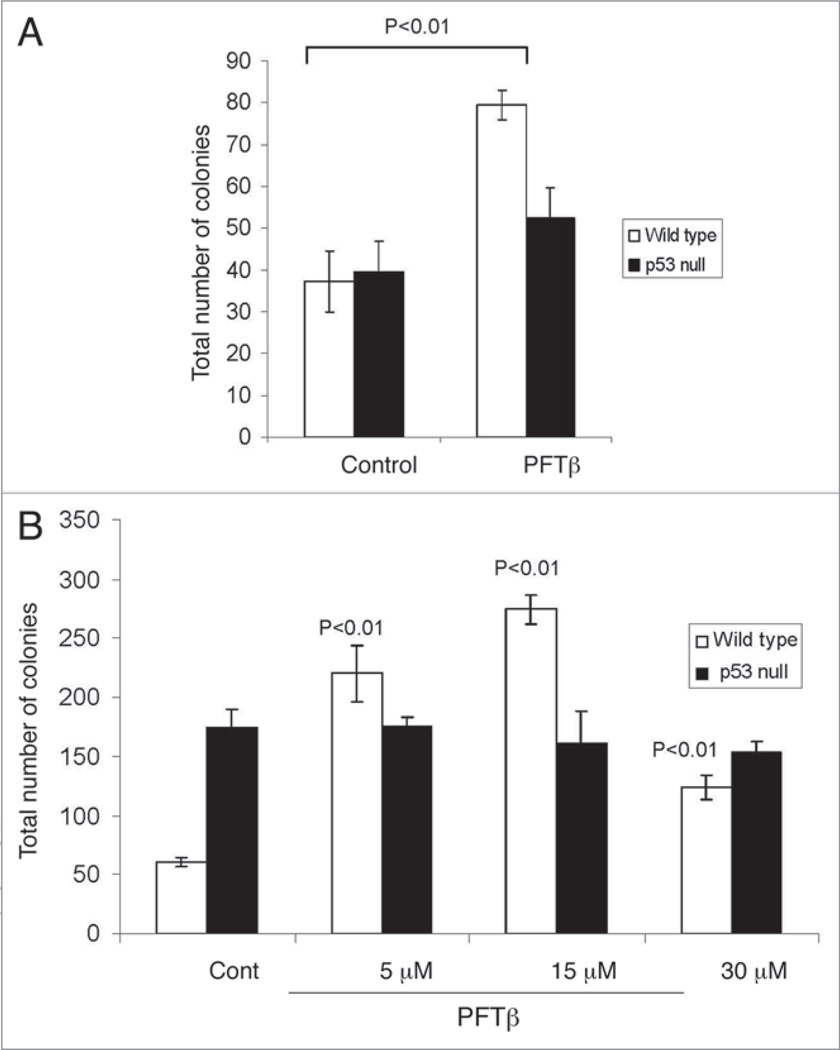
To directly test the effect of PFTβ on amplification of HPCs, we treated mice with PFTβ without accompanying TBI using a treatment schedule similar to the schedule of GM-CSF administration that resulted in maximum stimulation of HSC and HPC proliferation.28 We injected C57Bl/6 mice with PFTβ (i.p., 25 mg/kg) or vehicle once a day for 5 consecutive days. On day 6, BM-MNCs were isolated from the PFTβ-treated and vehicle-treated mice and plated (4 x 104 cells/ml in 35 mm plates) for assessment of MEP and GMP colony formation 7 days later. We found that BM-MNCs from PFTβ-treated mice produced more than twice as many colonies (MEP + GMP) than BM-MNCs from vehicle-treated mice (Fig. 3A). This effect was p53-dependent since identical treatment of p53-null mice with PFTβ did not significantly increase formation of MEP and GMP colonies relative to treatment of the same mice with vehicle (Fig. 3A). These data indicate that PFTβ-mediated inhibition of p53 results in enhanced proliferation of HPCs in vivo.
Figure 3.
In the absence of IR, PFTβ treatment of mice or cultured BM-MNCs enhances HPC-derived MEP and GMP colony formation in a p53-dependent manner. (A) Wild type (open bars) and p53-null (solid bars) mice were given 5 daily injections of PFTβ (25 mg/kg) or vehicle (“control”). On day 6, BM-MNCs were isolated from the mice and plated (4 × 104 cells/ml in 35 mm diameter plates) for assessment of MEP and GMP colony formation 7 days later. The mean number of colonies per plate (MEP + GMP) +/− SE (n = 5) is shown. (B) Application of PFTβ (5–30 µM) to wild type BM-MNCs in vitro increased the total number of MEP + GMP colonies in CFC assays as compared to application of vehicle (“cont”). Application of PFTβ (5–30 µM) to BM-MNCs from p53− / − mice did not affect the growth of colonies (GMP and MEP). The data are presented as mean number of colonies per plate +/− SE (n = 5).
To confirm that PFTβ has a direct effect on proliferation of HPCs, we added PFTβ (5–30 µM) in vitro to BM-MNCs and assessed its effect on colony formation. Application of PFTβ (5–30 µM) to BM-MNCs isolated from wild type C57Bl/6 mice resulted in an increase in MEP and GMP colony formation (Fig. 3B). In contrast, addition of PFTβ (5–30 µM) to BM-MNCs isolated from p53-null mice did not affect the growth of colonies (Fig. 3B), suggesting that this effect is p53-dependent. Thus, in the presence of PFTβ, the clonogenic potential of HPCs in vitro and in vivo is increased. This could be due to either inhibition of HPC death or stimulation of HPC proliferation (reduced dormancy/quiescence of HPCs under the conditions of cultivation).
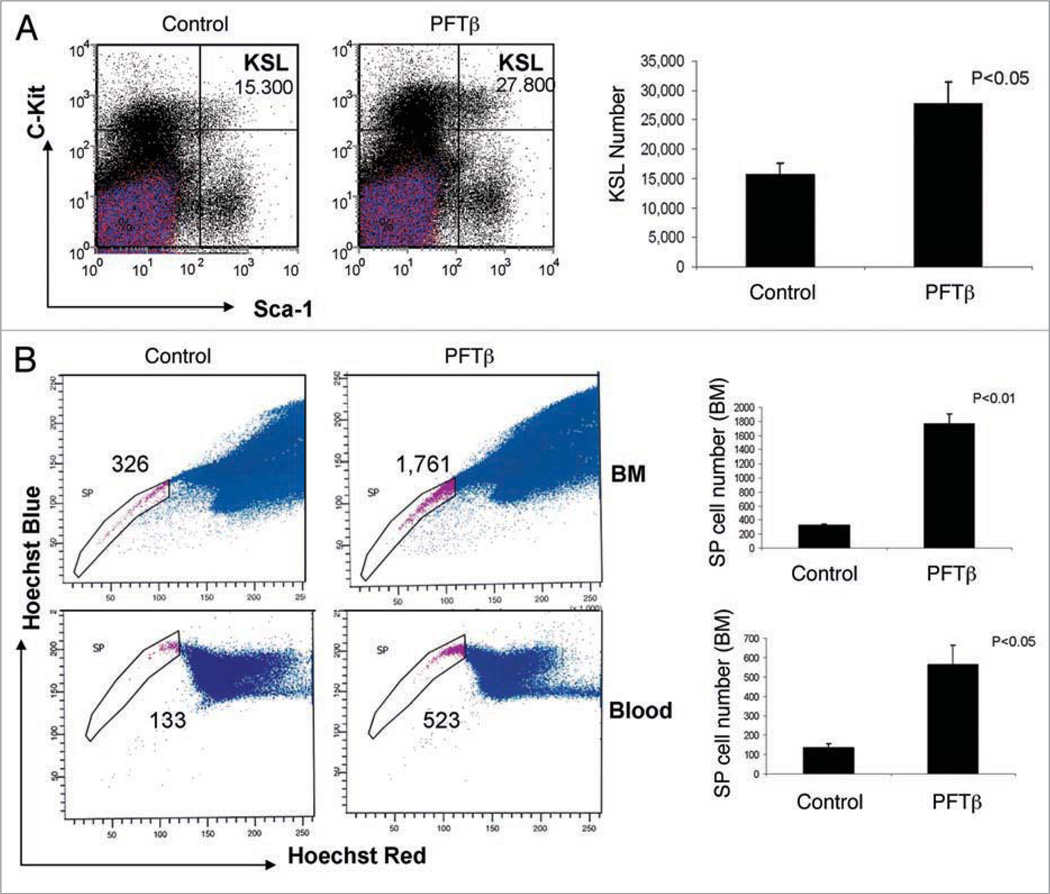
Since genetic inhibition of p53 (knockout, siRNA) was shown to increase self-renewal of HSCs,4 we proposed that chemical inhibition of p53 by PFTβ might also activate proliferation of HSCs. To test this hypothesis, we injected (i.p.) C57Bl/6 mice with PFTβ (25 mg/kg) or vehicle and used flow cytometry to quantify HSCs (KSL cells) within BM-MNCs collected 2, 3, 5 or 10 days post-injection. We found that even a single injection of PFTβ produced a statistically significant increase in the number of HSCs. Similar to the results that were obtained on days 2, 3 and 10 (data not shown), in BM-MNCs collected on day 5 post-injection, we detected a nearly 2-fold increase in the number of KSL cells for PFTβ-treated mice as compared to vehicle-treated controls (Fig. 4A).
Figure 4.
PFTβ treatment of mice results in increased numbers of HSCs in the BM. (A) 5 mice were given a single injection of PFTβ (25 mg kg−1 body weight) or vehicle (“control”) without accompanying IR. BM-MNCs were isolated on day 5 after injection and stained for flow cytometric detection of HSCs (Lin, c-Kit+, Sca1+ “KSL” cells). Left: The total number of KSL cells per mouse (4 bones) is shown in the upper right quadrants of the scatter plots (representative results from individual control and PFTβ-treated mice). Right: Bar graph showing the mean number of KSL cells per mouse (4 bones) +/− SE (n = 5). (B) 7 mice were injected with PFTβ (25 mg kg−1 body weight) or vehicle (“control”) without accompanying IR once a day for 5 days. BM-MNCs (top) and PBMCs (bottom) were isolated on day 6 and stained with Hoechst 33342. Side population (SP) cells equivalent to HSCs were detected by FACS using blue and red filters. Left: Representative scatter plots in which SP cells are shown gated and the number of cells per animal is indicated. Right: Bar graphs indicating the mean number of SP cells per animal +/− SE (n = 7).
HSCs demonstrate increased efflux of a number of ABC transporter-dependent substrates.29 HSCs can be identified based on this property; in particular, their ability to efflux the dye Hoechst 33342 at a greater rate than other cells within the BM, blood and solid tissues. For example, upon FACS analysis of Hoechst 33342-stained BM-MNCs, HSCs are detectable as a “side population” (SP) that produces a low-fluorescence “tail” in the scatter plot.29,30 We used SP analysis as an additional method to confirm the ability of PFTβ to increase the abundance of HSCs in the BM and to test whether this leads to an increased frequency of HSCs in the peripheral blood. C57Bl/6 mice were given 5 daily i.p. injections of PFTβ (25 mg/kg) or vehicle (mimicking a schedule used for HSC stimulation by GM-CSF). BM-MNCs and peripheral blood mononuclear cells (PBMCs) were isolated on day 6, stained with Hoechst 33342 and analyzed by FACS. For both BM-MNCs and PBMCs, we observed a five- to six-fold increase in SP cells in PFTβ-injected mice as compared to vehicle-injected mice (Fig. 4B). This indicates that PFTβ treatment of mice stimulated both proliferation of HSCs within the BM and their release into the blood stream.
Mouse BM-derived SP cells were shown to be functionally active since they provide transplantation enrichment that is similar to the enrichment achieved by purification of HSCs from BM using combinations of cell-surface markers.29 Thus, as an additional test of the-ability of PFTβ to stimulate HSCs (SP cells) within the BM, we evaluated the function of BM-MNCs isolated from PFTβ- or vehicle-injected mice using a short-term hematopoietic reconstitution assay. C57Bl/6 “donor” mice were given 5 daily i.p. injections of PFTβ (25 mg/kg) or vehicle (3 mice per group). On day 6, BM-MNCs were isolated and pooled within each group. We then injected (i.v.) 2 × 106 BM-MNCs into lethally irradiated (10 Gy, 3 Gy min−1) C57Bl/6 “recipient mice” (7 mice per group) 3 h after irradiation. Control mice that were irradiated but not injected with donor BM-MNCs died within approximately 2 weeks of irradiation. Transplanted mice were bled 16 and 90 days after injection of donor BM-MNCs and circulating total white blood cell (WBC), monocyte, neutrophil and lymphocyte counts were determined using a Hematology Cell Counter (see Materials and Methods). 16 days post-transplant, WBCs remained at very low levels in both groups of mice. However, by 90 days post-transplant the analyzed populations of WBCs were significantly reconstituted. At this time point, we detected increases in all analyzed circulating WBC populations in mice that received BM-MNCs from PFTβ-treated mice as compared to those that received BM-MNCs from vehicle-treated mice (for monocytes and neutrophils these increases were statistically significant, p < 0.05) (Fig. 5). Thus, PFTβ treatment improved BM-dependent WBC reconstitution in lethally irradiated mice. These results indicate that PFTβ-stimulated proliferation of HSCs (SP cells) in the BM produces stem cells that are fully functional.
Figure 5.
PFTβ-treated BM provides enhanced white blood cell reconstitution. 2 × 106 BM-MNCs from DMSO (control) or PFTβ (25 mg/kg)-treated (daily i.p. injections, 5 times) C57Bl/6 mice were transplanted into lethally irradiated (10 Gy, 3 Gy min−1) C57Bl/6 recipient mice 3 h after irradiation. Total circulating white blood cells and separate monocyte, neutrophil and lymphocyte counts were measured in peripheral blood collected from the mice 16 and 90 days post-transplantation. The results are expressed as the mean number of cells/µl of blood +/− SE (n = 7 mice/group). The mean number of cells in a control group of 7 untreated mice is indicated by the dash line (“norm”).
PFTβ does not affect radiation-induced tumor development in p53 hemizygous mice
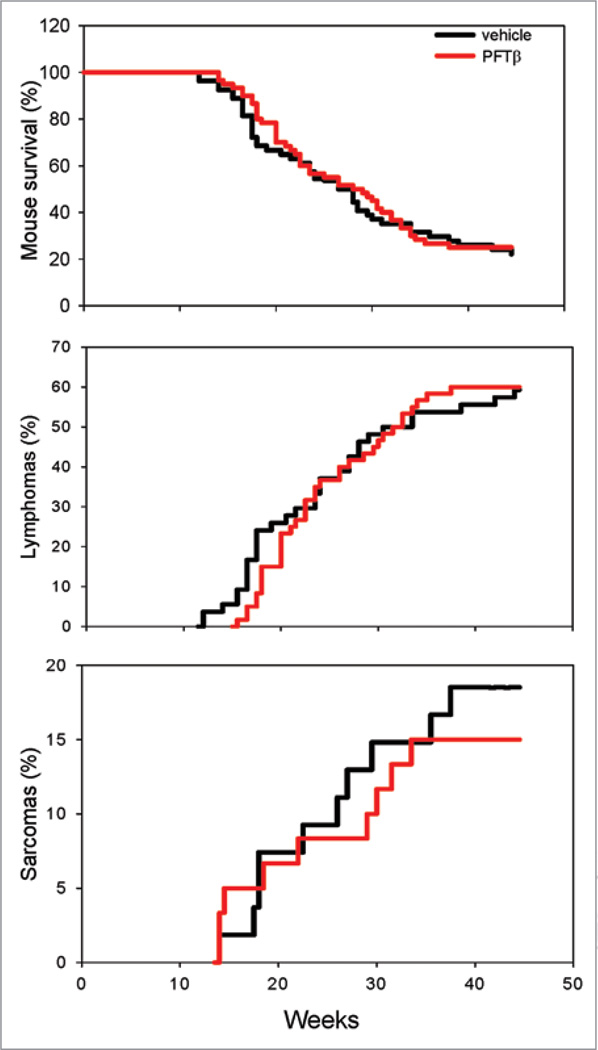
Temporary suppression of p53 has been suggested as a therapeutic strategy to prevent damage of normal tissues in anticancer therapy. This strategy is supported by our results described above demonstrating the radioprotective effect of PFTβ on the HP system. However, theoretical concerns about the safety of such therapies have been raised based on a number of facts. First, genetic p53-deficiency in mice and humans promotes tumor development1–3 and the vast majority of tumors are characterized by inactivation of the p53 pathway.31 Second, it was recently found that p53 can regulate self-renewal of HSCs and early HPCs and that p53-deficient mice have an increased HSC pool size.13 This is corroborated by our finding that inhibition of p53 by PFTβ stimulates HSC proliferation and results in an increased HSC population size (see above). Increased numbers of HSCs with enhanced proliferation capacity could, in theory, increase the risk of development of hematological tumors such as lymphomas. To address this concern, we tested the effect of PFTβ on development of radiation-induced tumors in cancer-prone p53+/− mice.21,22 TBI (1–4 Gy) significantly accelerates the carcinogenic process in p53 heterozygous mice and decreases their average lifespan.22,32 If left untreated, the majority of these mice develop tumors (mostly lymphomas and sarcomas) within sex (males and females)-matched groups of p53+/− mice were injected i.p. with PFTβ (25 mg/kg) or vehicle 30 min prior to 4 Gy TBI.32 Control and PFT-treated groups contained 55 and 60 mice, respectively. Mice were monitored 2 times a week for up to 12 months and the dynamics of tumor formation and mouse survival were determined. We observed that there was no difference in survival of control and PFTβ-treated mice in this model (Fig. 6). Since death in this model is generally attributed to cancer, the equivalent survival of the two groups of mice here suggests that PFTβ did not promote tumor formation. Indeed, we found that the timing and frequency of tumor appearance in this model were not affected by PFTβ injection. Taking into consideration all types of tumors, by 45 weeks post-TBI, 78% and 80% of vehicle-injected and PFTβ-injected mice had developed tumors, respectively (Fig. 6, Table 1). In addition, PFTβ did not affect the spectrum of tumor types that arose in irradiated p53+/−mice. Histological analysis of tumors showed that the majority of the tumors that developed were lymphomas (mostly thymus lymphomas) and that these occurred with equal frequency in the two groups of mice (58% in the control group and 60% in the PFTβ-treated group, Fig. 6). The prevalence of sarcoma tumors was also similar between the control group of mice (18%) and the PFTβ-treated group (15%) (Fig. 6). A number of other tumor types were less common (pleomorphic rhabdomyosarcoma, spindle cell sarcoma, osteosarcoma, angiosarcoma, adenocarcinomas, Table 1), but in all cases, they were detected with similar frequencies in control and PFTβ-treated mice. These data demonstrate that PFTβ had no effect on development of tumors in irradiated p53+/− mice, a widely used system for evaluation of carcinogenic effects. Thus, while temporary inhibition of p53 by PFTβ stimulates proliferation of HSCs and HPCs in vivo, it does not accelerate tumorigenesis.
Figure 6.
PFTβ does not change the frequency or timing of tumor development in p53+/− mice. p53+/− were injected i.p. with PFTβ (25 mg/kg, n = 60) or vehicle (n = 55) 30 min prior to 4 Gy TBI. Mouse survival, and sarcoma and lymphoma development (% of mice with tumors at different time points) were assessed.
Table 1.
Frequency of different types of tumors observed in PFTβ-treated and control (DMSO-treated) gamma-irradiated (4 Gy) p53+/− mice by 45 weeks post-irradiation
| Vehicle | PFTβ | |
|---|---|---|
| Number of mice | 55 (100%) | 60 (100%) |
| Total number of tumors | 43 (78%) | 48 (80%) |
| Carcinoma | 1 (2%) | 3 (5%) |
| Lymphoma (thymus and splenic) | 32 (58%) | 36 (60%) |
| Total sarcomas | 10 (18%) | 9 (15%) |
| Pleomorphic rhabdomyosarcoma | 3 (5%) | 4 (7%) |
| Spindle cell sarcoma | 3 (5%) | 4 (7%) |
| Osteosarcoma | 3 (5%) | 1 (1%) |
| Angiosarcoma | 1 (2%) | 0 (0%) |
| Mixoid sarcoma | 0 (0%) | 0 (0%) |
| Number of died mice (unknown reasons) | 2 (4%) | 1 (2%) |
| Number of survived mice (after 1 year) | 10 (18%) | 12 (20%) |
Discussion
The concept of using p53 inhibitors to protect normal tissues from p53-dependent apoptosis17 was validated by isolation of PFTs, small molecules that inhibit the transactivation function of p53 and protect against radiation-induced tissue damage.19 Since p53-deficient mice and humans (patients with Li-Fraumeni syndrome) suffer from frequent and early development of tumors, the safety of p53 inhibition as a therapeutic approach has been questioned. Adding to these concerns, recent work has shown that absence of basal p53 activity (p53 deficiency) stimulates expansion of hematopoietic stem cell (HSC) and early progenitor cell (HPC) populations that could increase the probability of tumor development.4
Both PFTα and its circularized derivative PFTβ protected mice from doses of IR that induce development of lethal hematopoietic (HP) syndrome in mice. The pathology of HP syndrome involves radiation-induced depletion of different populations of blood cells, including hematopoietic stem cells (HSC). The long-term survival of mice protected against lethal doses of IR by PFTα17 and PFTβ (Komarova EA and Gudkov AV, personal observations) suggests that the compound protects HSCs that are capable of fully reconstituting the HP system. It was shown that IR not only causes acute bone marrow suppression due to apoptotic cell loss, but also long-term residual HP injury due to induction of senescence in HSCs which limits their capacity for self-renewal.20 This, together with data showing that genetic inhibition of p53 leads to enhanced proliferation of HSCs4 and can thereby potentially affect tumor development, prompted us to determine whether chemical inhibition of p53 by PFTs affects proliferation of HSCs or increases cancer risk in mice.
We found that different populations of HP cells are targets of PFTβ action, including differentiated myeloid cells, HPCs and HSCs. PFTβ ameliorated the IR-induced reduction in HSC and HPC (megakaryocyte and myeloid progenitor cells) cell numbers. Even in the absence of IR (i.e., testing basal p53 activity), application of PFTβ to BM-MNCs in vitro or injection of PFTβ into mice in vivo stimulated proliferation of HPCs as illustrated by increased frequency of megakaryocyte and myeloid colony formation. Comparison of p53-wild type and -null systems demonstrated that this activity of PFTβ is indeed p53-dependent. PFTβ-mediated expansion of HSCs was confirmed by flow cytometric detection of HSCs within BM-MNCs in two ways: detection of KSL cells using antibodies against cell surface markers and detection of “side population” cells based on their increased efflux of the dye Hoechst 33342. Increased numbers of HSCs were also detected in the peripheral blood of PFTβ-treated mice. Since p53 contributes to HSC quiescence, its absence (due to siRNA-mediated knockdown or gene mutation/deletion) allows HSCs to enter the cell cycle more easily.12 The results of our current study show that temporary chemical inhibition of p53 by PFTβ has a similar effect leading to increased numbers of HSCs (and HPCs as well). Although we did not test the functional capacity of FACS-sorted HSCs from PFTβ-treated mice, transplantation of total BM cells from PFTβ-treated mice accelerated repopulation of white blood cell pools in lethally irradiated mice, which testifies to their functional capacity. Thus, stimulation of HPC and HSC proliferation is likely one mechanism by which PFTβ protects organisms (mice) from death caused by IR-induced HP syndrome.
Inhibition of p53 not only leads to increased HSC self-renewal,4 but also enhances the “stemness” of cells and conversion of differentiated cells into induced pluripotent stem cells (iPS cells).7–11 These recently discovered mechanisms of p53 activity might be involved in acceleration of tumor development in the organism. However, we found that despite its HSC-stimulatory effect, the p53 inhibitor PFTβ was not carcinogenic in mice. For this experiment, we tested the effect of PFTβ on IR-induced tumor formation in cancer-prone p53+/− mice, a standard model for determination of carcinogenicity. Temporary treatment with PFTβ (sufficient for protection of normal tissues from IR-induced damage) did not affect the frequency, timing or spectrum of tumor development in this model. Consistent with these results, a recent study from the lab of Gerard Evan demonstrated that p53 does not need to be at a constantly high level to exert its tumor suppressor function.33 This work provides evidence that the immediate p53 response to DNA damage is not necessary for tumor suppression and may actually be detrimental to the organism. Thus, absence of p53 immediately after DNA damage protects animals from radiation sickness, but does not prevent repair of the DNA damage. However, even transient restoration of p53 activity after resolution of the initial DNA damage can suppress tumor development. Therefore, temporary inhibition of p53 activity (achieved either by genetic means, as in the study from Gerard Evan, or pharmacologically, as in our injection of mice with PFTs) does not lead to increased cancer incidence if p53 function is later restored.33 This eliminates a significant theoretical safety concern and validates our approach of developing p53 inhibitors as safe and effective radioprotectants and stem cell stimulators for human use.
Materials and Methods
Mice
The colony of p53-knockout mice on a C57Bl/6 background was maintained in the lab of A. Gudkov by crossing p53+/−females with p53− / − males (purchased from Jackson Laboratories, Bar Harbor, ME) followed by genotyping of the progeny (PCR). Heterozygous p53+/− mice were generated by crossing p53− / − males with wild type p53 females. C57Bl/6J and Balb/c mice were purchased from Jackson Laboratories.
PFTβ treatment and gamma irradiation
Mice were treated in accordance with the animal care protocol approved by the Institutional Animal Care and Use Committee. C57Bl/6J, Balb/c and p53+/− mice (8–10 weeks of age) were given an intraperitoneal (i.p.) injection of PFTβ (ASH Stevens, Inc., Detroit, MI) in DMSO/PBS (1:4) solution or vehicle (DMSO/PBS solution), 0.2 ml per mouse. 30 min later, mice were gamma irradiated on a rotating platform using a Shepherd 4000-Ci cesium 137 source at a dose rate of 4 Gy min−1. Cultures of BM-MNC cells were irradiated using the same dose rate of 4 Gy min−1. Mice were euthanized if they developed visible tumors (tumor size greater than 2 cm in diameter) or looked morbid.
Isolation and enumeration of bone marrow cells
Bone marrow was isolated from 4 hind limb bones (femurs and tibias) of each mouse. The bones were dissected, and the marrow was flushed into cold IMDM, 2% FBS (Invitrogen, catalog #12440–053) using a 21-gauge needle. Nucleated cells were counted with a hematocytometer using trypan blue exclusion following lysis of red blood cells with 3% acetic acid in methylene blue (Stem Cell Technologies, Vancouver, BC, Canada).
Colony-forming cell (CFC) assay
C57Bl/6 wild type and p53 null mice were injected (i.p.) with vehicle (DMSO) or PFTβ (25 mg/kg) 30 min before TBI (4 Gy). Immediately after TBI or later (1–5 days), BM-MNCs were isolated and plated (4 × 104 cells/ ml in 35 mm diameter plates) in MethoCult M3231 medium (StemCell Technologies, catalog #03231) supplemented with 10 ng/ml recombinant mouse GM-CSF (StemCell Technologies, catalog #02735) and IMDM (Invitrogen, catalog #12440–053). PFTβ (with or without IR) was either injected into mice or applied directly to the BM-MNCs before their plating. Colony formation (MEP and GMP) was scored after 7 d incubation at 37°C in the presence of 5% CO2. MEP and GMP colonies were distinguished from each other based on their morphology (MEP colony have a condensed center and GMP colonies have a dispersed appearance).
Flow cytometric determination of HSC (KSL) number
Murine hematopoietic stem cells (KSL phenotype cells) were identified and evaluated by flow cytometric analysis of a single cell suspension of BM-MNCs (isolated from mice as described above). To discriminate populations of progenitor and mature hematopoietic cells (Lin-), we used antibodies against the following cell surface markers: CD3, CD5, CD45R/B220, CD11b, Erythroid marker and Ly6G (eBioscience, San Diego, CA). The antibody-labeled cells were stained with Peridinin-chlorophyll-a (PerCP)-streptavidin (BD Biosciences, San Diego, CA). FITC-conjugated anti-c-kit (clone 2B8), PE-conjugated anti-CD34 (RAM34) and APC-conjugated anti-Sca1 (D7, eBioscience, San Diego, CA) were used after incubation with blocking antibodies. 7-AAD- and PerCP-positive cells were gated out to allow calculation of only viable Lin− /low, c-kitpositive and Sca1positive cells. Acquisition was performed on a dual-laser FACSCalibur instrument (Beckton Dickinson Immunocytometry Systems, San Jose, CA) and data were analyzed using FCS Express 3 software (De Novo Software, Los Angeles, CA). The number of KSL cells is expressed as the mean number of KSL cells per animal (within BM-MNCs from 4 hind limb bones) +/− SE (n = 5 to 7).
Staining and analysis of side population (SP) cells
We performed SP detection and analysis as previously described.29 Briefly, BM-MNCs or peripheral blood mononuclear cells (white blood cells) isolated by centrifugation through Ficoll from whole blood were enumerated as described above, then resuspended at 1 × 106 cells/ml in DMEM+ (high-glucose Dulbecco modified eagle medium), 2% FBS (Invitrogen) and incubated with Hoechst 33342 (5 µg/ml) (Bis-benzimide; Sigma, St. Louis, MO) for 90 min at 37°C. Cells were then centrifuged and resuspended in cold HBSS+ and kept on ice for subsequent analysis. Analysis was performed on a MoFlo flow cytometer (Cytomation) equipped with a 351 nm laser for UV. Blue Hoechst fluorescence was collected with a 450/20 filter, and red Hoechst/PI fluorescence was collected via a 675 ESLP filter.
Short-term repopulation
8-week-old “donor” mice were injected once a day for 5 consecutive days with PFTβ (25 mg/kg) or an equal volume of DMSO solution (control). On day 6, BM-MNCs were isolated from both groups of mice by flushing the femurs and tibias and a single-cell suspension of BM-MNCs was prepared (see above). “Recipient” C57Bl/6J mice were irradiated (10 Gy at a dose rate of 3 Gy min−1) and reconstituted 3 h later with approximately 2 × 106 “donor” BM-MNCs by intravenous injection into the tail vein (7 mice/group). Non-reconstituted irradiated mice were included as controls in the experiment and typically had to be euthanized between days 8 and 14 post-irradiation due to bone marrow aplasia. Recipient mice were bled 16 and 90 days after transplantation and circulating total white blood cell counts and separate monocyte, neutrophil and lymphocyte counts were determined through analysis of 40 µl of blood using a System 9118+ Hematology Series Cell Counter (Biochem Immunosystems, Allentown, PA). The results are expressed as the mean number of cells/µl +/− SD (n = 7 mice/ group). A group of 7 untreated and unirradiated mice was used as a control group to establish “normal” levels of each cell type in the peripheral blood.
Tumor morphology
Tumor tissues were processed using a standard protocol. Tumor sections were stained with H&E and analyzed by a pathologist.
Acknowledgements
We thank Patricia Stanhope Baker for help with manuscript preparation and Kellee Greene for technical assistance. This work was supported by grants from National Institutes of Health CA75179 (to A.V.G.) and GM075226 (to M.P.A.).
References
- 1.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 2.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 3.Varley JM, McGown G, Thorncroft M, Santibanez-Koref MF, Kelsey AM, Tricker KJ, et al. Germ-line mutations of TP53 in Li-Fraumeni families: an extended study of 39 families. Cancer Res. 1997;57:3245–3252. [PubMed] [Google Scholar]
- 4.Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, et al. p53 regulates hematopoietic stem cell quiescence. Cell stem cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature. 2009;460:1085–1086. doi: 10.1038/4601085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 7.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 13.Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53− / − p16Ink4a− / − p19Arf− / − multipotent progenitors. Nature. 2008;453:228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 14.Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol. 2003;31:521–527. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, Young NS. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008;36:1236–1243. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komarova EA, Gudkov AV. Could p53 be a target for therapeutic suppression? Semin Cancer Biol. 1998;8:389–400. doi: 10.1006/scbi.1998.0101. [DOI] [PubMed] [Google Scholar]
- 18.Gudkov AV, Komarova EA. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun. 2005;331:726–736. doi: 10.1016/j.bbrc.2005.03.153. [DOI] [PubMed] [Google Scholar]
- 19.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French J, Storer RD, Donehower LA. The nature of the heterozygous Trp53 knockout model for identification of mutagenic carcinogens. Toxicol Pathol. 2001;29:24–29. doi: 10.1080/019262301753178456. [DOI] [PubMed] [Google Scholar]
- 22.Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8:66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- 23.Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, Gudkov AV. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastrointestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 24.Spangrude GJ, Aihara Y, Weissman IL, Klein J. The stem cell antigens Sca-1 and Sca-2 subdivide thymic and peripheral T lymphocytes into unique subsets. J Immunol. 1988;141:3697–3707. [PubMed] [Google Scholar]
- 25.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science (New York, NY) 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 26.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 27.van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci USA. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filshie RJ. Cytokines in haemopoietic progenitor mobilisation for peripheral blood stem cell transplantation. Curr Pharm Des. 2002;8:379–394. doi: 10.2174/1381612023396122. [DOI] [PubMed] [Google Scholar]
- 29.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 32.Mitchel RE, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]