Abstract
Adipose tissue contains an abundant source of multipotent mesenchymal cells termed “adipose-derived stromal cells” (ASCs) that hold potential for regenerative medicine. However, the heterogeneity inherent to ASCs harvested using standard methodologies remains largely undefined, particularly in regards to differences across donors. Identifying the subpopulations of ASCs predisposed toward differentiation along distinct lineages holds value for improving graft survival, predictability, and efficiency. Human ASCs (hASCs) from three different donors were independently isolated by density-based centrifugation from adipose tissue and maintained in culture or differentiated along either adipogenic or osteogenic lineages using differentiation media. Undifferentiated and differentiated hASCs were then analyzed for the presence of 242 human surface markers by flow cytometry analysis. By comprehensively characterizing the surface marker profile of undifferentiated hASCs using flow cytometry, we gained novel insights into the heterogeneity underlying protein expression on the surface of cultured undifferentiated hASCs across different donors. Comparison of the surface marker profile of undifferentiated hASCs with hASCs that have undergone osteogenic or adipogenic differentiation allowed for the identification of surface markers that were upregulated and downregulated by osteogenic or adipogenic differentiation. Osteogenic differentiation induced upregulation of CD164 and downregulation of CD49a, CD49b, CD49c, CD49d, CD55, CD58, CD105, and CD166 while adipogenic differentiation induced upregulation of CD36, CD40, CD146, CD164, and CD271 and downregulation of CD49b, CD49c, CD49d, CD71, CD105, and CD166. These results lend support to the notion that hASCs isolated using standard methodologies represent a heterogeneous population and serve as a foundation for future studies seeking to maximize their regenerative potential through fluorescence-activated cell sorting-based selection before therapy.
Introduction
The International Fat Applied Technology Society adopted the term “adipose-derived stromal cells” (ASCs) to identify a plastic-adherent, multipotent cell population isolated from adipose tissue.1 Current methods for ASC isolation utilize collagenase digestion and centrifugal separation to isolate vascular and stromal cells from primary adipocytes.2 While some authors have claimed that ASCs represent a homogenous pure population of stem cells, recent evidence suggests that ASCs are actually a heterogeneous mixture containing both stem and more committed progenitor cells.1
ASCs represent an abundant source of multipotent adult stem cells that are suitable for aesthetic, tissue engineering, and regenerative medicine applications. Soft tissue defects are a common problem after trauma, burns, and oncologic surgery. Many studies have demonstrated successful adipogenic differentiation of ASCs in vitro followed by transplantation for the correction of soft tissue defects.3,4 Biomimetic scaffolds have emerged as an effective means to improve adipose graft survival and proliferation. von Heimburg et al. transplanted ASC-seeded scaffolds subcutaneously and showed improved neovascularization, higher proliferation rates of mature adipocytes, and enhanced penetration of adipose precursor cells, as compared with controls.5
In addition to adipogenic differentiation, ASCs are able to differentiate into osteoblast-like cells in the presence of dexamethasone, ascorbate, β-glycerophosphate, and vitamin D3.6 Levi et al. showed that critical-sized 4-mm calvarial defects in the parietal bone of adult male nude mice can be efficiently healed with human ASCs (hASCs).7 The therapeutic potential of ASCs as a source for new bone tissue was fully realized in a case of autologous ASC transplantation for the treatment of a 7-year-old girl suffering from widespread calvarial defects after severe head injury. CT scans showed new bone formation and near complete calvarial continuity 3 months postreconstruction.8 ASCs meet a clear need for an accessible, abundant, and autologous source of cells for the correction of skeletal defects.
Efforts to characterize ASC surface marker expression have revealed that CD29, CD44, CD71, CD90, and CD105 are common to both ASCs and bone marrow-derived mesenchymal stem cells (BM-MSCs), while ASCs differentially express CD10, CD13, and CD73.9 A recent study by Baer et al. comprehensively characterized the surface marker profile of cultured hASCs isolated from 13 different female donors.10 Our study both confirms and builds on these results by comprehensively characterizing the surface marker profiles of undifferentiated hASCs as well as hASCs that have undergone either osteogenic or adipogenic differentiation using lyoplates that contain 242 purified monoclonal antibodies and corresponding isotype controls. These lyoplates allow for a high-throughput screening of hundreds of surface markers by flow cytometry analysis. This analysis provides a searchable database of cell surface marker expression on undifferentiated hASCs and under conditions that promote their osteogenic or adipogenic differentiation. We identify several surface markers with increased or decreased expression on hASCs undergoing either adipogenic or osteogenic differentiation. These differentially expressed markers may allow for enrichment of distinct hASC subpopulations with enhanced differentiation capacity before cell-based therapy for the regeneration of soft and hard tissue defects.
Materials and Methods
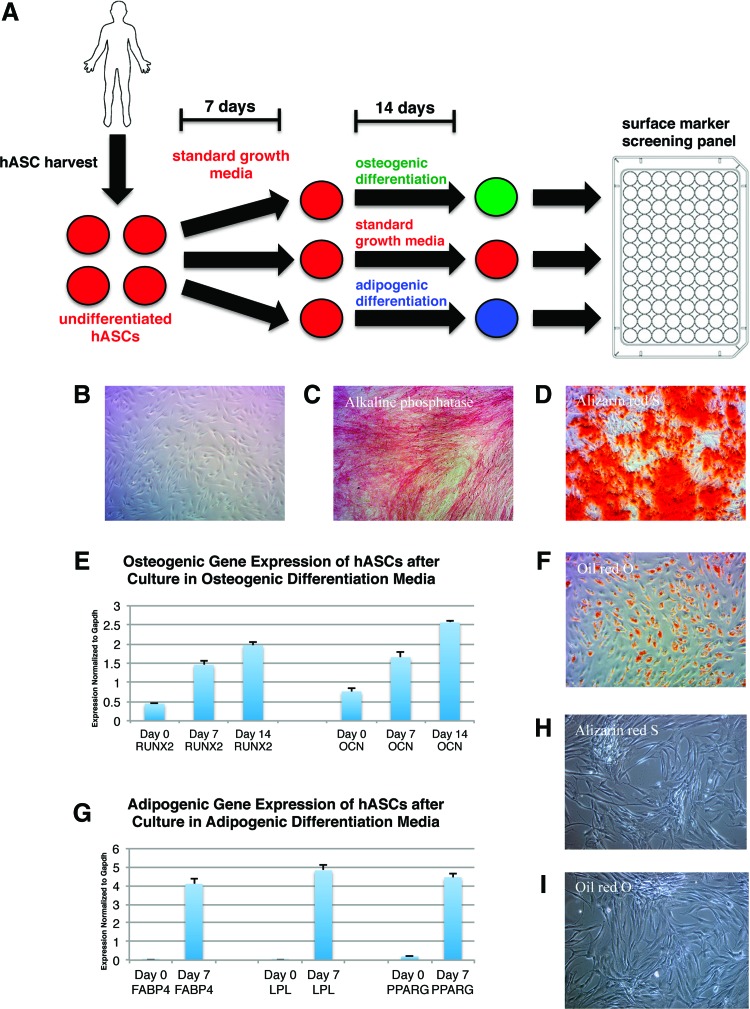
For a schematic of the experimental overview, see Figure 1A.
FIG. 1.
Osteogenic and adipogenic differentiation of human adipose-derived stromal cells (hASCs). (A) Schematic showing experimental overview for the identification of surface markers differentially regulated after osteogenic or adipogenic differentiation. (B) Light microscope image of undifferentiated hASCs from a single donor after 21 days in culture. (C) Alkaline phosphatase staining in hASCs harvested from a single donor on day 7 of osteogenic differentiation. (D) Alizarin red S staining in hASCs harvested from a single donor on day 14 of osteogenic differentiation. (E) Quantitative real-time–polymerase chain reaction (qRT-PCR) of RUNX2 and Osteocalcin (OCN) expression in hASCs harvested from a single donor on day 7 and 14 of osteogenic differentiation. Errors bars represent one standard deviation with an n=3 wells. (F) Oil red O staining in hASCs harvested from a single donor on day 7 of adipogenic differentiation. (G) qRT-PCR of FABP4, LPL, and PPARG expression in hASCs harvested from a single donor on day 7 of adipogenic differentiation. Errors bars represent one standard deviation with an n=3 wells. (H) Alizarin red S staining on hASCs maintained in vitro in plain medium (no differentiation components added) for 21 days from a single donor. (I) Oil red O staining on hASCs maintained in vitro in plain medium (no differentiation components added) for 21 days from a single donor. Color images available online at www.liebertpub.com/tea
Tissue procurement and isolation of hASCs
Raw lipoaspirate samples were obtained by suction-assisted liposuction from three healthy female donors (ages 35, 42, 44) after acquiring informed consent in accordance with Stanford University Institutional Review Board guidelines. Cells from each donor (n=3) were treated as distinct biological replicates. hASCs were harvested from fat as previously described.11 Raw lipoaspirate was allowed to separate by gravity sedimentation, and the blood layer was aspirated. Fat was treated with 0.075% collagenase type I (Sigma-Aldrich, St. Louis, MO) in Hank's balanced salt solution (Cellgro, Manassas, VA) for 1 h in a 37°C water bath with gentle agitation at 150 rpm. The stromal vascular fraction (SVF) containing the hASCs was then pelleted by centrifugation at 1200 g for 5 min and isolated by aspiration of supernatant. Digest activity was inactivated by resuspending SVF in an equal volume of standard cell culture growth medium (Dulbecco's modified Eagle's medium [DMEM] with GlutaMAX [Invitrogen Corp., Carlsbad, CA]), 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin, and it was then filtered through a 100 μm cell strainer to remove large undigested fragments. Cells were pelleted again, re-suspended in standard cell culture growth medium, seeded onto 15 cm tissue culture plates (5,000,000 cells/plate) and six-well plates (100,000 cells/well), and incubated at 37°C with an atmosphere of 5% carbon dioxide. Cells were expanded in standard cell culture growth medium and passaged with Accutase (Life Technologies, Grand Island, NY). Control undifferentiated hASCs were maintained in culture for a total of 21 days.
In vitro osteogenic differentiation of hASCs
For osteogenic differentiation, cells were grown to at least 70% confluency before being treated with osteogenic differentiation medium (ODM), which consisted of DMEM with 10% FBS, 1% penicillin/streptomycin, 100 μg/mL ascorbic acid, and 10 mM β-glycerophosphate. Alkaline phosphatase staining was performed on cells in six-well plates at day 7 of differentiation. Alizarin red staining was performed at day 14 to assess extracellular mineralization. RNA was collected at days 0, 7, and 14 for osteogenic gene expression analysis by quantitative real-time–polymerase chain reaction (qRT-PCR). hASCs undergoing osteogenic differentiation were cultured for 7 days in standard cell culture growth medium and 14 days in ODM. For primer sequences, see Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea).
In vitro adipogenic differentiation of hASCs
For adipogenic differentiation, cells were grown to 90% confluency and then treated with adipogenic differentiation medium (ADM) containing DMEM, 10% FBS, 1% penicillin/streptomycin, 10 μg/mL insulin, 1 μM dexamethasone, 0.5 mM methylxanthine, and 200 μM indomethacin. Oil red O staining was performed on cells in six-well plates at day 7 of differentiation. RNA was collected at day 0 and 7 for adipogenic gene expression analysis by qRT-PCR. hASCs undergoing adipogenic differentiation were cultured for 7 days in standard cell culture growth medium and 14 days in ADM. For primer sequences, see Supplementary Table S1.
Cell surface marker screening of hASCs
hASCs harvested as previously described were analyzed using BD Lyoplate™ Human Cell Surface Marker Screening Panel (cat. 560747; BD Biosciences, Franklin Lakes, NJ) containing 242 purified monoclonal antibodies and corresponding isotype controls. The manufacturer's staining protocol was followed with slight modifications. hASCs were plated into U-bottom 96-well plates at a density of 5.0×105 cells per well in fluorescence-activated cell sorting (FACS) buffer containing 10 μg/mL DNAse. Primary antibody incubation was carried out in 100 μL volume for 30 min on ice followed by 2×in FACS buffer washes. Next, cells were incubated with biotinylated secondary antibodies (goat anti-mouse 1:200, goat anti-rat 1:200) in 100 μL volume for 30 min on ice followed by 3× FACS buffer washes. Tertiary incubation with Alexa Fluor 647 Streptavidin (1:4000) was carried out in 100 μL volume for 30 min on ice followed by 3× FACS buffer washes. DAPI was used as the viability dye. Analysis was performed using flow cytometer BD LSR Fortessa with a High-Throughput Sampler.
Reverse transcription and qRT–PCR
RNA from cultured cells was extracted using Trizol (Life Technologies) and an RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA) as per the manufacturer's protocol. Isolated RNA was quantified using Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA) and then reverse transcribed using TaqMan Reverse Transcription Reagents (Invitrogen). Expression of the osteogenic genes (RUNX2, OCN) and adipogenic genes (FABP4, LPL, PPARG) was determined by qRT-PCR using the Applied Biosystems Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) and the SYBR Green PCR Master Mix (Applied Biosytems). Target quantities were normalized to endogenous β-actin quantities using the standard curve method. Primers for RUNX2, OCN, FABP4, LPL, PPARG, and β-actin were constructed based on their PrimerBank (Massachusetts General Hospital, Boston, MA) sequences (Supplementary Table S2).
Evaluation of osteogenic potential of CD55− subpopulation
For evaluation of the in vitro osteogenic potential of the CD55− subpopulation of ASCs, CD55− cells were isolated from the SVF of freshly obtained lipoaspirate via FACs. Unsorted SVF cells and CD55− ASCs were separately seeded onto six-well plates and cultured in traditional growth medium consisting of DMEM with 10% FBS and 0.01% Primocin (InvivoGen, San Diego, CA). Triplicate wells were used for all assays.
Once cells had reached 80–90% confluency, cells were cultured in ODM consisting of traditional growth medium supplemented with 10 mM β-glycerophosphate and 100 μg/mL ascorbic acid. Alkaline phosphatase staining was performed at 7 days, and then stain intensity was quantified on ImageJ by measuring the mean gray value for each well. Alizarin red S staining was performed at 14 days, and then photometric quantification of extracellular mineralization was performed.12 Briefly, 2 mL of a solution of 20% methanol and 10% acetic acid was added to each well with Alizarin red S stain. After 15 min of gentle rocking at room temperature, 1 mL of resultant solution was collected and optical density was red at 450 nm. Osteogenic gene expression was analyzed at days 0, 7, and 14 via qRT–PCR.
Results
hASCs harvested from abdominal lipoaspirates of three healthy female donors were either maintained as undifferentiated hASCs in culture (Fig. 1B) or differentiated along osteogenic and adipogenic lineages. The osteogenic differentiation of hASCs using standard ODM was verified by alkaline phosphatase staining on day 7 (Fig. 1C), Alizarin red S staining on day 14 (Fig. 1D), and qRT-PCR analysis of RUNX2 and OCN expression for each donor (Fig. 1E). Only data from a single donor are shown. Adipogenic differentiation using standard ADM over a period of 7 days was verified by Oil red O staining on day 7 (Fig. 1F) and qRT-PCR analysis of FABP4, LPL, and PPARG expression for each donor (Fig. 1G). Only data from a single donor are shown. Negative controls for Alizarin red S (Fig. 1H) and Oil red O (Fig. 1I) staining on hASCs maintained in vitro for 21 days in plain medium (no differentiation components added) were also included. These data demonstrate that hASCs do not spontaneously differentiate when maintained in vitro and are in concordance with previous studies showing that the rate of spontaneous differentiation in vitro is negligible.13–15
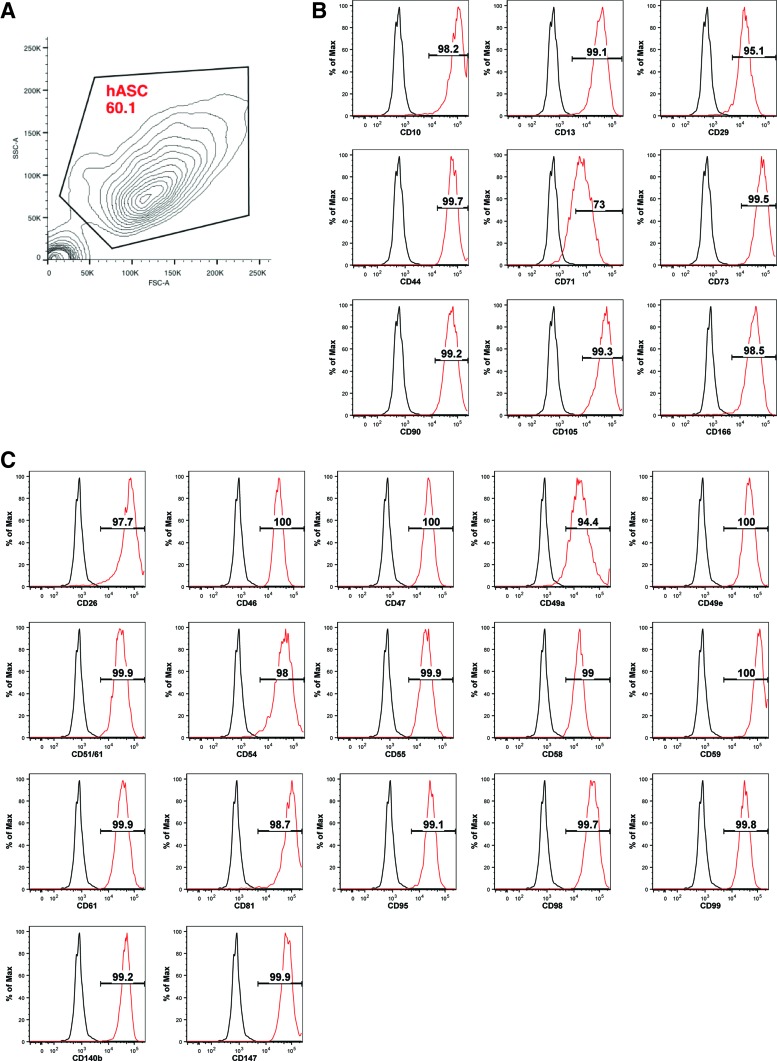
After confirming adipogenic and osteogenic differentiation, undifferentiated and differentiated hASCs were lifted from tissue culture plates using Accutase. In comparison to Trypsin-EDTA, Accutase offers gentler dissociation and reduced surface protein degradation,16 potentially allowing for a more accurate assessment of surface marker expression. Previous studies analyzing hASC surface marker expression have used Accutase based on this rationale.10 hASCs were then analyzed by flow cytometry using a surface marker screening panel containing purified monoclonal antibodies that are specific to 242 human surface proteins. To control for changes in surface marker expression due to culture conditions, both undifferentiated and differentiated hASCs were cultured for equal periods of time (21 days) (Fig. 1A). FACS analysis gating allowed for the elimination of debris and selection of cultured undifferentiated hASCs (Fig. 2A). Lyoplate analysis confirmed expression of the commonly cited ASC markers CD10, CD13, CD29, CD44, CD71, CD73, CD90, CD105, and CD166 on the surface of cultured, undifferentiated hASCs (Fig. 2B). Surface marker analysis of cultured, undifferentiated hASCs also revealed broad expression (>90%) within a narrow range across all donors (n=3) of several markers, including CD26, CD46, CD47, CD49a, CD49e, CD51/61, CD54, CD55, CD58, CD59, CD61, CD81, CD95, CD98, CD99, CD140b, and CD147 (Fig. 2C). These results are consistent with recent findings by Baer et al.10
FIG. 2.
Validation of known surface markers on undifferentiated hASCs by lyoplate analysis. (A) Gating strategy to eliminate debris and select for cultured undifferentiated hASCs represented by contour plot. (B) fluorescence-activated cell sorting (FACS) analysis of CD10, CD13, CD29, CD44, CD71, CD73, CD90, CD105, and CD166 expression on undifferentiated hASCs isolated from a single donor cultured for 21 days. Gates representing percentage of the population positive for each marker drawn based on isotype controls (red, undifferentiated; black, isotype control). (C) FACS analysis of CD26, CD46, CD47, CD49a, CD49e, CD51/61, CD54, CD55, CD58, CD59, CD61, CD81, CD95, CD98, CD99, CD140b, CD147, and CD166 expression on undifferentiated hASCs isolated from a single donor cultured for 21 days. Gates representing percentage of the population positive for each marker drawn based on isotype controls (red, undifferentiated; black, isotype control). Color images available online at www.liebertpub.com/tea
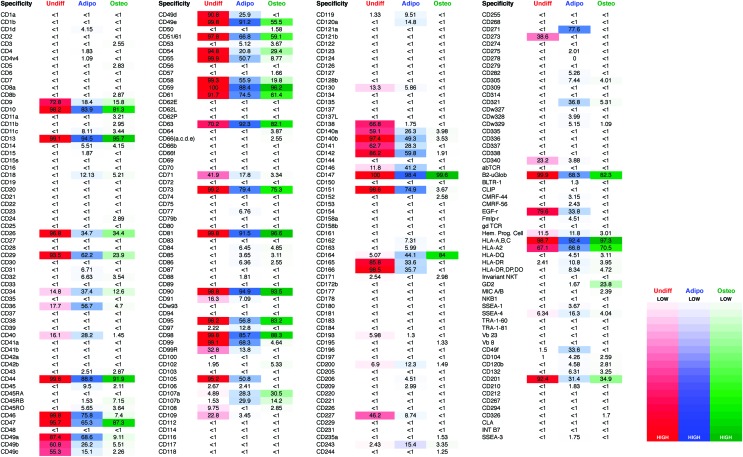
To identify surface markers induced on adipogenic and osteogenic differentiation, we compared the surface marker expression of undifferentiated hASCs with that of adipogenic or osteogenic differentiated hASCs. In comparison to undifferentiated ASCs, osteogenic differentiation induced upregulation of CD164 (Fig. 3A) and downregulation of CD49a, CD49b, CD49c, CD49d, CD55, CD58, CD105, and CD166 (Fig. 3B). Adipogenic differentiation induced expression of CD36, CD40, CD146, CD164, and CD271 (Fig. 3A) and downregulation of CD49b, CD49c, CD49d, CD71, CD105, and CD166 (Fig. 3C). The mean percentage of positive cells for each surface marker assayed across each of the three donor hASC populations can be found in Figure 4. For a searchable database of mean surface marker expression across donors with gene ID and CD nomenclature, see Supplementary Table S2.
FIG. 3.
Differentially expressed hASC surface markers after adipogenic or osteogenic differentiation. (A) FACS analysis of CD36, CD40, CD146, CD164, and CD271 expression on the surface of undifferentiated hASCs, osteogenically differentiated hASCs, and adipogenically differentiated hASCs isolated from the same donor (red, undifferentiated; blue, adipogenic; green, osteogenic). Gates representing percentage of the population positive for each marker drawn based on isotype controls. (B) FACS analysis of CD49a, CD49b, CD49c, CD49d, CD55, CD58, CD105, and CD166 on undifferentiated hASCs and osteogenically differentiated hASCs isolated from the same donor (red, undifferentiated; green, osteogenic). Gates representing percentage of the population positive for each marker drawn based on isotype controls. (C) FACS analysis of CD49b, CD49c, CD49d, CD71, CD105, and CD166 on undifferentiated hASCs and adipogenically differentiated hASCs isolated from the same donor (red, undifferentiated; blue, adipogenic). Gates representing percentage of the population positive for each marker drawn based on isotype controls. Color images available online at www.liebertpub.com/tea
FIG. 4.
Lyoplate analysis of surface marker expression on undifferentiated and differentiated hASCs. FACS-based expression analysis of 242 surface markers using the BD Lyoplate™ Human Surface Marker Screening Panel on cultured undifferentiated hASCs, osteogenically differentiated hASCs, and adipogenically differentiated hASCs (red, undifferentiated; blue, adipogenic; green, osteogenic). These values represent the mean percentage of positive cells across the three donor populations for each surface marker in the screen. Color images available online at www.liebertpub.com/tea
To determine whether differential expression of a surface marker on differentiated hASCs versus undifferentiated hASCs might hold functional significance, we examined a marker with marked differential expression identified in our screen. CD55 was expressed on 99.9% of undifferentiated, cultured hASCs, 74.6% of undifferentiated, uncultured hASCs, and only 8.56% of osteogenically differentiated hASCs (Fig. 3A). This observation suggested that sorting for CD55-negative cells might increase osteogenic differentiation capacity. We compared unsorted, uncultured hASCs with uncultured CD55− hASCs for Alizarin red S and alkaline phosphatase staining as well as RUNX2, OPN, and OCN gene expression by qRT-PCR analysis. CD55− hASCs demonstrated significantly elevated levels of alkaline phosphatase staining at 7 days and significantly elevated Alizarin red S staining at 14 days in comparison to unsorted hASCs (Fig. 5A, B). Similarly, CD55− hASCs showed significantly elevated OPN gene expression at day 0 and 7, and OCN gene expression at day 7 and 14 (Fig. 5C, D) as compared with unsorted hASCs. These data serve as a strong proof of concept for the validity of screening for differentiation-based changes in surface marker expression on hASCs for the purposes of identifying subpopulations with enhanced or diminished differentiation potential.
FIG. 5.
Analysis of osteogenic differentiation of CD55+ subpopulation. (A) Quantification of alkaline phosphatase stain intensity on ImageJ for unsorted and CD55− hASCs on day 7 of osteogenic differentiation (*p<0.01). (B) Quantification of Alizarin red S staining on photometric analysis of unsorted and CD55− hASCs on day 14 of osteogenic differentiation. Representative images of Alizarin red S staining on unsorted and CD55− hASCs at day 14 of osteogenic differentiation (*p<0.01). (C) qRT-PCR of OPN expression in unsorted and CD55− hASCs harvested from a single donor on day 0, 7, and 14 of osteogenic differentiation. Errors bars represent one standard deviation with an n=3 wells (*p and **p<0.01). (D) qRT-PCR of OCN expression in unsorted and CD55− hASCs harvested from a single donor on day 14 of osteogenic differentiation. Errors bars represent one standard deviation with an n=3 wells (*p and **p<0.01).
To identify donor-specific differences in marker expression, we looked for markers displaying high variability in their expression among donors in cultured, undifferentiated hASCs. We identified the top 20 markers with the broadest ranges across the three donors (Table 1). For these same markers, we also looked at variability in their expression among donor hASCs after adipogenic and osteogenic differentiation (Table 1). In general, variability in the expression of surface markers among donors decreased on both adipogenic and osteogenic differentiation for 14 out of these 20 markers (Table 1). This is not surprising given that differentiation along a given lineage should lead to a convergence of phenotype, even among a population as hetereogeneous as hASCs.
Table 1.
Analysis of Variability in hASC Surface Marker Expression Across Human Donors
| Surface marker | Undifferentiated | Adipogenic | Osteogenic |
|---|---|---|---|
| CD34 | 14.4 (4.9–25.1) | 43.3 (18.9–50) | 12 (10.2–15.6) |
| CD36 | 22.8 (4.7–25.6) | 54 (53.4–62.7) | 3.62 (3.5–6.98) |
| CD49c | 74 (17.6–74.3) | 15.4 (13.9–16) | 2.29 (2.18–2.31) |
| CD91 | 20.82 (0.58–27.5) | 8.55 (3.47–9.25) | 0.654 (0.046–0.884) |
| CD99R | 29.4 (27.4–41.6) | 17.5 (1.69–22.2) | 0.0453 (0.0351–0.397) |
| CD107a | 4.89 (0–9.69) | 25.8 (20.8–38.3) | 28.8 (26.5–36.2) |
| CD108 | 13.6 (0.089–15.6) | 0.055 (0.054–0.767) | 2.92 (2.02–3.61) |
| CD109 | 14.5 (14.2–39.7) | 3.81 (1.69–4.85) | 0.025 (0.015–0.068) |
| CD130 | 14.1 (5.72–20.1) | 5.18 (3.66–8.74) | 0.0595 (0.0175–0.13) |
| CD138 | 61.5 (61.2–77.7) | 1.95 (0.63–2.67) | 0.111 (0.0202–0.19) |
| CD140a | 61.7 (48.7–66.9) | 22.1 (13.6–43.2) | 4.32 (0.76–6.86) |
| CD141 | 55 (43.7–89.4) | 26.5 (21.7–36.7) | 0.696 (0.168–0.825) |
| CD165 | 95.4 (62.2–99.8) | 37.4 (22.8–40.6) | 0.0707 (0.0228–0.0991) |
| CD193 | 5.43 (0.713–11.8) | 1.32 (0.651–1.93) | 0.259 (0.051–0.323) |
| CD200 | 5.23 (2.77–12.7) | 9.59 (6.69–20.6) | 1.58 (1.13–1.76) |
| CD227 | 53.8 (27.6–57.2) | 9.01 (6.55–10.66) | 0.301 (0.117–2.02) |
| CD273 | 36.7 (31.3–47.8) | 0.363 (0.133–0.563) | 0.0965 (0.0457–0.275) |
| CD340 | 19.7 (13.1–36.8) | 3.75 (1.33–6.56) | 0.205 (0.115–0.929) |
| HLA-A2 | 99.9 (1.40–100) | 100 (0.401–100) | 84 (29.1–98.4) |
The mean expression values (% population positive) and the range of expression for surface markers displaying the highest variability in expression on cultured, undifferentiated hASCs across the three donors.
hASCs, human adipose-derived stromal cells.
Discussion
The regenerative potential of hASCs resides in their ability to secrete an array of cytokines/growth factors and to be differentiated into a variety of cell types using established lineage-specific factors. Selecting for hASCs subpopulations predisposed toward osteogenic or adipogenic differentiation on the basis of surface marker expression holds value for improving graft survival, predictability, and efficiency. Here, by comprehensively characterizing the surface marker profiles of both differentiated and undifferentiated hASCs, we have identified surface markers either induced or repressed on osteogenic/adipogenic differentiation and uncovered some of the heterogeneity in protein expression among hASCs. This strategy may be applied to other cell types (i.e., BM-MSCs) used in tissue engineering and regenerative medicine for the identification of candidate surface markers predicting enhanced differentiation capacity.
The cell surface receptor CD105 (endoglin) was predicted to enhance hASC osteogenesis through negative enrichment by our screening approach. CD105 expression is believed to correlate with increased stem cell capacity in both adipose- and bone marrow-derived mesenchymal cells,7,17,18 and previous work demonstrated that CD105 depletion enhances both in vitro and in vivo osteogenesis by selecting for an hASC subpopulation with decreased TGF-B1/Smad2 signaling.19 In the lyoplate analysis, CD105 was broadly expressed on undifferentiated hASCs across all three donors (mean=95.2%) but was almost nonexistent on the surface of osteogenically differentiated hASCs (mean=0.913%). Although retrospective, this observation lends further support to the validity of this screening approach for the identification of markers impacting differentiation potential. The validity of enriching or depleting for a single surface marker has also been demonstrated for CD90 in the literature.20
Several markers (CD36, CD40, CD146, CD164, CD271) were upregulated on the surface of hASCs in response to adipogenic differentiation. CD36, a class B scavenger receptor family member, was expressed on 54% of adipogenically differentiated hASCs in comparison to 22.8% of undifferentiated hASCs and 3.5% of osteogenically differentiated hASCs. CD36 binds a large set of ligands, including collagen, thrombospondin, low-density lipoprotein, and long-chain fatty acid.21–23 Previous studies have suggested that CD36 promotes adipocyte differentiation and adipogenesis,24 a finding that is consistent with our data. CD40, another marker upregulated by adipogenic differentiation, is known to contribute to crosstalk between lymphocytes and adipocytes.25,26 CD40 was expressed on 28.8% of hASCs differentiated down the adipogenic lineage, 18.7% of undifferentiated hASCs, and substantially downregulated by osteogenic differentiation (1.2%). CD40 on the surface of human adipocytes regulates adipocytokine production by T lymphocytes via heterotypic contact and may contribute to obesity-related inflammation and insulin resistance.27 Given these findings, it may be therapeutically advantageous to select for the CD40-negative subset of adipogenically differentiated hASCs for adipose tissue engineering in diabetic patients.
Another surface marker, CD146, was expressed on 41.7% of adipogenically differentiated hASCs but only 11.4% of undifferentiated hASCs and <1% of osteogenically differentiated hASCs. CD146 has been implicated in the regulation of stromal vascular progenitors found within adult human adipose tissue. In adipose tissue, perivascular stromal cells are organized in two discrete layers, with the outermost consisting of CD146+ pericytes. The CD146+ subset has been suggested to represent a population transitional between pericytes and adipose stromal cells.28 Furthermore, soluble CD146 displays angiogenic properties and has been shown to promote neovascularization in ischemic models.29 Given these findings and our data showing CD146 upregulation in adipogenically differentiated hASCs, CD146 enrichment of hASCs may represent a viable strategy before transplantation to facilitate angiogenesis in conjunction with adipogenesis in recipient sites with poor blood supply, such as in irradiated tissue or in individuals with a reduced capacity for neovascularization, such as elderly and diabetic patients.
CD271 was expressed on 75% of adipogenically differentiated hASCs, but was almost nonexistent on osteogenically differentiated hASCs (0.2%) and undifferentiated hASCs (1.35%). CD271, also known as low-affinity nerve growth factor receptor, has primarily been studied in the context of the nervous system where it stimulates neuronal cells to survive and differentiate.30 A recent study found that CD271 defines a subset of multipotent bone marrow stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties.31 Although CD271 has not been linked to adipogenic processes, our finding that CD271 is broadly induced by adipogenic differentiation suggests a functional role for the marker in adipocyte differentiation and adipogenesis. However, from a translational perspective, given the low abundance (1.4%) of a CD271+ population within undifferentiated hASCs, CD271 represents a poor candidate for adipogenic enrichment of freshly harvested, undifferentiated hASCs.
In comparison to adipogenic differentiation, relatively few markers were significantly upregulated by osteogenic differentiation. One of these, CD164, was expressed on 91% of osteogenically differentiated hASCs, in comparison to 40.7% of adipogenically hASCs and only 5.46% of undifferentiated hASCs. CD164, also known as endolyn, is a cell adhesion molecule expressed by human hematopoietic progenitors and bone marrow stromal cells. In these cell types, CD164 functions as a negative regulator of hematopoiesis.32 Although the functional significance of CD164 in hASCs has not been established, the CD164+ subset of hASCs may represent a population with enhanced osteogenic differentiation potential, given the significant induction of CD164 on the surface of osteogenically differentiated hASCs.
In conclusion, ASCs are an abundant autologous source for multipotent mesenchymal cells, making them an ideal cell type for applications in aesthetic surgery, tissue engineering, and regenerative medicine to repair tissue defects. Here, we identify several surface markers with increased expression on hASCs that have undergone either adipogenic or osteogenic differentiation. These differentially expressed markers may allow, as in the case of CD105, for prospective selection of distinct hASC subpopulations with enhanced differentiation capacity before transplantation for the repair of osseous and adipose tissue defects. Most importantly, the screening methodology documented here may also be applied to other cell types for applications in regenerative medicine and tissue engineering.
Supplementary Material
Acknowledgments
This work was supported in part by the Hagey Laboratory for Pediatric Regenerative Medicine, The Oak Foundation (to M.T.L.), NIH grant U01 HL099776 (to M.T.L), and the Gunn/Olivier fund (to M.T.L.). G.G.W was supported by the Stanford School of Medicine, the Stanford Medical Scientist Training Program, and NIGMS training grant GM0736.
Authors' Contributions
Graham G. Walmsley: conception and design, data collection, analysis and interpretation, writing the article, critical revision of the article; David A. Atashroo: data collection; Zeshaan N. Maan: data collection; Michael S. Hu: data collection; Elizabeth R. Zielins: data collection; Jonathan M. Tsai: data collection; Dominik Duscher: data collection; Kevin Paik: data collection, critical revision of the article; Ruth Tevlin: critical revision of the article, figure creation; Owen Marecic: data collection and revision; Derrick C. Wan: critical revision of the article; Geoffrey C. Gurtner: critical revision of the article; Michael T. Longaker: conception and design, analysis and interpretation, critical revision of the article, obtaining funding.
Disclosure Statement
No competing financial interests exist.
References
- 1.Baer P.C., and Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012, 812693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho J.H., Ma W.H., Tseng T.C., Chen Y.F., Chen M.H., and Lee O.K. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A 17, 255, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Rajashekhar G., Traktuev D.O., Roell W.C., Johnstone B.H., Merfeld-Clauss S., Van Natta B., Rosen E.D., March K.L., and Clauss M. IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells 26, 2674, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Yu G., Floyd Z.E., Wu X., Hebert T., Halvorsen Y.D., Buehrer B.M., and Gimble J.M. Adipogenic differentiation of adipose-derived stem cells. Methods Mol Biol 702, 193, 2011 [DOI] [PubMed] [Google Scholar]
- 5.von Heimburg D., Zachariah S., Low A., and Pallua N. Influence of different biodegradable carriers on the in vivo behavior of human adipose precursor cells. Plastic Reconstr Surg 108, 411, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Kroeze R.J., Knippenberg M., and Helder M.N. Osteogenic differentiation strategies for adipose-derived mesenchymal stem cells. Methods Mol Biol 702, 233, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Levi B., James A.W., Nelson E.R., Vistnes D., Wu B., Lee M., Gupta A., and Longaker M.T. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 5, e11177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lendeckel S., Jodicke A., Christophis P., Heidinger K., Wolff J., Fraser J.K., Hedrick M.H., Berthold L., and Howaldt H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32, 370, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Mizuno H., Tobita M., and Uysal A.C. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30, 804, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Baer P.C., Kuci S., Krause M., Kuci Z., Zielen S., Geiger H., Bader P., and Schubert R. Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev 22, 330, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., and Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7, 211, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Panetta N.J., Gupta D.M., Kwan M.D., Wan D.C., Commons G.W., and Longaker M.T. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg 124, 65, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Pansky A., Roitzheim B., and Tobiasch E. Differentiation potential of adult human mesenchymal stem cells. Clin Lab 53, 81, 2007 [PubMed] [Google Scholar]
- 14.de Girolamo L., Sartori M.F., Albisetti W., and Brini A.T. Osteogenic differentiation of human adipose-derived stem cells: comparison of two different inductive media. J Tissue Eng Regen Med 1, 154, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ogawa R., Mizuno H., Hyakusoku H., Watanabe A., Migita M., and Shimada T. Chondrogenic and osteogenic differentiation of adipose-derived stem cells isolated from GFP transgenic mice. J Nippon Med Sch 71, 240, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Quan Y., Yan Y., Wang X., Fu Q., Wang W., Wu J., Yang G., Ren J., and Wang Y. Impact of cell dissociation on identification of breast cancer stem cells. Cancer Biomark 12, 125, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Aslan H., Zilberman Y., Kandel L., Liebergall M., Oskouian R.J., Gazit D., and Gazit Z. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells 24, 1728, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Rada T., Reis R.L., and Gomes M.E. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev 7, 64, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Levi B., Wan D.C., Glotzbach J.P., Hyun J., Januszyk M., Montoro D., Sorkin M., James A.W., Nelson E.R., Li S., Quarto N., Lee M., Gurtner G.C., and Longaker M.T. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem 286, 39497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung M.T., Liu C., Hyun J.S., Lo D.D., Montoro D.T., Hasegawa M., Li S., Sorkin M., Rennert R., Keeney M., Yang F., Quarto N., Longaker M.T., and Wan D.C. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19, 989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baillie A.G., Coburn C.T., and Abumrad N.A. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol 153, 75, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Nicholson A.C., Frieda S., Pearce A., and Silverstein R.L. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol 15, 269, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Tandon N.N., Kralisz U., and Jamieson G.A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem 264, 7576, 1989 [PubMed] [Google Scholar]
- 24.Christiaens V., Van Hul M., Lijnen H.R., and Scroyen I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim Biophys Acta 1820, 949, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Chatzigeorgiou A., Lyberi M., Chatzilymperis G., Nezos A., and Kamper E. CD40/CD40L signaling and its implication in health and disease. Biofactors 35, 474, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Chatzigeorgiou A., Phieler J., Gebler J., Bornstein S.R., and Chavakis T. CD40L stimulates the crosstalk between adipocytes and inflammatory cells. Horm Metab Res 45, 741, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Poggi M., Jager J., Paulmyer-Lacroix O., Peiretti F., Gremeaux T., Verdier M., Grino M., Stepanian A., Msika S., Burcelin R., de Prost D., Tanti J.F., and Alessi M.C. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia 52, 1152, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Zimmerlin L., Donnenberg V.S., Pfeifer M.E., Meyer E.M., Peault B., Rubin J.P., and Donnenberg A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77, 22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harhouri K., Kebir A., Guillet B., Foucault-Bertaud A., Voytenko S., Piercecchi-Marti M.D., Berenguer C., Lamy E., Vely F., Pisano P., Ouafik L., Sabatier F., Sampol J., Bardin N., Dignat-George F., and Blot-Chabaud M. Soluble CD146 displays angiogenic properties and promotes neovascularization in experimental hind-limb ischemia. Blood 115, 3843, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Buxser S., Puma P., and Johnson G.L. Properties of the nerve growth factor receptor. Relationship between receptor structure and affinity. J Biol Chem 260, 1917, 1985 [PubMed] [Google Scholar]
- 31.Kuci S., Kuci Z., Kreyenberg H., Deak E., Putsch K., Huenecke S., Amara C., Koller S., Rettinger E., Grez M., Koehl U., Latifi-Pupovci H., Henschler R., Tonn T., von Laer D., Klingebiel T., and Bader P. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica 95, 651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zannettino A.C., Buhring H.J., Niutta S., Watt S.M., Benton M.A., and Simmons P.J. The sialomucin CD164 (MGC-24v) is an adhesive glycoprotein expressed by human hematopoietic progenitors and bone marrow stromal cells that serves as a potent negative regulator of hematopoiesis. Blood 92, 2613, 1998 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.