Abstract
In this study, the prevalence and intensity of Schistosoma haematobium infection was determined among school-age children living in the Middle and Lower Awash Valley, Afar Regional State of Ethiopia. Between February and May 2014, urine samples were collected from 885 school-age children (5–16 years of age) from the Middle (n = 632; 4 villages) and Lower (n = 253; 3 villages) Awash Valley. All samples were processed using urine filtration to detect and quantify S. haematobium eggs. In addition, a subset of the urine samples was tested for hematuria using a urine dipstick (n = 556). The overall prevalence was 20.8% (95% Confidence Interval (CI) = 18.1%, 23.5%), based on urine filtration but the prevalence considerably varied across villages both in the Middle (from 12.5% to 37.0%) and Lower Awash Valley (from 0 to 5.3%). The overall mean urine egg count (UEC) among the infected children was 4.0 eggs/10 ml of urine (95% CI = 2.43, 5.52). The infection intensity varied from 0.4 eggs/10 ml of urine to 7.7 eggs/10 ml of urine in the Middle Awash Valley, and from 0 to 1.1 eggs/10 ml of urine in Lower Awash Valley. Age and sex were not associated with S. haematobium infection based on the multivariable logistic regression model. The prevalence of hematuria was 56.3% (95% CI = 52.2%, 60.4%) among a subset of the study participants (556) examined using the urine dipstick. The prevalence of hematuria also varies with villages from 8.3% to 93.2%. In conclusion, the prevalence of S. haematobium infection in the Middle Awash Valley was high and it varies across villages. Hence, children living in the present study villages of the Middle Awash Valley need to be treated with praziquantel to reduce morbidity and disrupt transmission.
Background
Urinary schistosomiasis is a common public health problem in the world caused by infection with Schistosoma haematobium [1]. Individuals may acquire the disease during contact with water containing cercaria of the parasite [2]. S. haematobium is responsible for majority of deaths due to schistosomiasis in the world [3]. The disease is particularly prevalent in sub-Saharan Africa where it is estimated to affect 112 million people [3,4]
S. haematobium infection causes haematuria, dysuria, lesions of the bladder, kidney failure, bladder cancer, [5–9]. Infection also interferes with nutrient uptake and can lead to undernutrition, growth and cognitive development retardation, and pose a serious threat to children’s health, education and productivity [10–13]. The disease is responsible for the death of 150,000 people in sub-Saharan Africa annually due to infection-related bladder problems [3,4].
In Ethiopia, S. haematobium infection has been known to be prevalent in northeastern part of Ethiopia following the Awash River Valley, in eastern Ethiopia around Wabi Shebele River and in Kurmuk areas bordering Ethiopia and the Sudan [14–17]. Previous studies in the Afar area documented a prevalence of S. haematobium ranging from 3.1% to 52.0% [17–20]. Nevertheless, since 1995 there has been limited work on the distribution and prevalence of the disease in the area. As the lifestyle of the communities and environmental conditions favouring survival of the parasite and transmission to human host could vary with time, current information about prevalence of the disease in suspected areas is vital to design appropriate control strategies including preventive chemotherapy of schistosomiasis. Therefore, the objective of the current study was to determine the prevalence and intensity of S. haematobium infection among school-age children living in different villages of the Amibara District (Middle Awash Valley) as well as in Dubti and Asaita Districts from Lower Awash Valley, Afar Regional State, northeastern Ethiopia.
Methods
Study area and population
The study was conducted in 7 purposely-selected villages in the Middle and Lower Awash Valley, Afar Regional State, northeastern Ethiopia (Fig 1). In the Middle Awash Valley, study participants were recruited from four villages of the Amibara District. They are situated about 300 to 350 km from Addis Ababa, and between 720 and 726 m above sea level. The mean annual rainfall and temperature are 654 mm3 and 25.6°C, respectively. The majority of the study participants living in the study villages are pastoralists who rely on livestock rearing. A minority are practicing small-scale irrigation to cultivate maize, onion and tomato following the Awash River. There is a large-scale irrigation based cotton farm in the Amibara District of the Middle Awash Valley. The villages in the Lower Awash Valley are located in Asaita District (Berga Debora) and in Dubti District (Farma and Dubti Town). They are situated about 584 (Dubti) to 628 (Asaita) km from Addis Ababa and at altitude between 350 and 381 m above sea level. The mean annual rainfall and temperature are 160 mm3 and 32°C, respectively. The pastoralists in the Asaita and Dubti areas practice small-scale irrigation-based cultivation of maize and rear animals. There is a large-scale irrigation-based sugar cane plantation in Dubti area.
Fig 1. Map of the study region.
Map of Afar region Map of Ethiopia.
The study villages were selected based on their endemicity for S. haematobium infection [17–20], location near to Awash River, availability of irrigation canals and swamps. Children living in the study villages spend times in the Awash River, irrigation canals and swamps playing, swimming and keeping animals which may expose them to S. haematobium. Thus, all school age children (5 to 16 years of age) who were living in the selected villages between February and May 2014 were eligible for the study.
Sample size and study design
The sample size was calculated assuming random sampling and an approximate normality in the distribution of the proportion of events (small population size which was not much larger than the sample size), 95% confidence interval (CI) (Zα/2 = 1.96), 5% margin of error and design effect of 2.5. The design effect was computed as the ratio of true sampling variance to the variance based on assumption of random sampling for proportion 50% of sample size 384. Current status of S. haematobium infection in Afar region is not known. Thus, the prevalence of S. haematobium was assumed 50% and the sample size was estimated to be 960. Then, 960 was proportionally divided among the 7 villages based on the size of school age children in each village.
However, the number of school age children living in most of the villages were less than the estimated sample size for the villages. Moreover, due to the nomadic nature of the communities some children did not show up during the study period. Thus, all school age children living in the villages (885) who were willing to participate were included in the study.
After obtaining informed consent from parents/guardians and assent from children, the study participants were given a labelled plastic container (200 ml capacity) and asked to provide a urine sample between 10:00 am and 2:00 pm. About 10 ml of individual’s urine sample was processed by urine filtration method and microscopically examined for S. haematobium eggs on the spot [21]. Then, S. haematobium infection intensity was determined as light (1–49 egg/10 ml urine) and heavy (≥50 eggs/10 ml urine) [5]. A subset of the urine samples was also tested for the presence of blood as an indicator of S. haematobium infection using Combur 10 Test reagent strips (Human GmbH-Max-Planck-Ring 21, Germany) following the manufacturer’s instructions. Briefly, after immersing the test strip in the urine sample for 1 to 2 second, the strips were taken out and left over the bench for about 1 min. Finally, the blood area on the test strip was compared with corresponding reference values (color fields). The results were recorded as 0 (negative), ca.5-10 (weak), ca.50 (moderate) and ca.250 (strong) erythrocytes/μl urine or a haemoglobin concentration out of ca. 10, ca. 50, ca. 250 Ery/μl as per the manufacturer's instruction. The test strip has a minimum sensitivity of 5 to 10 erythrocytes/μl urine corresponding to 0.015 mg haemoglobin or myoglobin/dl urine. Flecked discolorations in the test field indicate intact erythrocytes. A questionnaire was also used to collect information about the education status, places of residence and age of the study participants.
Statistical data analysis
Epidemiology of S. haematobium
Data were entered into excel-sheet 2007 and analysed using STATA version 11 (Stata Corporation, College Station, Texas, USA). Prevalence and intensity of S. haematobium infection was calculated for each village, age and sex group. Prevalence of infection was estimated by dividing the number of individuals diagnosed positive by the urine filtration technique with the total number of children examined. The infection intensity was determined by means of urine egg counts (UEC), expressed as number of eggs per 10 ml of urine. Results of individuals who were not infected with S. haematobium (zero values) were considered when calculating the mean UEC. Multivariable logistic regression analysis was used to estimate the association of age, sex, place of residence and education status with the prevalence of S. haematobium infection. Multivariable ordinal logistic regression analysis was used to estimate the association between age, sex, place of residence and education status and class intensity of S. haematobium infection. Zero-inflated negative binomial regression analysis was used to test the difference in the mean UEC between sex or age groups, place of residence and education status.
Agreement between diagnostic techniques
The agreement in qualitative and quantitative test results between the urine filtration and dipstick was assessed. The qualitative agreement in test results was determined using Kappa statistic. Kappa values were interpreted as poor (<0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80) and perfect (0.81–1.00) [22]. The quantitative agreement was determined using an ordinal logistic regression. In this regression model the outcome variable was the intensity of the colour urine regent strip test, the predictor was the UEC. 95% CI values were estimated for the odds ratio and mean difference values. The level of significance was set at p<0.05.
Ethical Consideration
The study was conducted after the study protocol was ethically approved by the Institutional Review Board (IRB) of Aklilu Lemma Institute of Pathobiology, Addis Ababa University. Permission to conduct the study was also obtained from the head of the district Health Offices and the principals of the schools and leaders of the villages. Verbal consent for the children was obtained from their parents/guardians. As the study population was mainly illiterate the IRB endorsed oral consenting of the parents or guardians of the children. Seeing that the study involved minimal risk the committee did not require tape recording or any other form of preserving the consenting processes. The children participated in the study on voluntary basis after being informed about the purposes of the study and giving their assent. Children who were positive for S. haematobium infection were treated with praziquantel (at 40 mg/kg body weight).
Results
In total, 885 out of 994 eligible individuals provided urine samples. The mean age was 9.8 year (age range: 5–16 years) and majority of the children were males (57.3%). The sample size and the corresponding distribution of age and sex across the different villages are summarized in Table 1. Children living in the Amibara District of the Middle Awash (n = 632) as well as Asaita and Dubti Districts of Lower Awash Valley (n = 253) were examined for S. haematobium using filtration method.
Table 1. Prevalence of S. haematobium infection among school-aged children in Awash Valley, Afar Regional State, northeastern Ethiopia, February to May 2014.
| Variable | Number examined | Percent positive (95% CI) | P-value* | |
|---|---|---|---|---|
| Age in years | 885 | 20.8 (18.1, 23.5) | 0.283 | |
| Gender | Female | 378 | 21.7 (17.5, 25.9) | |
| Male | 507 | 20.1 (16.6, 23.6) | 0.391 | |
| Villages | Ambash | 40 | 12.5 (1.8, 23.2) | |
| Buri | 189 | 24.9 (18.6, 31.1) | ||
| Hassoba | 254 | 37.0 (31.0, 43.0) | ||
| Hanledebe | 149 | 23.5 (16.6, 30.4) | <0.001 | |
| Farma | 57 | 5.3 (0.0, 11.2) | ||
| Dubti | 111 | 0.0 (0.0, 0.0) | ||
| Asaita | 84 | 0.0 (0.0, 0.0) | ||
| Non-school attenders | 179 | 29.7 (22.3, 35.7) | ||
| Education | Elementary | 628 | 17.8 (14.8, 20.8) | 0.663 |
| Junior | 78 | 25.6 (15.6, 35.5) | ||
| Valley | Lower Awash | 253 | 1.2 (0.0, 2.5) | <0.001 |
| Middle Awash | 632 | 28.6 (25.1, 32.2) |
*P-value: adjusted for age, sex, villages and education status: results are generated form one multivariable logistic regression model.
Elementary = grade 1 to 6; Junior: grade 7 to 8.
Prevalence and intensity of S. haematobium infection using urine filtration method
The prevalence for the different villages, sex, Valleys are summarized in Table 1. Out of 885 children, 20.8% (95% CI = 18.1, 23.5) were found positive for S. haematobium infection. The prevalence of S. haematobium infection was similar when compared between males and females. The odds of S. haematobium infection were also similar when compared between school attenders and non-school attenders. However, the difference in the prevalence of S. haematobium infection was significant among the villages and in the Middle and Lower Awash Valleys.
Out of 184 children found positive for S. haematobium infection, 90.2% had light intensity of infection and 9.8% had heavy intensity of infection (Table 2). The mean UEC among the 885 children examined was 4.0 eggs/10 ml (95% CI = 2.4, 5.5) of urine. There was significant variation in the mean UEC of S. haematobium among children who lived in different villages, in the Middle and Lower Awash Valley and had different education status. However, the mean UEC and intensity classes of S. haematobium were comparable among males and females of different age groups.
Table 2. Intensity of S. haematobium infection among school-age children in Middle Awash Valley, Afar Regional State, northeastern Ethiopia, February to May 2014.
| Variable | Number examined | Mean UEC (95% CI) | P-value* | Number of light infections | Number of heavy infection | P-value** | |
|---|---|---|---|---|---|---|---|
| Age in Years | 885 | 4.0 (2.4, 5.5) | 0.165 | 166 | 18 | 0.593 | |
| Gender | Female | 378 | 4.7 (1.7, 7.8) | 0.916 | 73 | 9 | 0.766 |
| Male | 507 | 3.4 (2.0, 4.8) | 93 | 9 | |||
| Villages | Ambash | 40 | 0.4 (0.0, 0.7) | 4 | 1 | ||
| Buri | 189 | 4.5 (1.2, 7.9) | 43 | 4 | |||
| Hassoba | 254 | 7.7 (4.4, 11.0) | 83 | 11 | |||
| Hanledebe | 149 | 4.2 (-1.7, 9.9) | <0.001 | 33 | 2 | 0.872 | |
| Farma | 57 | 1.1 (-1.0, 3.1) | 2 | 1 | |||
| Dubti | 111 | 0.0 (0.0, 0.0) | 0 | 0 | |||
| Asaita | 84 | 0.0 (0.0, 0.0) | 0 | 0 | |||
| Non-school | 179 | 8.0 (2.3, 13.6) | 44 | 8 | |||
| attenders | |||||||
| Education | Elementary | 628 | 2.8 (1.5, 4.1) | 0.001 | 103 | 9 | 0.154 |
| Junior | 78 | 4.4 (-1.6, 10.3) | 19 | 1 | |||
| Valley | Lower Awash | 253 | 0.2 (-0.2, 0.7) | 0.004 | 2 | 164 | 0.258 |
| Middle Awash | 632 | 5.5 (3.3, 7.6) | 164 | 17 |
P-value*: adjusted for age, sex, villages and education status: results are generated form one Zero-inflated negative binomial regression.
P-value**: adjusted for age, sex, villages and education status: results are generated form one multivariable ordinal logistic regression model.
Elementary = grade 1 to 6; Junior: grade 7 to 8.
Agreement in diagnostic techniques
A total of 556 samples were examined using both urine dipstick and filtration methods (Table 3). Based on the results of the urine dipstick, 56.3% of the children were positive for hematuria or S. haematobium infection. About 65.6% of the children positive for infection showed weak colour reaction (ca.5-10), 13.0% showed moderate colour reaction (ca.50) and 16.0% showed strong colour reaction (ca.250). Prevalence of S. haematobium based on the strip reagent analysis varies significantly among villages (93.2% to 8.3%) and education status (61.9% to 88.5%) (p<0.001). The prevalence of S. haematobium infection based on the strip reagent analysis was not associated with age and sex. However, the prevalence of infection was higher in females (76.1%) than in males (50.6%) when the analysis was limited to children in the 12 to 16 year age groups. About 24.5% of the 556 children were positive for S. haematobium when examined using filtration method, and most of the cases (133) were from Middle Awash Valley.
Table 3. Prevalence of S. haematobium infection among 556 school-age children in the Middle and Lower Awash Valley, Afar Regional State, northeastern Ethiopia, February to May 2014.
| Urine filtration Method | Urine dipstick analysis | ||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 228 | 192 | 420 |
| Positive | 15 | 121 | 136 |
| Total | 243 | 313 | 556 |
Majority (89.0%) of the children found positive for S. haematobium based on filtration method were also diagnosed as positive for hematuria by the strip (Fig 2). However, 61.3% of the children detected as positive for hematuria based on the reagent strip had no eggs of S. haematobium in their urine based on the filtration technique. Majority (88.5%, 170/192) of cases detected as positive for hematuria by the dipstick, but negative for the S. haematobium by the filtration method had weak colour reaction (ca.5-10). The prevalence of hematuria infection based on dipstick was comparable between males (54.0%) versus females (59.3%) and children of age 5 to 9 (56.5%) and 10 to 16 (56.1%) years. The agreement between the two methods in diagnosing children for the presence of hematuria and S. haematobium egg was fair [Kappa = 0.3]. The mean UEC estimated with the filtration method was significantly positively associated (predicts) with the intensity grade of colour reaction or hematuria with the reagent analysis [Adjusted Odds Ratio = 1.94, 95% CI = 1.33, 2.55].
Fig 2. Comparison of the status of S. haematobium infection based on filtration and dipstick among 556 school-age children in the Middle and Lower Awash Valleys, Afar Regional State, northeastern Ethiopia, from February to May 2014.
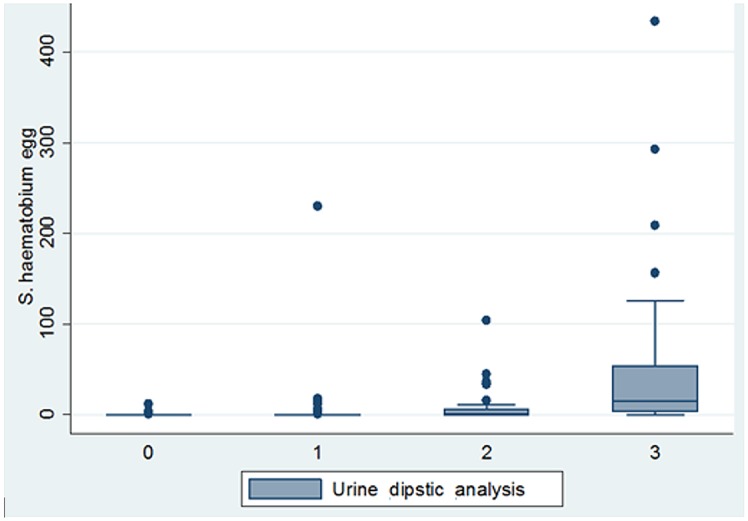
Urine dipstick analysis: 0 = Negative, 1 = weak (ca.5-10), 2 = moderate (ca.50), 3 = strong (ca.250).
Discussion
The present study was conducted to determine the prevalence of S. haematobium infection among school-age children living in selected villages in the Middle and Lower Awash Valley, Afar Regional State of Ethiopia. The prevalence of S. haematobium infection among children in Hassoba, Buri and Hanledebe villages in the Middle Awash Valley was 37%, 25%, and 23.5%, respectively as diagnosed using filtration method. Only three children in Lower Awash Valley (Farma village) were positive for S. haematobium infection as diagnosed with filtration method. Previous similar study in children living in the two villages of the current study area (Hassoba and Buri) documented a prevalence of 24.5% for S. haematobium infection [17]. On the other hand, the prevalence of S. haematobium infection in Hassoba was found to be 47.6% in 2007 [23]. The decline in the prevalence of urogenital schistosomiasis in the study area might be attributed to the drying out of most swamps which support intermediate snail hosts of S. haematobium (Bulinus abyssinicus) and distribution praziquantel drug at the health posts located in the region. Furthermore, the fact that more children are now enrolled at school might have also minimized the chance of exposure of children to cercariae infested swamps as children spend most of their time at school.
Due to difference in behaviour that affects the rate of contact with cercariae infested water, the magnitude of prevalence and intensity of S. haematobium infection is expected to vary with age and sex. Studies documented an increased prevalence of S. haematobium infection and mean egg count with an increase in the age of children and in males than in females [24–28]. However, in the current study there was no sex and age-related differences in the prevalence and intensity of infection among children of Middle Awash Valley unlike reports of previous studies [29–31]. Due to differences in environmental setting, culture and religious practices, risk factors of S. haematobium infection may vary with localities.
There was significant difference in the prevalence and intensity of S. haematobium infection between children who lived in Middle and Lower Awash Valley and in different villages of the Valley. The intensity of infection was also higher in children who did not attend school compared to those who attended elementary school in the Middle Awash Valley. There are swamps that support intermediate host of S. haematobium in Hassoba, Hanledebe and Buri. The school-age children may get infected in these swamps. As a result, children living in these villages will continue to be infected and re-infected. Particularly, the rate of infection with the parasite may increase in children who do not attend school as they spend most of their times in swamps when they help their parents with herding outside school activities.
According to WHO guideline of Preventive Chemotherapy in Human Helminthiasis, school-age children living in all of the villages in the Middle Awash Valley (where the prevalence of infection with the parasite lies between 10% and 50%) requires mass drug administration with praziquantel every two years to control schistosomiasis morbidity [32]. However, an integrated approach that may involve provision of clean water, sanitation and hygiene programs; education and behaviour change programs; snail control and development of primary health care systems in the villages would be vital to effectively eliminate urogenital schistosomiasis in the area. Further community based study in these villages would also be vital to determine the status of S. haematobium infection and risk factors in other age groups to consider them for preventive chemotherapy.
The prevalence of S. haematobium infection was 24.5% (136/556) in children living in the Middle and Lower Awash Valley based on the filtration method, but increased to 56.3% (313/556) when samples were diagnosed with reagent strip test. Diagnosis of S. haematobium infection using filtration method may miss cases especially during light infection [33, 34]. Indeed more than 90% of S. haematobium infection cases in the current study had light intensity of infection and majority (88.5%) of cases detected as positive by the dipstick, but missed by the filtration method had weak colour reaction (ca.5-10). On the other hand, the urine analysis dipstick method will be affected by menstruation and other genitourinary infections [35]. This may contribute to the increased prevalence of infection with the parasite in the region. Indeed in the current study the prevalence of hematuria was significantly higher in females than in males in children with age from 13 to 16 years, but this difference was not maintained in children younger than 13 years. Overall, the results of the current study confirm that the dipstick method could be used for rapid screening of S. haematobium infection in the region [36]. Ethiopia has launched a National Deworming Programme Targeting School age Children in January 7th, 2015. In this regard, the current finding would be important as it indicates districts and villages in Afar regions where the residents (particularly children) are at risk of schistosomiasis and deworming with praziquantel would be necessary.
The current result is based on urine samples collected only on a single day. On the other hand, the amount of S. haematobium egg released in urine could vary with days [37]. Had samples collected on different days were examined, the probability of missing light infections could decrease. Thus, the prevalence of S. haematobium infection in the current study areas might have been underestimated based on the results of the filtration method. Another limitation of the present study is that not all villages suspected for S. haematobium infection in the Middle and Lower Awash were covered during the survey. Hence, further surveys including all villages in the Middle and Lower Awash Valley are recommended. Female students who had experienced menstruation within recent days before examination were not excluded in the current study. This might have caused overestimation of the prevalence of S. haematobium infection in females based on the dipstick method.
In conclusion, S. haematobium infection in the Amibara District of the Middle Awash Valley is fairly high and showed variation in magnitude of prevalence among villages. However, S. haematobium infection was not common among children in Asaita and Dubti villages of the Lower Awash Valley. According to WHO guideline, school-age children living in endemic villages of the Middle Awash Valley need to be treated with praziquzntel once every two years and other supplementary measures such as provision of clean water and health education are also indicated for eventual elimination of the disease.
Acknowledgments
We would like to acknowledge all principals and teachers of schools in the current study villages for assisting during data collection. We also thank all the study participants for their cooperation during specimens and data collection. This study was carried out as part of the project entitled “Assessment of anthelmintic drug efficacy of single-dose praziquantel against schistosomiasis in selected endemic settings in Ethiopia.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Aklilu Lemma Institute of Pathobiology, Addis Ababa. University(www.aau.edu.et/aaubeta/alipb/). AD, ML and BE received the fund from Addis Ababa University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006; 6:411–425. [DOI] [PubMed] [Google Scholar]
- 2. Inobaya MT, Olveda RM, Chau TNP, Olveda DU, Ross AGP. Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med. 2014; 5: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pennington LF, Hsieh MH. Immune Response to Parasitic Infections, Bentham e books; 2014; pp. 93–124. [Google Scholar]
- 4. Van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003; 86:125–139. [DOI] [PubMed] [Google Scholar]
- 5. WHO. Prevention and Control of Schistosomiasis and Soil transmitted Helminthiasis- Report of a WHO Expert Committee. Tech Rep Ser. 2002; 912: 1–57. [PubMed] [Google Scholar]
- 6. Fenwick A, Savioli L, Engels D, Bergquist NR, Todd MH. Drugs for the Control of Parasitic Diseases: Current Status and Development in Schistosomiasis. Trends Parasitol. 2003; 19:509–515. [DOI] [PubMed] [Google Scholar]
- 7. Stephenson L. The impact of schistosomiasis on human nutrition. Parasitol. 1993; 107:S107–23. [DOI] [PubMed] [Google Scholar]
- 8. Farid Z. Schistosomes with terminal-spined eggs: pathological and clinical aspects In: Jordan P, Webbe G, Sturrock RF, editors. Human schistosomiasis. CAB International; 1993. pp.159–193. [Google Scholar]
- 9. Poggensee G, Feldmeier H. Female genital schistosomiasis: facts and hypotheses. Acta Trop. 2001; 79:193–210. [DOI] [PubMed] [Google Scholar]
- 10. Casmo V, Augusto G, Nala R, Sabonete A, Carvalho-Costa FA. The effect of hookworm infection and urinary schistosomiasis on blood hemoglobin concentration of schoolchildren living in northern Mozambique. Rev Inst Med Trop Sao Paulo. 2014; 56(3):219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deribew K1, Tekeste Z, Petros B, Huat LB. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed. 2013; 3(4):307–310. 10.1016/S2221-1691(13)60068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacko M, Magnussen P, Keita AD, Traoré MS, Landouré A, Doucouré A et al. Impact of Schistosoma haematobium infection on urinary tract pathology, nutritional status and anaemia in school-aged children in two different endemic areas of the Niger River Basin, Mali. Acta Trop. 2011; 120:S142–150. 10.1016/j.actatropica.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 13. Koukounari A, Gabrielli AF, Toure S, Bosque-Oliva E, Zhang Y, Sellin B et al. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007; 196(5):659–669. [DOI] [PubMed] [Google Scholar]
- 14. Lemma A. Bilharziasis in the Awash Valley: epidemiological study with special emphasis on its future economic and public health importance. Ethiopia Med J. 1969; 7:147–176. [Google Scholar]
- 15. DeSole G, Lemma A, Mazengia B. Schistosoma haematobium in the Wabi Shebele Valley of Ethiopia. Am J Trop Med Hyg. 1978; 27:928–930. [DOI] [PubMed] [Google Scholar]
- 16. Ali A, Lo CT, Ayele T. Schistosoma haematobium in western Ethiopia. Ethiop Med J. 1986; 24:73–78. [PubMed] [Google Scholar]
- 17. Deribew K, Tekeste Z, Petros B. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed. 2013; 3(4): 307–310. 10.1016/S2221-1691(13)60068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jemaneh L, Shewakena F, Tedla S, Erko B, Birrie H. Evaluation of reagent strips for detection of Schistosoma haematobium infection in the lower Awash Valley. Ethiopia Med J. 1993; 31(2):137–150. [PubMed] [Google Scholar]
- 19. Jemaneh L1, Tedla S, Birrie H. The use of reagent strips for detection of urinary schistosomiasis infection in the middle Awash Valley, Ethiopia. East Afr Med J. 1994; 71(10):679–683. [PubMed] [Google Scholar]
- 20. Kloos H, Polderman AM, Desole G, Lemma A. Haematobium schistosomiasis among seminomadic and agricultural Afar in Ethiopia. Trop Geogr Med. 1977; 29(4):399–406. [PubMed] [Google Scholar]
- 21. Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976; 54:159–162. [PMC free article] [PubMed] [Google Scholar]
- 22. Landis JR, Koch GG. "The measurement of observer agreement for categorical data". Biometrics. 1977; 33 (1): 159–174. [PubMed] [Google Scholar]
- 23. Ayele B, Erko B, Legesse M, Hailu A, Medhin G. Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar Ethiopia. Parasite. 2008; 15:69–75. [DOI] [PubMed] [Google Scholar]
- 24. Bradley DJ, McCullough FS. Egg output stability and the epidemiology of Schistosoma haematobium Part II. An analysis of the epidemiology of endemic Schistosoma haematobium . Trans R Soc Trop Med Hyg. 1973; 67: 491–500. [DOI] [PubMed] [Google Scholar]
- 25. Hammad TA, Gabr NS, Hussein MH, Orieby A, Shawky E, Strickland GT. Determinants of infection with schistosomiasis haematobia using logistic regression. Am J Trop Med Hyg. 1997; 57: 464–468. [DOI] [PubMed] [Google Scholar]
- 26. Ndyomugyenyi R, Minjas JN. Urinary schistosomiasis in schoolchildren in Dar-es-Salaam, Tanzania, and the factors influencing its transmission. Ann Trop Med Parasitol. 2001; 95: 697–706. [DOI] [PubMed] [Google Scholar]
- 27. Mafiana CF, Ekpo UF, Ojo DA. Urinary schistosomiasis in preschool children in settlements around Oyan Reservoir in Ogun State, Nigeria: implications for control. Trop Med Int Health. 2003; 8: 78–82. [DOI] [PubMed] [Google Scholar]
- 28. Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006; 368: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 29. Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006; 75: 83–92. [PMC free article] [PubMed] [Google Scholar]
- 30. Rudge JW, Stothard JR, Basa´n˜ez MG, Mgeni AF, Khamis I, Khamis AN et al. Microepidemiology of urinary schistosomiasis in Zanzibar: Local risk factors associated with distribution of infections among schoolchildren and relevance for control. Acta Trop. 2008; 105: 45–54. [DOI] [PubMed] [Google Scholar]
- 31. Hatz CF, Vennervald BJ, Nkulila T, Vounatsou P, Kombe Y, Mayombana C et al. Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg. 1998; 59: 775–781. [DOI] [PubMed] [Google Scholar]
- 32. WHO (2006) Preventive chemotherapy in human helminthiasis, Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. World Health Organization, 2006. [Google Scholar]
- 33. van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, Deelder AM. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol. 2004; 42(12):5458–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamilton JV, Klinkert M, Doenhoff MJ. Diagnosis of schistosomiasis: antibody detection, with notes on parasitological and antigen detection methods. Parasitol. 1998; 117:S41–57 [DOI] [PubMed] [Google Scholar]
- 35. Poggensee G, Krantz I, Kiwelu I, Feldmeier H. Screening of Tanzanian women of childbearing age for urinary schistosomiasis: validity of urine reagent strip readings and self-reported symptoms. Bull World Health Organ. 2000; 78(4):542–548. [PMC free article] [PubMed] [Google Scholar]
- 36. King CH1, Bertsch D. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low-prevalence and previously-treated populations. PLOS Negl Trop Dis. 2013; 12:7: e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Etten L, Kremsner PG, Krijger FW, Deelder AM. Day-to-day variation of egg output and schistosome circulating antigens in urine of Schistosoma haematobium-infected school children from Gabon and follow-up after chemotherapy. Am J Trop Med Hyg. 1997; 57(3):337–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.