Abstract
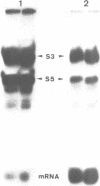
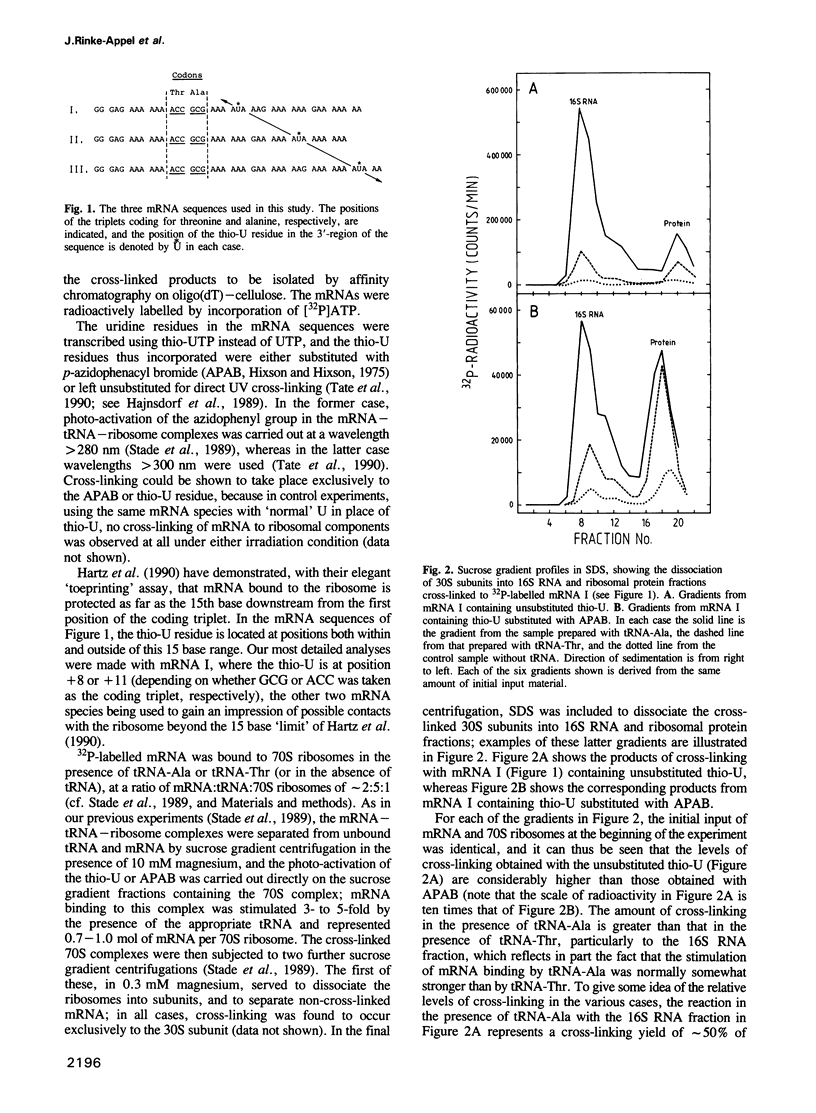
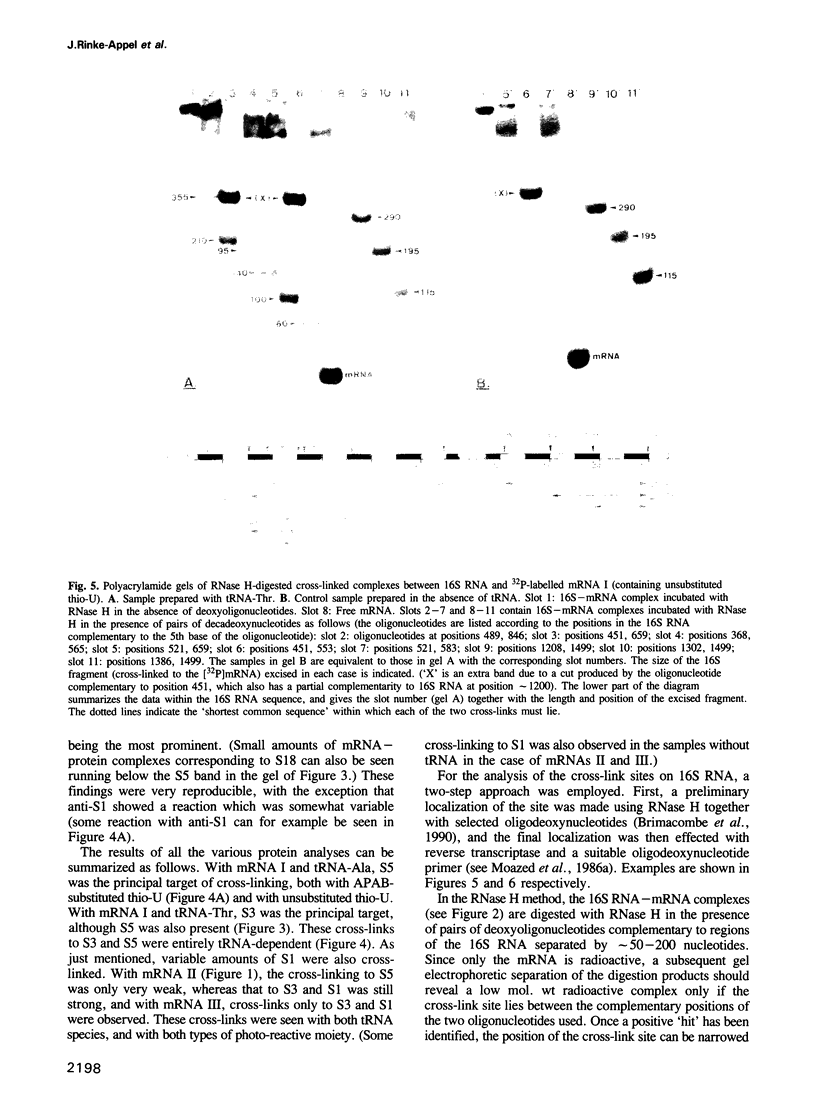
mRNA analogues approximately 40 bases long were prepared by T7 transcription from synthetic DNA templates. Each message contained the sequence ACC-GCG (coding for threonine and alanine, respectively), together with a single thio-U residue located at a variable position on the 3'-side of these coding triplets. The thio-U residue was either substituted with 4-azidophenacyl bromide to introduce a photo-reactive group, or was left unsubstituted for direct UV cross-linking. After binding to Escherichia coli 70S ribosomes in the presence of tRNA-Thr or tRNA-Ala, the thio-U residue or azidophenyl group was photo-activated and the products of cross-linking (which was exclusively to the 30S subunit) were analysed. Immunological analysis of the cross-linked proteins showed that S5 and S3, together with S1, were the targets of cross-linking at positions close to the decoding site, with the cross-linking to S3 and S1 persisting at positions further away. Analysis of the 16S RNA showed cross-links to the region of bases 1390-1400 in all cases, but in one instance (with the reactive nucleotide 11 bases from the decoding site) simultaneous cross-linking was observed to the latter region and to position 532; these two RNA regions are far apart in current three-dimensional models of the 30S subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Nucleotide residues of tRNA, directly interacting with proteins within the complex of the 30 S subunit of E. coli ribosome with poly(U) and NAcPhe-tRNA(Phe). FEBS Lett. 1989 Jan 30;243(2):299–302. doi: 10.1016/0014-5793(89)80149-8. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Stiege W., Kyriatsoulis A., Maly P. Intra-RNA and RNA-protein cross-linking techniques in Escherichia coli ribosomes. Methods Enzymol. 1988;164:287–309. doi: 10.1016/s0076-6879(88)64050-x. [DOI] [PubMed] [Google Scholar]
- Capel M. S., Kjeldgaard M., Engelman D. M., Moore P. B. Positions of S2, S13, S16, S17, S19 and S21 in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1988 Mar 5;200(1):65–87. doi: 10.1016/0022-2836(88)90334-8. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Evstafieva A. G., Shatsky I. N., Bogdanov A. A., Semenkov Y. P., Vasiliev V. D. Localization of 5' and 3' ends of the ribosome-bound segment of template polynucleotides by immune electron microscopy. EMBO J. 1983;2(5):799–804. doi: 10.1002/j.1460-2075.1983.tb01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornicki P., Nurse K., Hellmann W., Boublik M., Ofengand J. High resolution localization of the tRNA anticodon interaction site on the Escherichia coli 30 S ribosomal subunit. J Biol Chem. 1984 Aug 25;259(16):10493–10498. [PubMed] [Google Scholar]
- Gulle H., Hoppe E., Osswald M., Greuer B., Brimacombe R., Stöffler G. RNA-protein cross-linking in Escherichia coli 50S ribosomal subunits; determination of sites on 23S RNA that are cross-linked to proteins L2, L4, L24 and L27 by treatment with 2-iminothiolane. Nucleic Acids Res. 1988 Feb 11;16(3):815–832. doi: 10.1093/nar/16.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnsdorf E., Favre A., Expert-Bezançon A. New RNA-protein crosslinks in domains 1 and 2 of E. coli 30S ribosomal subunits obtained by means of an intrinsic photoaffinity probe. Nucleic Acids Res. 1989 Feb 25;17(4):1475–1491. doi: 10.1093/nar/17.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselman T., Camp D. G., Fox G. E. Phylogenetic evidence for tertiary interactions in 16S-like ribosomal RNA. Nucleic Acids Res. 1989 Mar 25;17(6):2215–2221. doi: 10.1093/nar/17.6.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Hixson S. H., Hixson S. S. P-Azidophenacyl bromide, a versatile photolabile bifunctional reagent. Reaction with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Sep 23;14(19):4251–4254. doi: 10.1021/bi00690a016. [DOI] [PubMed] [Google Scholar]
- Kang C. W., Cantor C. R. Structure of ribosome-bound messenger RNA as revealed by enzymatic accessibility studies. J Mol Biol. 1985 Jan 20;181(2):241–251. doi: 10.1016/0022-2836(85)90088-9. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Stöffler-Meilicke M., Stöffler G. Localization of the 3' end of 16S rRNA in Escherichia coli 30S ribosomal subunits by immuno electron microscopy. Mol Gen Genet. 1981;182(3):369–376. doi: 10.1007/BF00293924. [DOI] [PubMed] [Google Scholar]
- Melançon P., Lemieux C., Brakier-Gingras L. A mutation in the 530 loop of Escherichia coli 16S ribosomal RNA causes resistance to streptomycin. Nucleic Acids Res. 1988 Oct 25;16(20):9631–9639. doi: 10.1093/nar/16.20.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Schueler D., Brimacombe R. Selective isolation and detailed analysis of intra-RNA cross-links induced in the large ribosomal subunit of E. coli: a model for the tertiary structure of the tRNA binding domain in 23S RNA. Nucleic Acids Res. 1990 Aug 11;18(15):4325–4333. doi: 10.1093/nar/18.15.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell. 1986 Dec 26;47(6):985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Moazed D., Van Stolk B. J., Douthwaite S., Noller H. F. Interconversion of active and inactive 30 S ribosomal subunits is accompanied by a conformational change in the decoding region of 16 S rRNA. J Mol Biol. 1986 Oct 5;191(3):483–493. doi: 10.1016/0022-2836(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Montesano L., Glitz D. G. Wheat germ cytoplasmic ribosomes. Localization of 7-methylguanosine and 6-methyladenosine by electron microscopy of immune complexes. J Biol Chem. 1988 Apr 5;263(10):4939–4944. [PubMed] [Google Scholar]
- Oakes M. I., Lake J. A. DNA-hybridization electron microscopy. Localization of five regions of 16 S rRNA on the surface of 30 S ribosomal subunits. J Mol Biol. 1990 Feb 20;211(4):897–906. doi: 10.1016/0022-2836(90)90082-W. [DOI] [PubMed] [Google Scholar]
- Olson H. M., Lasater L. S., Cann P. A., Glitz D. G. Messenger RNA orientation on the ribosome. Placement by electron microscopy of antibody-complementary oligodeoxynucleotide complexes. J Biol Chem. 1988 Oct 15;263(29):15196–15204. [PubMed] [Google Scholar]
- Osswald M., Greuer B., Brimacombe R. Localization of a series of RNA-protein cross-link sites in the 23S and 5S ribosomal RNA from Escherichia coli, induced by treatment of 50S subunits with three different bifunctional reagents. Nucleic Acids Res. 1990 Dec 11;18(23):6755–6760. doi: 10.1093/nar/18.23.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T., Noller H. F. Dominant lethal mutations in a conserved loop in 16S rRNA. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1042–1046. doi: 10.1073/pnas.87.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G., Nierhaus K. H. Protein involved in the binding of dihydrostreptomycin to ribosomes of Escherichia coli. J Mol Biol. 1973 Nov 25;81(1):71–82. doi: 10.1016/0022-2836(73)90248-9. [DOI] [PubMed] [Google Scholar]
- Shen Z. H., Fox T. D. Substitution of an invariant nucleotide at the base of the highly conserved '530-loop' of 15S rRNA causes suppression of yeast mitochondrial ochre mutations. Nucleic Acids Res. 1989 Jun 26;17(12):4535–4539. doi: 10.1093/nar/17.12.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Stiege W., Stade K., Schüler D., Brimacombe R. Covalent cross-linking of poly(A) to Escherichia coli ribosomes, and localization of the cross-link site within the 16S RNA. Nucleic Acids Res. 1988 Mar 25;16(6):2369–2388. doi: 10.1093/nar/16.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate W., Greuer B., Brimacombe R. Codon recognition in polypeptide chain termination: site directed crosslinking of termination codon to Escherichia coli release factor 2. Nucleic Acids Res. 1990 Nov 25;18(22):6537–6544. doi: 10.1093/nar/18.22.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Elson D. A photoaffinity labelling study of the messenger RNA-binding region of Escherichia coli ribosomes. Nucleic Acids Res. 1978 Sep;5(9):3389–3407. doi: 10.1093/nar/5.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe M. R., Ohgi K., Glitz D. G. Ribosome structure. Localization of 7-methylguanosine in the small subunits of Escherichia coli and chloroplast ribosomes by immunoelectron microscopy. J Biol Chem. 1982 Aug 25;257(16):9822–9829. [PubMed] [Google Scholar]
- Trifonov E. N. Translation framing code and frame-monitoring mechanism as suggested by the analysis of mRNA and 16 S rRNA nucleotide sequences. J Mol Biol. 1987 Apr 20;194(4):643–652. doi: 10.1016/0022-2836(87)90241-5. [DOI] [PubMed] [Google Scholar]
- Vladimirov S. N., Babkina G. T., Venijaminova A. G., Gimautdinova O. I., Zenkova M. A., Karpova G. G. Structural arrangement of the decoding site of Escherichia coli ribosomes as revealed from the data on affinity labelling of ribosomes by analogs of mRNA--derivatives of oligoribonucleotides. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):245–256. doi: 10.1016/0167-4781(90)90063-8. [DOI] [PubMed] [Google Scholar]
- Walleczek J., Albrecht-Ehrlich R., Stöffler G., Stöffler-Meilicke M. Three-dimensional localization of the NH2- and carboxyl-terminal domain of ribosomal protein S1 on the surface of the 30 S subunit from Escherichia coli. J Biol Chem. 1990 Jul 5;265(19):11338–11344. [PubMed] [Google Scholar]