Abstract
Granulocyte macrophage colony stimulating factor (GM-CSF) is generally recognized as an inflammatory cytokine. Its inflammatory activity is primarily due its role as a growth and differentiation factor for granulocyte and macrophage populations. In this capacity, among other clinical applications, it has been used to bolster anti-tumor immune responses. GM-CSF-mediated inflammation has also been implicated in certain types of autoimmune diseases, including rheumatoid arthritis and multiple sclerosis. Thus, agents that can block GM-CSF or its receptor have been used as anti-inflammatory therapies. However, a review of literature reveals that in many situations GM-CSF can act as an anti-inflammatory/regulatory cytokine. We and others have shown that GM-CSF can modulate dendritic cell differentiation to render them “tolerogenic,” which, in turn, can increase regulatory T-cell numbers and function. Therefore, the pro-inflammatory and regulatory effects of GM-CSF appear to depend on the dose and the presence of other relevant cytokines in the context of an immune response. A thorough understanding of the various immunomodulatory effects of GM-CSF will facilitate more appropriate use and thus further enhance its clinical utility.
Introduction
Granulocyte macrophage colony-stimulating factor (GM-CSF) was first characterized by Burgess, Camakaris, and Metcalf as a soluble factor capable of differentiating bone marrow precursor cells into granulocytes and macrophages (Burgess and others 1977). It is now recognized as an immune modulatory cytokine produced by different cells, including macrophages, endothelial cells, alveolar epithelial cells, and T cells (Kelso and Metcalf 1985; Hamilton 2002; Fleetwood and others 2005; Ponomarev and others 2007). GM-CSF production can be regulated by cytokines, antigens, or other inflammatory agents. While inflammatory cytokines such as interleukin (IL)-1, IL-2, tumor necrosis factor-alpha (TNFα), and interferon-gamma (IFN-γ) can induce GM-CSF production and secretion, immune-regulatory cytokines such as IL-10 and transforming growth factor-beta (TGFβ) can suppress its production (Lari and others 2007; Hamilton 2008; Ogawa and others 2008).
GM-CSF plays a dominant role in the survival, proliferation, differentiation, and function of myeloid lineage cells (Hamilton 2008). Due to its pleiotropic effects on different cell lineages, the therapeutic potential of GM-CSF has been explored in different conditions such as inflammation, cancer, and autoimmunity (Hamilton 2008; Metcalf 2010; Di Gregoli and Johnson 2012; Zhan and others 2012). Consistent with this, some studies have suggested that GM-CSF causes, or is a part of, an inflammatory response (El-Behi and others 2011; van Nieuwenhuijze and others 2013), while others suggest that GM-CSF promotes immunological tolerance by acting as an immunoregulatory cytokine (Parmiani and others 2007; Kohanbash and others 2013). As has been shown for other cytokines that have both pro-inflammatory and regulatory properties, the effects of GM-CSF are likely dose and context dependent (Parmiani and others 2007; Shachar and Karin 2013). Here, we limit ourselves to providing a review and analyses of the literature that may help better understand how the apparent “duality” of GM-CSF function is brought about.
Nature and Properties of GM-CSF
GM-CSF is a glycoprotein consisting of 127 amino acids with 2 potential N-linked glycosylation sites (Kaushansky and others 1987; Cebon and others 1990). Although the glycosylation sites do not appear to be essential for GM-CSF activity in vitro or in vivo, they appear to affect GM-CSF's affinity for its receptor. The crystal structure of recombinant human GM-CSF has shown that it consists of 4 anti-parallel helical bundles (Walter and others 1992).
The GM-CSF receptor (GM-CSFR) consists of 2 distinct chains. The GM-CSF receptor α-chain (GMRα) directly binds GM-CSF, although with lower binding affinity (kd=2–8 nM) while displaying rapid dissociation kinetics (Gearing and others 1989; Park and others 1992). The GM-CSF receptor β-chain alone (GMRβ) does not exhibit any measurable binding affinity for GM-CSF. However, on its interaction with GMRα, it converts the pair into a higher-affinity receptor for the GM-CSF (Kd=30–100 pM) with slower dissociation kinetics (Cannistra and others 1990; Chiba and others 1990; Hayashida and others 1990; Park and others 1992). However, the GMR α-chain confers GM-CSF-binding specificity to GM-CSFR (Hara and Miyajima 1992; Takaki and others 1994); the GMR β- chain, in addition to its ability to associate with GMRα, can also associate with IL-3 and IL-5-specific receptor subunits and enhance the affinity of those ligand-receptor interactions. For this reason, IL-3, IL-5, and GM-CSF are considered members of the β common (βc) family of cytokines (Broughton and others 2012; Hercus and others 2013), and their sharing of this receptor subunit may explain the partial cross-reactivity for receptor binding displayed by these cytokines (Walker and others 1985; Lopez and others 1989). GMRα and the β chains are predominantly expressed in various cells of the hematopoietic lineage; however, they have been also found to be expressed in nonhematopoietic cell lines, such as small cell carcinomas, which bind endogenous GM-CSF, leading to the activation of downstream signaling pathways (Dedhar and others 1988; Baldwin and others 1989; Hercus and others 2009; van de Laar and others 2012).
GM-CSFR-mediated signaling appears to be initiated by trans-phosphorylation of Janus Kinase 2 (JAK2), which is associated with the βc receptor subunit. GM-CSF binding to the GMRα subunit facilitates its interaction with the GMRβ subunit, thus activating the GM-CSFR-mediated signaling (Hansen and others 2008; Hercus and others 2013). The activated JAK2 phosphorylates multiple tyrosine residues in the GMRβ, triggering the activation of downstream signaling cascade, including JAK2/STAT5, Ras/MAP-kinase, and PI3-kinase/Akt (Hercus and others 2009). While GM-CSF induced PI3K-Akt activation has been shown to protect cells from apoptosis (Guthridge and others 2004), ERK and STAT5 activation have been shown to be required for cell proliferation (Comalada and others 2004) and dendritic cell (DC) differentiation (Sebastian and others 2008), respectively. In addition, GM-CSF has been found to activate NF-κB directly through an interaction of the GMRβ with IKK2 (Ebner and others 2003; Perugini and others 2010) or indirectly through JAK2 activation (Guthridge and others 2004), leading to cell survival and proliferation (Ebner and others 2003).
Primary actions of GM-CSF include the regulation of cell survival, proliferation, and differentiation in granulocyte-macrophage populations (Metcalf 1988; Metcalf and Moore 1988). Withdrawal of GM-CSF from purified hematopoietic progenitor cells in vitro or from GM-CSF dependent cell lines leads to loss of cell viability (Metcalf and Merchav 1982). In the presence of GM-CSF, the cells survive and proliferate with extended life spans. GM-CSF-mediated prolongation of the half life of myeloid effector cells such as granulocytes and basophils in vivo may be important for augmenting host inflammatory responses (Begley and others 1986; Colotta and others 1992). Interestingly, GM-CSF has been shown to have a dose-dependent effect; at low concentrations, it has been shown to support cell survival without proliferation, while at higher concentrations, GM-CSF promotes both survival and proliferation (Guthridge and others 2006).
In addition to its effects on normal cells, a number of immortalized and growth factor-dependent cell lines proliferate with minimal differentiation in response to GM-CSF (Berdel and others 1990; Brizzi and others 1990). While GM-CSF can cause granulocyte lineage commitment at higher concentrations, it can cause monocyte lineage commitment (Metcalf 1980) at lower concentrations. Therefore, it is suggested that not only GM-CSF induces differentiation of specific myeloid cell types but also selectively guides the differentiation of a common progenitor along the granulocyte, macrophage, and DC development pathways in a concentration-dependent manner.
Role of GM-CSF in Inflammation and Autoimmunity
GM-CSF was first characterized as a pro-inflammatory cytokine due to its ability to stimulate plasminogen-dependent fibrinolysis activity in mouse macrophages (Hamilton and others 1980). Ever since, it has been consistently depicted as a pro-inflammatory cytokine that is important in many cellular processes such as DC activation, granulocyte survival, and enhancement of macrophage and microglial functions (Colotta and others 1992; Fischer and Reichmann 2001; Fleetwood and others 2005; Francisco-Cruz and others 2014). In a typical inflammatory response, DCs and macrophages play critical roles in linking the innate and adaptive immune responses. DCs are among the first cells to capture, process, and present antigens to naïve T cells, while concurrently expressing the co-stimulatory molecules necessary for the activation of T cells and initiation of the inflammatory response. The interaction between the antigen presenting cells (APCs) and T cells is influenced by GM-CSF through upregulation of the major histocompatibility complex class II molecules (MHC class-II), necessary for antigen presentation, and by enhancing the secretion of cytokines, including TNFα, IL-6, and IL-23, (Miller and others 2002; Mausberg and others 2009). Other studies have shown that GM-CSF treatment in conjunction with microbial stimuli can support the generation of potent effector DCs that are capable of secreting a variety of pro-inflammatory cytokines that guide the differentiation of T cells during the immune response into effector T cells (Min and others 2010).
Dysregulation of GM-CSF expression has been implicated in the pathogenesis of autoimmune inflammatory diseases (Cornish and others 2009). Several studies have suggested that GM-CSF can indeed exacerbate autoimmune diseases such as rheumatoid arthritis (RA), a chronic inflammatory disorder characterized by joint pain and deterioration (Fiehn and others 1992; Alsalameh and others 1994; Berenbaum and others 1994; Burmester and others 2013). The chronic pain and gradual destruction of the joints is mediated by macrophages, through the production of a wide range of inflammatory cytokines, such as TNFα, IL-1β, and IL-6 (Bischof and others 2000; Cook and others 2001). Two distinct macrophage lineages have been described: M1 lineage, which is involved in inflammation and host defense, and M2, which is characterized as anti-inflammatory and involved in tissue repair processes (Mantovani and others 2004). GM-CSF, which is found at higher levels in patients with RA, has been suggested to promote the development of M1 macrophages (Verreck and others 2004). Preclinical studies to establish a connection between GM-CSF and RA found that GM-CSF injections in a mouse model of collagen-induced arthritis (CIA) (Seki and others 1988) exacerbated the disease (Campbell and others 1997; Campbell and others 1998), while treating mice with CIA with neutralizing antibodies against GM-CSF prevented disease progression. The importance of GM-CSF in exacerbating CIA has been further substantiated by the observed resistance of GM-CSF knockout mice to CIA (Campbell and others 1998).
Inflammatory activity of the GM-CSF has also been implicated in the pathogenesis of Experimental Autoimmune Encephalomyelitis (EAE), an autoimmune disorder of the central nervous system (CNS) that serves as a model of Multiple Sclerosis (McQualter and others 2001; Ponomarev and others 2007). While EAE is widely believed to be a T-cell-mediated autoimmune disease (Kroenke and others 2008), T-cell activation is unlikely due to the direct effects of GM-CSF on mouse T cells as they do not generally express the GMRα (Rosas and others 2007). However, recent reports have suggested different mechanisms through which GM-CSF can drive inflammation in the CNS (Codarri and others 2011; El-Behi and others 2011) (Fig. 1).
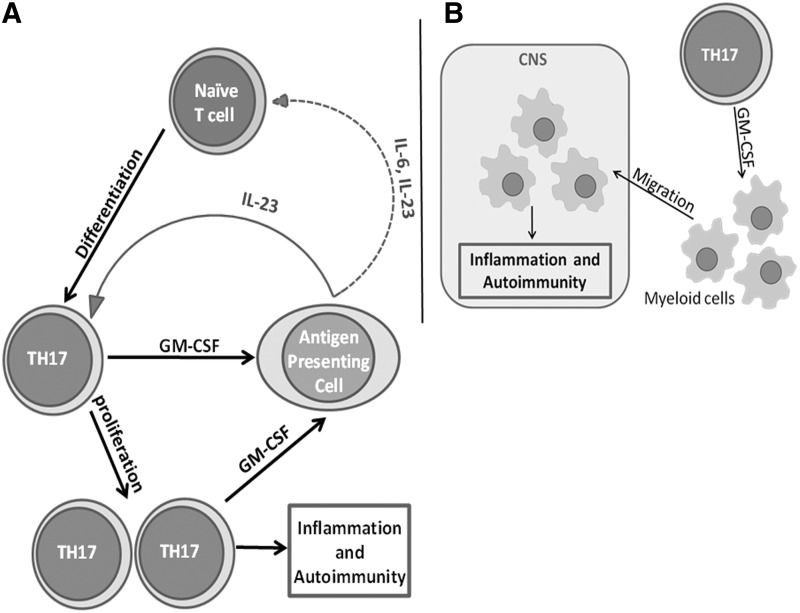
FIG. 1.
The pro-inflammatory role of granulocyte macrophage colony stimulating factor (GM-CSF) in experimental autoimmune encephalomyelitis. (A) GM-CSF produced by TH17 cells induces antigen presenting cells to secrete interleukin (IL)-23 (and IL-6), which supports the maintenance (and differentiation) of TH17 cells in a feedback mechanism, resulting in inflammation and autoimmunity. (B) GM-CSF secreted by TH17 induces the migration of myeloid cells into the central nervous system (CNS) and causes inflammation and autoimmunity.
IL-17 producing Th17 cells have been strongly implicated in the pathogenesis of both EAE and multiple sclerosis (Muls and others 2012; Kang and others 2013). Further, TGF-β and IL-6 have been implicated in the generation of Th17 cells (Bettelli and others 2006). GM-CSF has been implicated in both the regulation of pro-inflammatory activity of TH17 cells and the differentiation of TH17 cells indirectly through the stimulation of IL-6 secretion by APCs (Sonderegger and others 2008; Ko and others 2014). The cytokine IL-23 has also been shown to be critical in the “terminal differentiation” of TH17 cells (McGeachy and others 2009). Recent studies have shown that IL-23 can induce GM-CSF secretion by Th17 cells, which, in turn, can stimulate the production of IL-23 by APCs (El-Behi and others 2011). GM-CSF-mediated induction of IL-23 as well as IL-6 and other pro-inflammatory cytokines by APCs could result in continued activation as well as in de novo generation of Th17 effector cells. Thus, the IL-23/GM-CSF positive-feedback loop could help perpetuate and sustain the inflammatory cycle (El-Behi and others 2011) (Fig. 1A).
An alternative model suggests a different mechanism by which GM-CSF can drive inflammation. GM-CSF has been identified as the cytokine necessary for the recruitment of myeloid cells into the CNS and the development of EAE (Codarri and others 2011). GM-CSF-deficient mice are resistant to developing many experimental autoimmune diseases, including EAE, myocarditis, and CIA (Campbell and others 1998; McQualter and others 2001; Sonderegger and others 2008). The CNS of GM-CSF-deficient mice primed with EAE-specific antigen exhibited lower expression of MHC class II in microglia and had fewer infiltrating macrophages compared with similarly immunized WT mice. Although the expression of the GM-CSF receptor on microglia was not required for EAE induction, the accumulation of microglia was critical and was dependent on GM-CSF-mediated signaling (Fig. 1B). However, the precise mechanism by which GM-CSF promotes the infiltration of macrophages into the CNS is yet to be investigated (Codarri and others 2011).
In the same study, GM-CSF-secreting CD4+ T cells were shown to express retinoic acid receptor-related orphan receptor-γt (RORγt), which earlier had been shown to be critical for Th17 cell function (Ivanov and others 2006). Further, while RORγt-deficient T cells failed to produce GM-CSF, induction of RORγt expression resulted in GM-CSF production. Taken together, Th17-related transcription factor RORγt appears to be involved in the regulation of GM-CSF expression. Collectively, these data support the claim that GM-CSF is an important pro-inflammatory mediator in certain autoimmune disorders.
Role of GM-CSF in Immune Tolerance
In contrast to the role of GM-CSF as a pro-inflammatory cytokine, recent studies have described GM-CSF as a cytokine involved indirectly in the induction of immunological tolerance and anti-inflammatory responses. Unlike IL-10 and TGFβ, both of which demonstrate physiological anti-inflammatory and immunosuppressive functions, the GM-CSF has not been considered a tolerogenic or immunosuppressive cytokine (Hillyer and others 2006). However, GM-CSF has been shown to facilitate T-cell-mediated tolerance by inducing “tolerogenic” DCs (Steinman and others 2003). DCs differentiate from a population of precursor cells, and they undergo either terminal differentiation or maturation for efficient antigen presentation. This maturation process is characterized by enhanced expression of MHC class II, B7 family co-stimulatory molecules, and an array of pro-inflammatory cytokines such as IL-12, which enhances the inflammatory response (Inaba and others 1994; Koch and others 1996; Inaba and others 2000). Antigen presentation by matured DCs leads to T-cell activation and pro-inflammatory responses. The critical role of DC development in T-cell activation has been further established by studying the effects of low antigen dose in vivo in the absence of the maturation signals. Low levels of antigens without the accompanying maturation signals can result in incomplete maturation of DCs, which fail to activate T cells and result in immune tolerance (Hawiger and others 2001; Gilliet and others 2002). It has been suggested that these maturation-resistant DCs can effectively induce tolerance by failing to deliver the required “strength of signal” to activate T cells. Thus, DCs in this context may be described as “tolerogenic” (Steinman and others 2003).
Tolerogenic DCs can induce peripheral tolerance through a variety of mechanisms. Sub-optimal antigen presentation by tolerogenic DCs can directly cause T-cell hypo-responsiveness and/or anergy (Kriegel and others 2012; Mayer and others 2012; Raich-Regue and others 2012). Alternatively, tolerogenic DCs can expand or induce regulatory T-cells, which, in turn, can suppress the T-effector cells (Bhattacharya and others 2011). Thus, the means by which tolerogenic DCs induce peripheral tolerance is well understood, but how DC precursors develop into tolerogenic DCs is relatively unstudied.
The basic understanding of DC-mediated tolerance stems from the notion that semi-developed/matured DCs are responsible for developing T-cell tolerance (Rutella and others 2006). Various cytokines, including TNFα, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), and GM-CSF, can modulate DC development into a tolerogenic phenotype (Rutella and Lemoli 2004; Rutella and others 2004; Gangi and others 2005; Li and others 2005; Verginis and others 2005). While bone marrow progenitor cells treated with low-dose GM-CSF can develop into tolerogenic immature DCs, cells treated with a higher dose of GM-CSF can develop into mature pro-inflammatory DCs (Lutz and others 2000). These low-dose GM-CSF-derived tolerogenic DCs have been reported to be maturation resistant when challenged with standard DC maturation agents such as LPS and TNFα (Berger and others 2009; Guindi and others 2012). Furthermore, these cells failed to upregulate the expression of co-stimulatory molecules or increase the secretion of IL-12 on appropriate stimulation. Interestingly, when these “immature” DCs were adoptively transferred, they were able to home to T-cell areas of the lymphoid organs in the recipient mice (Lutz and others 2000). Moreover, these immature DCs were able to induce T-cell hypo-responsiveness in vitro and prolong allograft survival in vivo, both definitive indications of immune tolerance (Lutz and others 2000).
A related but alternative mechanism of T-cell tolerance that involves “semi-mature DCs” has been proposed (Verginis and others 2005). GM-CSF exposed bone marrow cells have been shown to develop into semi-mature DCs, which are characterized by increased levels of expression of MHC class-II and CD80/86 co-stimulatory molecules, but lower levels of expression/production of inflammatory cytokines such as TNFα, IL-12, IL-1β, and IL-6 (Verginis and others 2005). These semi-matured DCs pulsed with autoantigen could cause selective activation of autoantigen-specific CD4+CD25+ regulatory T-cells that expressed Glucocorticoid-induced TNF-Receptor (GITR), Cytotoxic T-lymphocyte antigen-4 (CTLA-4), and the transcription factor Foxp3. These regulatory T-cells secreted IL-10 and suppressed proliferation of antigen-specific CD4+CD25− T-effector cells. In addition, adoptive transfer of antigen pulsed semi-matured DCs prevented the induction of Experimental Autoimmune Thyroiditis (EAT) by increasing autoantigen-specific Tregs (Verginis and others 2005).
Direct administration of “low-dose” GM-CSF into mice can prevent as well as suppress ongoing EAT (Vasu and others 2003). GM-CSF-induced suppression of EAT was associated with a selective expansion of CD4+CD25+ T regulatory cells that suppressed autoantigen-specific responses through increased production of IL-10 (Gangi and others 2005). Furthermore, treatment with low-dose GM-CSF can reverse experimental autoimmune myasthenia gravis (EAMG) in C57BL/6 mice and prevent the development of type-1 diabetes (T1D) in NOD mice (Gangi and others 2005; Sheng and others 2006; Cheatem and others 2009). These studies revealed that low-dose GM-CSF acted on DC precursors in vivo and caused expansion of a subset of CD11C+CD8α− DCs (Cheatem and others 2009). These CD8α− DCs expressed very low to negligible levels of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6, but they expressed higher levels of TGFβ (Ganesh and others 2009). Adoptive transfer of these “tolerogenic” DCs from GM-CSF treated donor mice to recipient mice followed by immunization with mTg led to an expansion of Foxp3+ Tregs in the draining lymph nodes and prevented the development of EAT (Ganesh and others 2009).
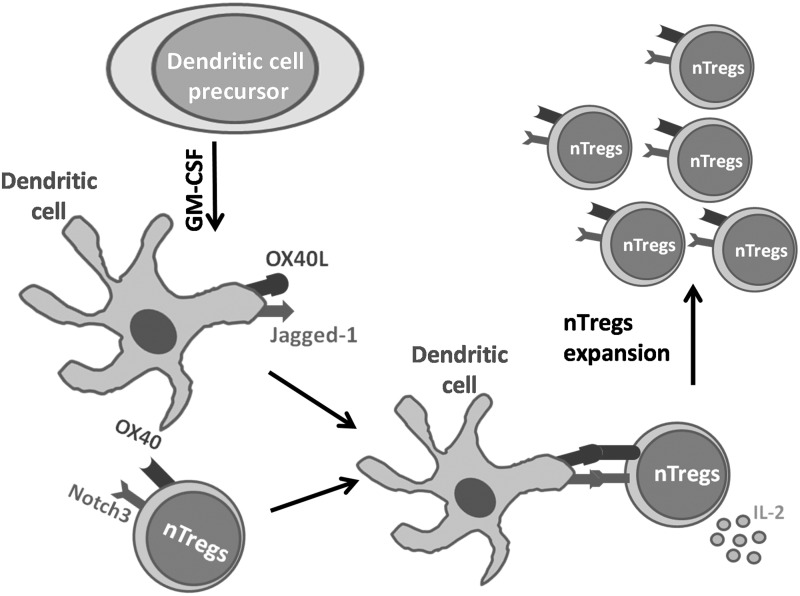
Subsequent studies with bone marrow cells showed that GM-CSF could differentiate precursor cells ex vivo into Bone Marrow-Derived Dendritic cells (G-BMDCs) that could expand natural Tregs and differentiate adaptive Tregs through a different mechanism (Bhattacharya and others 2011). In the first case, G-BMDCs caused selective proliferation of only CD4+Foxp3+ Tregs, and not CD4+Foxp3− Teff, in co-cultures with total CD4+ T-cells. Further, this Treg proliferation occurred even in the presence of G-BMDCs derived from MHC class-II−/− mice and thus was TCR independent. This represented a unique mechanism of G-BMDC-induced Treg proliferation that did not require canonical antigen presentation/activation. In a search for cell surface molecules expressed in G-BMDCs that could interact with Tregs and cause their proliferation, we identified 2 molecules, namely the TNF-family ligand OX40L and the notch-family ligand jagged-1 (Jag-1), to be critically important (Bhattacharya and others 2011; Gopisetty and others 2013) (Fig. 2). Adoptive transfer of only OX40L+Jag-1+ G-BMDCs led to in vivo Treg expansion, increased production of IL-4 and IL-10, and suppression of EAT in the recipient mice. The cognate receptor for OX40L is OX40 and it is constitutively expressed on Tregs (Vu and others 2007). Furthermore, we found that the most likely cognate receptor for Jag-1 contributing to Treg expansion was Notch3 (Gopisetty and others 2013), which is preferentially expressed in Tregs (Anastasi and others 2003). Based on these observations, we speculate that OX40 and Notch3-mediated signaling pathways may be co-operatively interacting to aid in TCR-independent Treg proliferation.
FIG. 2.
GM-CSF induces differentiation of precursor cells into tolerogenic dendritic cells. GM-CSF induces differentiation of bone marrow-derived precursor cells into dendritic cells that co-express OX40L and Jagged-1. On cell–cell interaction with Foxp3+ nTregs, these ligands signal through their cognate receptors, OX40 and Notch3, respectively, driving the proliferation of nTregs in the presence of IL-2.
In addition to the capacity of G-BMDCs to expand natural Tregs in a TCR-independent manner, G-BMDCs secreted high levels of TGFβ, which along with TCR stimulation could convert T-effectors into adaptive Foxp3+ Tregs. Supplementation of anti-CD3 stimulated CD4+CD25− T-cell cultures with supernatant from G-BMDC cultures, and it resulted in robust conversion of Teff into induced T-regulatory cells (iTregs) (Bhattacharya and others 2011). Thus, G-BMDCs can contribute to peripheral tolerance by both expanding the pool of natural Tregs and inducing antigen-specific iTregs (Bhattacharya and others 2011). Other unpublished data from our laboratory suggest that low-dose GM-CSF directly contributes to the development of OX40L+Jag-1+ DCs in vivo, and thus may be responsible for maintaining physiological Treg homeostasis. This would suggest a nonredundant role for GM-CSF in maintaining immune tolerance.
Divergent Effects of GM-CSF Noted in Preclinical and Clinical Studies
GM-CSF is either being used or targeted in a variety of treatment protocols for a wide range of diseases, including cancer, autoimmune diseases, and sepsis-related immune-suppression (Salgia and others 2003; Meisel and others 2009; Eroglu and others 2011; Peng and others 2012; Rowin and others 2012; Behrens and others 2014; Kaufman and others 2014). Most of these treatments assume a pro-inflammatory function for GM-CSF.
Dual roles of GM-CSF in cancer
Since cytokines play a major role in modulating anti-tumor immune response (Candido and Hagemann 2013), cytokines such as IL-2 (Forni and others 1985; Forni and others 1988) and GM-CSF (Dranoff 2002, 2003) have been used in cancer treatment protocols for eliciting a strong anti-tumor immune response. In one preclinical study, modified B16 melanoma cells engineered to express GM-CSF elicited a more potent anti-tumor response than the nonexpressing cells in C57BL/6 mice, (Dranoff and others 1993). In another study, closer examination of tumor histology showed an association of increased expression of GM-CSF with enhanced infiltration of APCs such as DCs and macrophages into the tumor (Armstrong and others 1996). An increase in numbers of infiltrating APCs in the tumor may be due to GM-CSF's ability to prime target cells to enhance the synthesis of IL-8, a chemoattractant for neutrophils and MIP-1alpha, a chemoattractant for monocytes and lymphocyte subpopulations (Roberge and others 1998). Consistent with these observations, when vaccinated with irradiated autologous melanoma cells engineered to secrete GM-CSF, 11 of 16 patients with metastatic melanoma showed extensive tumor destruction (Soiffer and others 1998). Similarly, autologous tumor cells modified by adenoviral vectors to secrete GM-CSF were successfully used to vaccinate patients with nonsmall-cell-lung carcinoma (NSCLC) (Salgia and others 2003). In another clinical study, in advanced-stage NSCLC patients, tumor vaccines secreting higher levels of GM-CSF correlated with a better survival outcome (Nemunaitis and others 2004).
In a different approach, DCs pulsed with a specific tumor antigen in the presence of GM-CSF alone or GM-CSF+IL-4 have been used as cancer vaccines for melanoma, renal cell carcinoma, malignant glioma, and metastatic prostate cancer (Murphy and others 1996; Nestle and others 1998; Holtl and others 1999; Thurner and others 1999; Yu and others 2001). In multiple clinical trials, metastatic prostate cancer patients injected with autologous APCs precultured with a fusion protein (Sipuleucel-T; approved by the FDA for the treatment of metastatic prostate cancer) of prostatic acid phosphatase (PAP; a protein expressed by prostate cancer cells), and GM-CSF showed prolonged survival (Higano and others 2009; Kantoff and others 2010). However, similar approaches using tumor-lysate-pulsed autologous DCs generated with GM-CSF/IL-4 resulted in either a partial protection in patients with metastatic renal carcinoma (Kim and others 2007) or lung carcinoma (Chang and others 2005), or as shown in another study failed to elicit an adequate immune response as noted in metastatic melanoma patients (Redman and others 2008), In addition, vaccines using GM-CSF-secreting tumor cells have demonstrated unpredictable outcomes. In a phase III clinical trial for metastatic prostate cancer, vaccination with GM-CSF producing prostate tumor cells had adverse outcomes with regard to patient survival (Lassi and Dawson 2010). Similarly, the use of GM-CSF as an adjuvant for cell-based vaccination in patients with melanoma had negative outcomes on survival (Faries and others 2009). Thus, it appears that the outcome of GM-CSF-based strategies for treating cancer is dose dependent and may be influenced by the overall context of the immune response (Bendandi 2009; Eggermont 2009).
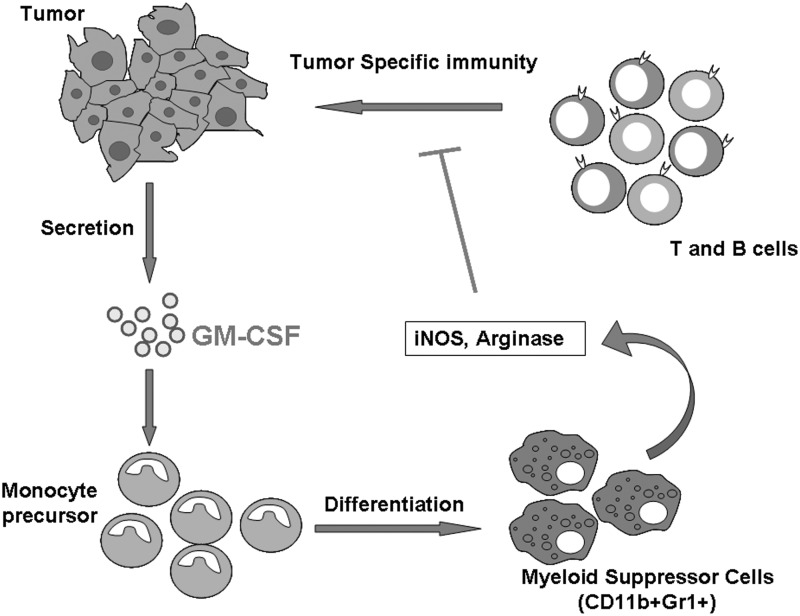
The reasons for these diverse outcomes is not clear. Interestingly, some studies suggest that one of the strategies that tumor cells use for immune evasion is to produce GM-CSF (Tsuchiya and others 1988; Bronte and others 1999). Human prostate cancer cells have been shown to express both GM-CSF and its receptor (Rokhlin and others 1996), and their levels of expression have been found to correlate with the stage of advancement of gliomas (Mueller and others 1999). Interestingly, GM-CSF concentrations were found to be higher in the local glioma environment than in the periphery (Kohanbash and others 2013). This study further suggested that GM-CSF can cause suppression of T-cell function through the expression of arginase in a suppressor myeloid subpopulation (Kohanbash and others 2013). In a tumor-vaccine study using modified tumor cells expressing GM-CSF, serum level of GM-CSF that was above a threshold level induced the development of CD11b+Gr-1+ myeloid suppressor cells that impaired antigen-specific T-cell responses (Serafini and others 2004; Serafini and others 2006). Other studies have shown that GM-CSF secretion by tumor cells can lead to the development of an inhibitory population of CD11b+Gr-1+ cells, which can result in functional impairment of CD8+ T-cells (Gallina and others 2006). Collectively, these data suggest that high concentrations of GM-CSF may induce a myeloid suppressor population that can negatively regulate immune activation (Fig. 3). Although CD11b+Gr-1+ cells present an immature phenotype when cultured with GM-CSF alone, they can be matured with a combination of GM-CSF and IL-4. This suggests that GM-CSF can cause divergent outcomes based not only on the dose but also on the presence of other cytokines (Bronte and others 1999). Studies involving patients with melanoma or colon cancer have shown that using a sub-cutaneous injection of GM-CSF at a dose above100 μg is ineffective in enhancing an anti-tumor response (Parmiani and others 2007). In contrast, studies using lower doses of GM-CSF, (i.e., 40–80 μg) have shown increased T-effector-cell response as an overall marker of immune system activation (Parmiani and others 2007). Collectively, these studies suggest that GM-CSF could act in an inflammatory or immunosuppressive manner, depending on dose and the overall cytokine milieu.
FIG. 3.
Secretion of GM-CSF by tumor cells leads to immune evasion. Tumor cell-secreted GM-CSF differentiates precursor cells into myeloid suppressor cells, which inhibit host immune response through different mechanisms, including secretion of iNOS and Arginase.
Use of GM-CSF in inflammation and immunosuppression
Although Crohn's Disease (CD) is characterized as an inflammatory disease affecting the gut, treatment with GM-CSF has been successfully used to cause disease remission (Dieckgraefe and Korzenik 2002). It has recently been proposed that the CD develops as a result of immunodeficiency (Roth and others 2011). GM-CSF is a potent growth and differentiation factor for myeloid cells, which are responsible for anti-microbial activity. Therefore, neutralization of GM-CSF by anti-GM-CSF autoantibodies has been proposed to be associated with disease progression and relapse (Dabritz and others 2013). A phase I-II trial of GM-CSF in pediatric CD had encouraging results (Kelsen and others 2010). Similarly, in another phase I-II trial in Japanese patients with CD, GM-CSF therapy improved disease activity scores (Takazoe and others 2009). However, a systematic analyses of multiple randomized trials revealed that GM-CSF treatment in CD did not yield better outcomes relative to the placebo-treated group (Roth and others 2012)
GM-CSF has also been used in the treatment of sepsis-associated immunosuppression, which is characterized by a tolerogenic cytokine milieu, T-cell anergy and hypo-responsiveness, and reduced monocyte lineage cell activity (Hutchins and others 2014). GM-CSF treatment resulted in a favorable outcome as indicated by the restoration of immune competence with an accompanying increase in both lymphoid and myeloid subpopulations, including CD4+ T cells, CD8+ T cells, and neutrophils (Meisel and others 2009). Furthermore, patients in the GM-CSF treatment group exhibited enhanced levels of TNFα (Meisel and others 2009). These data support a general pro-inflammatory role for GM-CSF. However, in one study on preterm Small for Gestational Age (SGA) babies, GM-CSF treatment did not improve sepsis-free survival (Marlow and others 2013).
Collectively, these experiences suggest that inflammation or immunosuppression may not merely depend on the presence or absence of GM-CSF but may depend on the overall immune environment in the host.
Use of GM-CSF in treating autoimmune diseases
As previously discussed in this review, GM-CSF has been linked to several autoimmune disorders, primarily acting in a pro-inflammatory manner through M1 macrophages and Th17 effector cells. When exploring putative treatment modalities for autoimmune disorders such as RA, a common approach involves blocking antibodies for GM-CSF (Behrens and others 2014). MOR103, a human monoclonal antibody against GM-CSF, has been shown to have moderately positive benefits in patients with RA (Behrens and others 2014). In a phase Ib/IIa, double-blind, placebo-controlled, dose-escalation trial, Behrens and others found a statistically significant improvement in the moderate and high dosage treatment groups as assessed by the disease activity scores. Supporting evidence for this approach has come from studies in which monoclonal antibody Mavrilimumab, which blocks GMRα, was found to significantly ameliorate the severity of RA in a phase II, randomized, double-blind, placebo-controlled, and dose-escalation trial (Burmester and others 2011; Burmester and others 2013).
In contrast, our laboratory combined clinical assessment scores and an analysis of the relevant lymphoid populations in a patient with myasthenia gravis (MG) who underwent experimental GM-CSF treatment to show that GM-CSF has a potential role in stimulating peripheral tolerance as the means to ameliorating MG (Rowin and others 2012). Three weeks after cessation of GM-CSF treatment, a marked decline in the clinical score was observed (Rowin and others 2012). Before treatment with GM-CSF, the Tregs from this patient had lower levels of Foxp3 expression, as compared with Tregs from healthy controls. On treatment with GM-CSF, the Foxp3 expression was increased and sustained, and Foxp3+CD4+CD25+ Tregs were capable of limiting the proliferative capacities of CD4+ T effector cells (Rowin and others 2012). This is consistent with our observation on the effects of GM-CSF on peripheral blood cells in vitro (Thiruppathi and others 2012) and our earlier in vivo studies in a mouse model of EAMG (Sheng and others 2006; Meriggioli and others 2008; Sheng and others 2008; Sheng and others 2011). Thus, it is important to consider the immunological status of the patient and progression of the autoimmune disorder in determining the potential clinical utility of GM-CSF.
GM-CSF itself is implicated directly in at least one autoimmune disease. Pulmonary Alveolar Proteinosis, an autoimmune disorder characterized by lipid deposition in the alveoli of the lungs, has been associated with GM-CSF deficiency (Venkateshiah and others 2006). In clinical trials, administration of GM-CSF either subcutaneously (Venkateshiah and others 2006) or via inhalation (Tazawa and others 2014) has shown promising results. Some studies suggest that autoantibodies against GM-CSF may be responsible for disease pathology (Costabel and Guzman 2005). It is believed that GM-CSF-dependent development of alveolar macrophages is defective in patients, leading to a defect in the pulmonary clearance of surfactants (Sakagami and others 2010). Consistent with this notion, improvement in lung health in patients with Pulmonary Alveolar Proteinosis has been shown to correlate with reduced anti-GM-CSF autoantibody levels (Ohashi and others 2012).
Other Cytokines That Exhibit Duality of Function
GM-CSF is not the only cytokine that exhibits divergent effects, as many other cytokines have also been shown to have divergent effects (Shachar and Karin 2013). TGFβ is generally characterized as a regulatory cytokine because of its role in the conversion of peripheral T-effector cells into adaptive Tregs through the induction of Foxp3 expression. (Chen and others 2003; Li and others 2007). TGFβ-mediated conversion of adaptive Tregs (Mucida and others 2009) is enhanced in a milieu that is rich in retinoic acid. These Tregs play an important role in establishing antigen-specific immunological tolerance. However, it is now known that the functional effects of TGFβ are context dependent. In mouse, TGFβ synergizes with IL-6 while in human TGFβ synergizes with cytokines such as IL-6, IL-1β, and IL-21, to induce Th17 cells that have been implicated in both inflammation and autoimmunity (Korn and others 2007; Rubtsov and Rudensky 2007; Manel and others 2008; Volpe and others 2008; Yang and others 2008; Benwell and Lee 2010; Maddur and others 2012). These findings suggest that the functional property of TGFβ whether as an “inflammatory” or a “regulatory” cytokine is not intrinsic but dependent on the presence of other factors.
IL-10 is believed to be an anti-inflammatory cytokine and has been shown to suppress the function of immune T cells (Chaudhry and others 2011) and the inflammatory function of macrophages (Bogdan and others 1991). Moreover, the suppressive activity of Foxp3+ Tregs is dependent on their capacity to secrete IL-10 (Gangi and others 2005). However, IL-10 has also been shown to enhance the expression of MHC class-II molecules in B cells, thereby enhancing their ability to present antigens (Go and others 1990) and produce autoantibodies (Llorente and others 1995).
The best example of a cytokine with profound duality of function is perhaps IL-2. Originally described as a T-cell growth factor, it has been used in cancer therapy to support the growth of immune T cells (Rosenberg and Lotze 1986). However, in mice, deficiency of either IL-2 or its receptor results in lymphoproliferative phenotype, often causing autoimmune-like symptoms (Sadlack and others 1993; Suzuki and others 1995). It is now believed that although IL-2 contributes, it is not essential for effector T-cell proliferation. In contrast, a nonredundant function of IL-2 is to serve as a survival factor for Foxp3+ Tregs (Tang and others 2008). A number of recent preclinical and clinical studies have shown that a low dose of IL-2 can support Treg survival and growth while being unable to support significant T-effector proliferation (Grinberg-Bleyer and others 2010; Koreth and others 2011; Saadoun and others 2011).
Even well-characterized Th1-type cytokines such as IL-1β with an established role in inflammation and immunity (Dinarello 2011) have been shown to have immunomodulatory effects. IL-1β has been shown to promote Foxp3+ expression in activated T cells in the presence of IL-2 and TGFβ (Ganesh and others 2011), thus exhibiting a context-dependent regulatory activity.
Conclusion
In summary, studies have shown that although GM-CSF and many other cytokines are assumed to be drivers of inflammatory responses, depending on the dose, the microenvironment, and the presence of other cytokines, some of them can act as regulatory cytokines. However, the underlying mechanisms that determine the pro-inflammatory versus the regulatory effects of GM-CSF are not fully elucidated. One of the factors that contributes to the differential effects of GM-CSF appears to be the dose of the cytokine, whereby a lower dose of GM-CSF maintains a tolerogenic subpopulation of myeloid cells that are involved in Treg homeostasis. At higher doses, GM-CSF can cause myeloproliferation, leading to a robust immune response (Nemunaitis and others 2004). At still higher doses, and above a critical physiological threshold, GM-CSF differentiates myeloid precursors into an immunosuppressive phenotype (Serafini and others 2004). Alternatively, the GM-CSF action may be context dependent in that it is known to differentiate myeloid precursors into inflammatory DCs (Naik and others 2006), similar to those found during Listeria monocytogenes infection (Zhan and others 1998; Dominguez and others 2011). It is possible that the population of monocytic precursor cells that are exposed to GM-CSF use adequate antigen load as a necessary stimulus for their development into inflammatory DCs. In contrast, without the necessary environmental stimulus, GM-CSF could enable monocyte precursors to develop into a population of immature DCs that can exacerbate the immunosuppression noted in the tumor microenvironment. Thus, the intrinsic property of GM-CSF is neither “inflammatory” nor “regulatory” and its true function is likely determined by the dose, presence or absence of other relevant cytokines and the overall context of the immune response.
Acknowledgments
This study was supported by grants # 1R41AI085677-01 and R01 AI107516-01A1 to Dr. Bellur S. Prabhakar from the National Institutes of Health.
Author Disclosure Statement
The authors have no competing financial interests.
References
- Alsalameh S, Firestein GS, Oez S, Kurrle R, Kalden JR, Burmester GR. 1994. Regulation of granulocyte macrophage colony stimulating factor production by human articular chondrocytes. Induction by both tumor necrosis factor-alpha and interleukin 1, downregulation by transforming growth factor beta and upregulation by fibroblast growth factor. J Rheumatol 21(6):993–1002 [PubMed] [Google Scholar]
- Anastasi E, Campese AF, Bellavia D, Bulotta A, Balestri A, Pascucci M, Checquolo S, Gradini R, Lendahl U, Frati L, Gulino A, Di Mario U, Screpanti I. 2003. Expression of activated Notch3 in transgenic mice enhances generation of T regulatory cells and protects against experimental autoimmune diabetes. J Immunol 171(9):4504–4511 [DOI] [PubMed] [Google Scholar]
- Armstrong CA, Botella R, Galloway TH, Murray N, Kramp JM, Song IS, Ansel JC. 1996. Antitumor effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res 56(9):2191–2198 [PubMed] [Google Scholar]
- Baldwin GC, Gasson JC, Kaufman SE, Quan SG, Williams RE, Avalos BR, Gazdar AF, Golde DW, DiPersio JF. 1989. Nonhematopoietic tumor cells express functional GM-CSF receptors. Blood 73(4):1033–1037 [PubMed] [Google Scholar]
- Begley CG, Lopez AF, Nicola NA, Warren DJ, Vadas MA, Sanderson CJ, Metcalf D. 1986. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood 68(1):162–166 [PubMed] [Google Scholar]
- Behrens F, Tak PP, Ostergaard M, Stoilov R, Wiland P, Huizinga TW, Berenfus VY, Vladeva S, Rech J, Rubbert-Roth A, Korkosz M, Rekalov D, Zupanets IA, Ejbjerg BJ, Geiseler J, Fresenius J, Korolkiewicz RP, Schottelius AJ, Burkhardt H. 2014. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis [Epub ahead of print]; DOI: 10.1136/annrheumdis-2013-204816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendandi M. 2009. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer 9(9):675–681 [DOI] [PubMed] [Google Scholar]
- Benwell RK, Lee DR. 2010. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol 134(2):178–187 [DOI] [PubMed] [Google Scholar]
- Berdel WE, Danhauser-Riedl S, Steinhauser G, Rastetter J. 1990. Stimulation of clonal growth of human colorectal tumor cells by IL-3 and GM-CSF. Modulation of 5-FU cytotoxicity by GM-CSF. Onkologie 13(6):437–443 [DOI] [PubMed] [Google Scholar]
- Berenbaum F, Rajzbaum G, Amor B, Toubert A. 1994. Evidence for GM-CSF receptor expression in synovial tissue. An analysis by semi-quantitative polymerase chain reaction on rheumatoid arthritis and osteoarthritis synovial biopsies. Eur Cytokine Netw 5(1):43–46 [PubMed] [Google Scholar]
- Berger TG, Schulze-Koops H, Schafer M, Muller E, Lutz MB. 2009. Immature and maturation-resistant human dendritic cells generated from bone marrow require two stimulations to induce T cell anergy in vitro. PLoS One 4(8):e6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238 [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. 2011. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol 89(2):235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof RJ, Zafiropoulos D, Hamilton JA, Campbell IK. 2000. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF-1 and granulocyte macrophage (GM)-CSF: evidence of macrophage infiltration and local proliferation. Clin Exp Immunol 119(2):361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C, Vodovotz Y, Nathan C. 1991. Macrophage deactivation by interleukin 10. J Exp Med 174(6):1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzi MF, Avanzi GC, Veglia F, Clark SC, Pegoraro L. 1990. Expression and modulation of IL-3 and GM-CSF receptors in human growth factor dependent leukaemic cells. Br J Haematol 76(2):203–209 [DOI] [PubMed] [Google Scholar]
- Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. 1999. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 162(10):5728–5737 [PMC free article] [PubMed] [Google Scholar]
- Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. 2012. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 250(1):277–302 [DOI] [PubMed] [Google Scholar]
- Burgess AW, Camakaris J, Metcalf D. 1977. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem 252(6):1998–2003 [PubMed] [Google Scholar]
- Burmester GR, Feist E, Sleeman MA, Wang B, White B, Magrini F. 2011. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-alpha, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis 70(9):1542–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester GR, Weinblatt ME, McInnes IB, Porter D, Barbarash O, Vatutin M, Szombati I, Esfandiari E, Sleeman MA, Kane CD, Cavet G, Wang B, Godwood A, Magrini F, Group ES. 2013. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis 72(9):1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Bendele A, Smith DA, Hamilton JA. 1997. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis 56(6):364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. 1998. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol 161(7):3639–3644 [PubMed] [Google Scholar]
- Candido J, Hagemann T. 2013. Cancer-related inflammation. J Clin Immunol 33 (Suppl. 1):S79–S84 [DOI] [PubMed] [Google Scholar]
- Cannistra SA, Groshek P, Garlick R, Miller J, Griffin JD. 1990. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proc Natl Acad Sci U S A 87(1):93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebon J, Nicola N, Ward M, Gardner I, Dempsey P, Layton J, Duhrsen U, Burgess AW, Nice E, Morstyn G. 1990. Granulocyte-macrophage colony stimulating factor from human lymphocytes. The effect of glycosylation on receptor binding and biological activity. J Biol Chem 265(8):4483–4491 [PubMed] [Google Scholar]
- Chang GC, Lan HC, Juang SH, Wu YC, Lee HC, Hung YM, Yang HY, Whang-Peng J, Liu KJ. 2005. A pilot clinical trial of vaccination with dendritic cells pulsed with autologous tumor cells derived from malignant pleural effusion in patients with late-stage lung carcinoma. Cancer 103(4):763–771 [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34(4):566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatem D, Ganesh BB, Gangi E, Vasu C, Prabhakar BS. 2009. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol 131(2):260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. 2003. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198(12):1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Tojo A, Kitamura T, Urabe A, Miyazono K, Takaku F. 1990. Characterization and molecular features of the cell surface receptor for human granulocyte-macrophage colony-stimulating factor. Leukemia 4(1):29–36 [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. 2011. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12(6):560–567 [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80(8):2012–2020 [PubMed] [Google Scholar]
- Comalada M, Xaus J, Sanchez E, Valledor AF, Celada A. 2004. Macrophage colony-stimulating factor-, granulocyte-macrophage colony-stimulating factor-, or IL-3-dependent survival of macrophages, but not proliferation, requires the expression of p21(Waf1) through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol 34(8):2257–2267 [DOI] [PubMed] [Google Scholar]
- Cook AD, Braine EL, Campbell IK, Rich MJ, Hamilton JA. 2001. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res 3(5):293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. 2009. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol 5(10):554–559 [DOI] [PubMed] [Google Scholar]
- Costabel U, Guzman J. 2005. Pulmonary alveolar proteinosis: a new autoimmune disease. Sarcoidosis Vasc Diffuse Lung Dis 22 (Suppl. 1):S67–S73 [PubMed] [Google Scholar]
- Dabritz J, Bonkowski E, Chalk C, Trapnell BC, Langhorst J, Denson LA, Foell D. 2013. Granulocyte macrophage colony-stimulating factor auto-antibodies and disease relapse in inflammatory bowel disease. Am J Gastroenterol 108(12):1901–1910 [DOI] [PubMed] [Google Scholar]
- Dedhar S, Gaboury L, Galloway P, Eaves C. 1988. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A 85(23):9253–9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckgraefe BK, Korzenik JR. 2002. Treatment of active Crohn's disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet 360(9344):1478–1480 [DOI] [PubMed] [Google Scholar]
- Di Gregoli K, Johnson JL. 2012. Role of colony-stimulating factors in atherosclerosis. Curr Opin Lipidol 23(5):412–421 [DOI] [PubMed] [Google Scholar]
- Dinarello CA. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117(14):3720–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez PM, Lopez-Bravo M, Kalinke U, Ardavin C. 2011. Statins inhibit iNOS-mediated microbicidal potential of activated monocyte-derived dendritic cells by an IFN-beta-dependent mechanism. Eur J Immunol 41(11):3330–3339 [DOI] [PubMed] [Google Scholar]
- Dranoff G. 2002. GM-CSF-based cancer vaccines. Immunol Rev 188:147–154 [DOI] [PubMed] [Google Scholar]
- Dranoff G. 2003. GM-CSF-secreting melanoma vaccines. Oncogene 22(20):3188–3192 [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. 1993. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 90(8):3539–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Bandion A, Binder BR, de Martin R, Schmid JA. 2003. GMCSF activates NF-kappaB via direct interaction of the GMCSF receptor with IkappaB kinase beta. Blood 102(1):192–199 [DOI] [PubMed] [Google Scholar]
- Eggermont AM. 2009. Immunostimulation versus immunosuppression after multiple vaccinations: the woes of therapeutic vaccine development. Clin Cancer Res 15(22):6745–6747 [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12(6):568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu Z, Kong KM, Jakowatz JG, Samlowski W, Fruehauf JP. 2011. Phase II clinical trial evaluating docetaxel, vinorelbine and GM-CSF in stage IV melanoma. Cancer Chemother Pharmacol 68(4):1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. 2009. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res 15(22):7029–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn C, Wermann M, Pezzutto A, Hufner M, Heilig B. 1992. [Plasma GM-CSF concentrations in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropathy]. Z Rheumatol 51(3):121–126 [PubMed] [Google Scholar]
- Fischer HG, Reichmann G. 2001. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol 166(4):2717–2726 [DOI] [PubMed] [Google Scholar]
- Fleetwood AJ, Cook AD, Hamilton JA. 2005. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol 25(5):405–428 [DOI] [PubMed] [Google Scholar]
- Forni G, Fujiwara H, Martino F, Hamaoka T, Jemma C, Caretto P, Giovarelli M. 1988. Helper strategy in tumor immunology: expansion of helper lymphocytes and utilization of helper lymphokines for experimental and clinical immunotherapy. Cancer Metastasis Rev 7(4):289–309 [DOI] [PubMed] [Google Scholar]
- Forni G, Giovarelli M, Santoni A. 1985. Lymphokine-activated tumor inhibition in vivo. I. The local administration of interleukin 2 triggers nonreactive lymphocytes from tumor-bearing mice to inhibit tumor growth. J Immunol 134(2):1305–1311 [PubMed] [Google Scholar]
- Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, Mata-Espinosa D, Marquina-Castillo B, Barrios-Payan J, Hernandez-Pando R. 2014. Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor. Med Oncol 31(1):774. [DOI] [PubMed] [Google Scholar]
- Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. 2006. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116(10):2777–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BB, Bhattacharya P, Gopisetty A, Sheng J, Vasu C, Prabhakar BS. 2011. IL-1beta promotes TGF-beta1 and IL-2 dependent Foxp3 expression in regulatory T cells. PLoS One 6(7):e21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. 2009. GM-CSF-induced CD11c+CD8a—dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol 21(3):269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangi E, Vasu C, Cheatem D, Prabhakar BS. 2005. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol 174(11):7006–7013 [DOI] [PubMed] [Google Scholar]
- Gearing DP, King JA, Gough NM, Nicola NA. 1989. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J 8(12):3667–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med 195(7):953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go NF, Castle BE, Barrett R, Kastelein R, Dang W, Mosmann TR, Moore KW, Howard M. 1990. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med 172(6):1625–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopisetty A, Bhattacharya P, Haddad C, Bruno JC, Jr., Vasu C, Miele L, Prabhakar BS. 2013. OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells. J Immunol 190(11):5516–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. 2010. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 207(9):1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindi C, Menard M, Cloutier A, Gaudreau S, Besin G, Larivee P, McDonald PP, Dupuis G, Amrani A. 2012. Differential role of NF-kappaB, ERK1/2 and AP-1 in modulating the immunoregulatory functions of bone marrow-derived dendritic cells from NOD mice. Cell Immunol 272(2):259–268 [DOI] [PubMed] [Google Scholar]
- Guthridge MA, Barry EF, Felquer FA, McClure BJ, Stomski FC, Ramshaw H, Lopez AF. 2004. The phosphoserine-585-dependent pathway of the GM-CSF/IL-3/IL-5 receptors mediates hematopoietic cell survival through activation of NF-kappaB and induction of bcl-2. Blood 103(3):820–827 [DOI] [PubMed] [Google Scholar]
- Guthridge MA, Powell JA, Barry EF, Stomski FC, McClure BJ, Ramshaw H, Felquer FA, Dottore M, Thomas DT, To B, Begley CG, Lopez AF. 2006. Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch. EMBO J 25(3):479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA. 2002. GM-CSF in inflammation and autoimmunity. Trends Immunol 23(8):403–408 [DOI] [PubMed] [Google Scholar]
- Hamilton JA. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8(7):533–544 [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Stanley ER, Burgess AW, Shadduck RK. 1980. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J Cell Physiol 103(3):435–445 [DOI] [PubMed] [Google Scholar]
- Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, McKinstry WJ, Lopez AF, Parker MW. 2008. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 134(3):496–507 [DOI] [PubMed] [Google Scholar]
- Hara T, Miyajima A. 1992. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J 11(5):1875–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194(6):769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Kitamura T, Gorman DM, Arai K, Yokota T, Miyajima A. 1990. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A 87(24):9655–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercus TR, Dhagat U, Kan WL, Broughton SE, Nero TL, Perugini M, Sandow JJ, D'Andrea RJ, Ekert PG, Hughes T, Parker MW, Lopez AF. 2013. Signalling by the betac family of cytokines. Cytokine Growth Factor Rev 24(3):189–201 [DOI] [PubMed] [Google Scholar]
- Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. 2009. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood 114(7):1289–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. 2009. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115(16):3670–3679 [DOI] [PubMed] [Google Scholar]
- Hillyer L, Dao B, Niemiec P, Lee S, Doidge M, Bemben I, Neyestani T, Woodward B. 2006. Elevated bioactivity of the tolerogenic cytokines, interleukin-10 and transforming growth factor-beta, in the blood of acutely malnourished weanling mice. Exp Biol Med (Maywood) 231(8):1439–1447 [DOI] [PubMed] [Google Scholar]
- Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. 1999. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol 161(3):777–782 [PubMed] [Google Scholar]
- Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. 2014. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med 20(4):224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain RN, Mellman I, Steinman RM. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med 191(6):927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, Muramatsu S, Hodes RJ, Steinman RM. 1994. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med 180(5):1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126(6):1121–1133 [DOI] [PubMed] [Google Scholar]
- Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, Li X. 2013. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci 16(10):1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. 2010. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422 [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Ruby CE, Hughes T, Slingluff CL., Jr. 2014. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K, O'Hara PJ, Hart CE, Forstrom JW, Hagen FS. 1987. Role of carbohydrate in the function of human granulocyte-macrophage colony-stimulating factor. Biochemistry 26(15):4861–4867 [DOI] [PubMed] [Google Scholar]
- Kelsen JR, Rosh J, Heyman M, Winter HS, Ferry G, Cohen S, Mamula P, Baldassano RN. 2010. Phase I trial of sargramostim in pediatric Crohn's disease. Inflamm Bowel Dis 16(7):1203–1208 [DOI] [PubMed] [Google Scholar]
- Kelso A, Metcalf D. 1985. Clonal heterogeneity in colony stimulating factor production by murine T lymphocytes. J Cell Physiol 123(1):101–110 [DOI] [PubMed] [Google Scholar]
- Kim SH, Yang EM, Park HJ, Ye YM, Lee HY, Park HS. 2007. Differential contribution of the CysLTR1 gene in patients with aspirin hypersensitivity. J Clin Immunol 27(6):613–619 [DOI] [PubMed] [Google Scholar]
- Ko HJ, Brady JL, Ryg-Cornejo V, Hansen DS, Vremec D, Shortman K, Zhan Y, Lew AM. 2014. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J Immunol 192(5):2202–2209 [DOI] [PubMed] [Google Scholar]
- Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med 184(2):741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanbash G, McKaveney K, Sakaki M, Ueda R, Mintz AH, Amankulor N, Fujita M, Ohlfest JR, Okada H. 2013. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer Res 73(21):6413–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ. 2011. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365(22):2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Anderson AC, Bettelli E, Oukka M. 2007. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol 191(1–2):51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Rathinam C, Flavell RA. 2012. Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cells. Proc Natl Acad Sci U S A 109(9):3457–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. 2008. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med 205(7):1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lari R, Fleetwood AJ, Kitchener PD, Cook AD, Pavasovic D, Hertzog PJ, Hamilton JA. 2007. Macrophage lineage phenotypes and osteoclastogenesis—complexity in the control by GM-CSF and TGF-beta. Bone 40(2):323–336 [DOI] [PubMed] [Google Scholar]
- Lassi K, Dawson NA. 2010. Update on castrate-resistant prostate cancer: 2010. Curr Opin Oncol 22(3):263–267 [DOI] [PubMed] [Google Scholar]
- Li G, Kim YJ, Broxmeyer HE. 2005. Macrophage colony-stimulating factor drives cord blood monocyte differentiation into IL-10(high)IL-12absent dendritic cells with tolerogenic potential. J Immunol 174(8):4706–4717 [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. 2007. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26(5):579–591 [DOI] [PubMed] [Google Scholar]
- Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P, Emilie D. 1995. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med 181(3):839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AF, Eglinton JM, Gillis D, Park LS, Clark S, Vadas MA. 1989. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A 86(18):7022–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. 2000. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol 30(7):1813–1822 [DOI] [PubMed] [Google Scholar]
- Maddur MS, Miossec P, Kaveri SV, Bayry J. 2012. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 181(1):8–18 [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9(6):641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686 [DOI] [PubMed] [Google Scholar]
- Marlow N, Morris T, Brocklehurst P, Carr R, Cowan FM, Patel N, Petrou S, Redshaw ME, Modi N, Dore C. 2013. A randomised trial of granulocyte-macrophage colony-stimulating factor for neonatal sepsis: outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed 98(1):F46–F53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausberg AK, Jander S, Reichmann G. 2009. Intracerebral granulocyte-macrophage colony-stimulating factor induces functionally competent dendritic cells in the mouse brain. Glia 57(12):1341–1350 [DOI] [PubMed] [Google Scholar]
- Mayer CT, Berod L, Sparwasser T. 2012. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front Immunol 3:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10(3):314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. 2001. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med 194(7):873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. 2009. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 180(7):640–648 [DOI] [PubMed] [Google Scholar]
- Meriggioli MN, Sheng JR, Li L, Prabhakar BS. 2008. Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis. Ann N Y Acad Sci 1132:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. 1980. Clonal analysis of proliferation and differentiation of paired daughter cells: action of granulocyte-macrophage colony-stimulating factor on granulocyte-macrophage precursors. Proc Natl Acad Sci U S A 77(9):5327–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. 1988. Colony stimulating factors and hemopoiesis. Ann Acad Med Singapore 17(2):166–170 [PubMed] [Google Scholar]
- Metcalf D. 2010. The colony-stimulating factors and cancer. Nat Rev Cancer 10(6):425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D, Merchav S. 1982. Effects of GM-CSF deprivation on precursors of granulocytes and macrophages. J Cell Physiol 112(3):411–418 [DOI] [PubMed] [Google Scholar]
- Metcalf D, Moore JG. 1988. Divergent disease patterns in granulocyte-macrophage colony-stimulating factor transgenic mice associated with different transgene insertion sites. Proc Natl Acad Sci U S A 85(20):7767–7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Pillarisetty VG, Shah AB, Lahrs S, Xing Z, DeMatteo RP. 2002. Endogenous granulocyte-macrophage colony-stimulating factor overexpression in vivo results in the long-term recruitment of a distinct dendritic cell population with enhanced immunostimulatory function. J Immunol 169(6):2875–2885 [DOI] [PubMed] [Google Scholar]
- Min L, Mohammad Isa SA, Shuai W, Piang CB, Nih FW, Kotaka M, Ruedl C. 2010. Cutting edge: granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J Immunol 184(9):4625–4629 [DOI] [PubMed] [Google Scholar]
- Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. 2009. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity 30(4):471–472; author reply 472–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. 1999. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol 155(5):1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muls N, Jnaoui K, Dang HA, Wauters A, Van Snick J, Sindic CJ, van Pesch V. 2012. Upregulation of IL-17, but not of IL-9, in circulating cells of CIS and relapsing MS patients. Impact of corticosteroid therapy on the cytokine network. J Neuroimmunol 243(1–2):73–80 [DOI] [PubMed] [Google Scholar]
- Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. 1996. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate 29(6):371–380 [DOI] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. 2006. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol 7(6):663–671 [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Sterman D, Jablons D, Smith JW, 2nd, Fox B, Maples P, Hamilton S, Borellini F, Lin A, Morali S, Hege K. 2004. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 96(4):326–331 [DOI] [PubMed] [Google Scholar]
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. 1998. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4(3):328–332 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Duru EA, Ameredes BT. 2008. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med 8(5):437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Sato A, Takada T, Arai T, Kasahara Y, Hojo M, Nei T, Nakayama H, Motoi N, Urano S, Eda R, Yokoba M, Tsuchihashi Y, Nasuhara Y, Ishii H, Ebina M, Yamaguchi E, Inoue Y, Nakata K, Tazawa R. 2012. Reduced GM-CSF autoantibody in improved lung of autoimmune pulmonary alveolar proteinosis. Eur Respir J 39(3):777–780 [DOI] [PubMed] [Google Scholar]
- Park LS, Martin U, Sorensen R, Luhr S, Morrissey PJ, Cosman D, Larsen A. 1992. Cloning of the low-affinity murine granulocyte-macrophage colony-stimulating factor receptor and reconstitution of a high-affinity receptor complex. Proc Natl Acad Sci U S A 89(10):4295–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. 2007. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 18(2):226–232 [DOI] [PubMed] [Google Scholar]
- Peng RQ, Ding Y, Zhang X, Liao Y, Zheng LM, Zhang XS. 2012. A pilot study of paclitaxel combined with gemcitabine followed by interleukin-2 and granulocyte macrophage colony-stimulating factor for patients with metastatic melanoma. Cancer Biol Ther 13(14):1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugini M, Brown AL, Salerno DG, Booker GW, Stojkoski C, Hercus TR, Lopez AF, Hibbs ML, Gonda TJ, D'Andrea RJ. 2010. Alternative modes of GM-CSF receptor activation revealed using activated mutants of the common beta-subunit. Blood 115(16):3346–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. 2007. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol 178(1):39–48 [DOI] [PubMed] [Google Scholar]
- Raich-Regue D, Naranjo-Gomez M, Grau-Lopez L, Ramo C, Pujol-Borrell R, Martinez-Caceres E, Borras FE. 2012. Differential effects of monophosphoryl lipid A and cytokine cocktail as maturation stimuli of immunogenic and tolerogenic dendritic cells for immunotherapy. Vaccine 30(2):378–387 [DOI] [PubMed] [Google Scholar]
- Redman BG, Chang AE, Whitfield J, Esper P, Jiang G, Braun T, Roessler B, Mule JJ. 2008. Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma. J Immunother 31(6):591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge CJ, McColl SR, Larochelle B, Gosselin J. 1998. Granulocyte-macrophage colony-stimulating factor enhances EBV-induced synthesis of chemotactic factors in human neutrophils. J Immunol 160(5):2442–2448 [PubMed] [Google Scholar]
- Rokhlin OW, Griebling TL, Karassina NV, Raines MA, Cohen MB. 1996. Human prostate carcinoma cell lines secrete GM-CSF and express GM-CSF-receptor on their cell surface. Anticancer Res 16(2):557–563 [PubMed] [Google Scholar]
- Rosas M, Gordon S, Taylor PR. 2007. Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. Eur J Immunol 37(9):2518–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT. 1986. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol 4:681–709 [DOI] [PubMed] [Google Scholar]
- Roth L, Macdonald JK, McDonald JW, Chande N. 2011. Sargramostim (GM-CSF) for induction of remission in Crohn's disease. Cochrane Database Syst Rev (11):CD008538. [DOI] [PubMed] [Google Scholar]
- Roth L, MacDonald JK, McDonald JW, Chande N. 2012. Sargramostim (GM-CSF) for induction of remission in Crohn's disease: a cochrane inflammatory bowel disease and functional bowel disorders systematic review of randomized trials. Inflamm Bowel Dis 18(7):1333–1339 [DOI] [PubMed] [Google Scholar]
- Rowin J, Thiruppathi M, Arhebamen E, Sheng J, Prabhakar BS, Meriggioli MN. 2012. Granulocyte macrophage colony-stimulating factor treatment of a patient in myasthenic crisis: effects on regulatory T cells. Muscle Nerve 46(3):449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rudensky AY. 2007. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol 7(6):443–453 [DOI] [PubMed] [Google Scholar]
- Rutella S, Bonanno G, Pierelli L, Mariotti A, Capoluongo E, Contemi AM, Ameglio F, Curti A, De Ritis DG, Voso MT, Perillo A, Mancuso S, Scambia G, Lemoli RM, Leone G. 2004. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-alpha. Eur J Immunol 34(5):1291–1302 [DOI] [PubMed] [Google Scholar]
- Rutella S, Danese S, Leone G. 2006. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 108(5):1435–1440 [DOI] [PubMed] [Google Scholar]
- Rutella S, Lemoli RM. 2004. Regulatory T cells and tolerogenic dendritic cells: from basic biology to clinical applications. Immunol Lett 94(1–2):11–26 [DOI] [PubMed] [Google Scholar]
- Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. 2011. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365(22):2067–2077 [DOI] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75(2):253–261 [DOI] [PubMed] [Google Scholar]
- Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, Keller G, Wood RE, Wert SE, Ikegami M, Whitsett JA, Luisetti M, Davies S, Krischer JP, Brody A, Ryckman F, Trapnell BC. 2010. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med 182(1):49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, Hodi FS, Jaklitsch M, Mentzer S, Swanson S, Lukanich J, Bueno R, Wain J, Mathisen D, Wright C, Fidias P, Donahue D, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Sugarbaker D, Mihm M, Dranoff G. 2003. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol 21(4):624–630 [DOI] [PubMed] [Google Scholar]
- Sebastian C, Serra M, Yeramian A, Serrat N, Lloberas J, Celada A. 2008. Deacetylase activity is required for STAT5-dependent GM-CSF functional activity in macrophages and differentiation to dendritic cells. J Immunol 180(9):5898–5906 [DOI] [PubMed] [Google Scholar]
- Seki N, Sudo Y, Yoshioka T, Sugihara S, Fujitsu T, Sakuma S, Ogawa T, Hamaoka T, Senoh H, Fujiwara H. 1988. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol 140(5):1477–1484 [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. 2006. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 16(1):53–65 [DOI] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. 2004. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 64(17):6337–6343 [DOI] [PubMed] [Google Scholar]
- Shachar I, Karin N. 2013. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leukoc Biol 93(1):51–61 [DOI] [PubMed] [Google Scholar]
- Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. 2006. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol 177(8):5296–5306 [DOI] [PubMed] [Google Scholar]
- Sheng JR, Li LC, Ganesh BB, Prabhakar BS, Meriggioli MN. 2008. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin Immunol 128(2):172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN. 2011. GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol 240–241:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, Hodi FS, Liebster L, Lam P, Mentzer S, Singer S, Tanabe KK, Cosimi AB, Duda R, Sober A, Bhan A, Daley J, Neuberg D, Parry G, Rokovich J, Richards L, Drayer J, Berns A, Clift S, Cohen LK, Mulligan RC, Dranoff G. 1998. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A 95(22):13141–13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. 2008. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J Exp Med 205(10):2281–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. 2003. Tolerogenic dendritic cells. Annu Rev Immunol 21:685–711 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science 268(5216):1472–1476 [DOI] [PubMed] [Google Scholar]
- Takaki S, Kanazawa H, Shiiba M, Takatsu K. 1994. A critical cytoplasmic domain of the interleukin-5 (IL-5) receptor alpha chain and its function in IL-5-mediated growth signal transduction. Mol Cell Biol 14(11):7404–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazoe M, Matsui T, Motoya S, Matsumoto T, Hibi T, Watanabe M. 2009. Sargramostim in patients with Crohn's disease: results of a phase 1–2 study. J Gastroenterol 44(6):535–543 [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28(5):687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa R, Inoue Y, Arai T, Takada T, Kasahara Y, Hojo M, Ohkouchi S, Tsuchihashi Y, Yokoba M, Eda R, Nakayama H, Ishii H, Nei T, Morimoto K, Nasuhara Y, Ebina M, Akira M, Ichiwata T, Tatsumi K, Yamaguchi E, Nakata K. 2014. Duration of benefit in patients with autoimmune pulmonary alveolar proteinosis after inhaled granulocyte-macrophage colony-stimulating factor therapy. Chest 145(4):729–737 [DOI] [PubMed] [Google Scholar]
- Thiruppathi M, Rowin J, Li Jiang Q, Sheng JR, Prabhakar BS, Meriggioli MN. 2012. Functional defect in regulatory T cells in myasthenia gravis. Ann N Y Acad Sci 1274:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. 1999. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 190(11):1669–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Igarashi M, Suzuki R, Kumagai K. 1988. Production of colony-stimulating factor by tumor cells and the factor-mediated induction of suppressor cells. J Immunol 141(2):699–708 [PubMed] [Google Scholar]
- van de Laar L, Coffer PJ, Woltman AM. 2012. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 119(15):3383–3393 [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuijze A, Koenders M, Roeleveld D, Sleeman MA, van den Berg W, Wicks IP. 2013. GM-CSF as a therapeutic target in inflammatory diseases. Mol Immunol 56(4):675–682 [DOI] [PubMed] [Google Scholar]
- Vasu C, Dogan RN, Holterman MJ, Prabhakar BS. 2003. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol 170(11):5511–5522 [DOI] [PubMed] [Google Scholar]
- Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, Kavuru MS. 2006. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest 130(1):227–237 [DOI] [PubMed] [Google Scholar]
- Verginis P, Li HS, Carayanniotis G. 2005. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol 174(11):7433–7439 [DOI] [PubMed] [Google Scholar]
- Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A 101(13):4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. 2008. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol 9(6):650–657 [DOI] [PubMed] [Google Scholar]
- Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. 2007. OX40 costimulation turns off Foxp3+ Tregs. Blood 110(7):2501–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F, Nicola NA, Metcalf D, Burgess AW. 1985. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell 43(1):269–276 [DOI] [PubMed] [Google Scholar]
- Walter MR, Cook WJ, Ealick SE, Nagabhushan TL, Trotta PP, Bugg CE. 1992. Three-dimensional structure of recombinant human granulocyte-macrophage colony-stimulating factor. J Mol Biol 224(4):1075–1085 [DOI] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454(7202):350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL. 2001. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61(3):842–847 [PubMed] [Google Scholar]
- Zhan Y, Lieschke GJ, Grail D, Dunn AR, Cheers C. 1998. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood 91(3):863–869 [PubMed] [Google Scholar]
- Zhan Y, Vega-Ramos J, Carrington EM, Villadangos JA, Lew AM, Xu Y. 2012. The inflammatory cytokine, GM-CSF, alters the developmental outcome of murine dendritic cells. Eur J Immunol 42(11):2889–2900 [DOI] [PubMed] [Google Scholar]