Introduction
The article by Peyser et al.1 published in this issue of Diabetes Technology & Therapeutics reports accuracy results in the hypoglycemic range for the Dexcom (San Diego, CA) G4 Platinum continuous glucose monitoring (CGM) system (software 505), which uses algorithmic signal processing to convert the raw electrochemical sensor data into calibrated blood glucose (BG) values. In this commentary, we match these results to previously published in silico experiments linking accuracy of CGM to frequency of hypoglycemia. The in silico experiments indicate that, compared with the original Dexcom G4 Platinum sensor, software 505 would result in (1) an approximately 25% reduction in biochemical severe hypoglycemia (defined as reference BG ≤39 mg/dL) during direct point-of-care use of CGM values for insulin dosing and (2) 45% reduction in biochemical severe hypoglycemia if all hypoglycemia alerts issued by the sensor are adequately attended to. In addition, the reported error levels of Dexcom G4 Platinum (software 505) are below the thresholds that would allow CGM use for insulin dosing decisions (i.e., below the thresholds for nonadjunct CGM use) determined by our in silico experiments.
The Optimization Problem of Diabetes
People with diabetes face a lifelong biobehavioral optimization problem: to maintain strict glycemic control without increasing their risk for hypoglycemia. Average glycemia (typically represented by hemoglobin A1c) and glucose variability are the measurable results from this optimization and the principal feedback to the patient for his or her maintenance of diabetes control. Many, now classic, studies have established links among intensive insulin therapy, hypoglycemia unawareness, and impaired counterregulation2–6 and concluded that recurrent hypoglycemia spirals into a “vicious cycle” implicated as the principal factor limiting intensive treatment of diabetes.7,8
Despite the progress of technology, hypoglycemia still remains a significant challenge to diabetes control.9 However, in a study of adolescents with hypoglycemia unawareness, epinephrine response was restored in most subjects with 4 weeks of CGM when the alarm threshold was set to 108 mg/dL.10 It was also shown that real-time CGM reduced significantly severe hypoglycemia while improving hemoglobin A1c in patients with established hypoglycemia unawareness.11 Moreover, virtually all contemporary studies of threshold12 or predictive13 low glucose suspend and closed-loop control (known as the artificial pancreas)14–18 report significant reduction of hypoglycemia achieved by real-time control algorithms using CGM data as their principal source of information. Thus, CGM data contain valuable information that, if sufficiently accurate and correctly interpreted by the patient or by automated algorithms, can help with optimization of diabetes control and with reducing the risk for hypoglycemia.
Hypoglycemia Reduction and the Frequency of BG Observations
Intuitively, the aggressiveness of glucose control in diabetes depends on the frequency of BG measurement. For example, if only the average glycemic state of a patient is available once every few months (as it would be with measurement of hemoglobin A1c alone), then control strategies could only target adjustment of long-term average glycemia, but would not be able to respond to daily or hourly variation in glucose level. Rapid BG changes would remain largely unnoticed, unless leading to acute complications such as severe hypoglycemia or diabetic ketoacidosis. Thus, the temporal resolution of glucose measurement to a large extent determines the aggressiveness of possible treatments. Episodic self-monitoring of BG (SMBG) typically includes several (e.g., two to five) BG readings per day; thus, the temporal resolution of SMBG allows for assessment of daily BG profiles, or weekly trends.
With the advent of CGM, it is now well accepted that BG fluctuations are a process in time characterized by the amplitude and the rate of BG changes. Contemporary CGM devices are capable of producing BG determinations every 5–10 min, which allows for detailed monitoring of glucose fluctuations on a temporal scale of minutes. Thus, the frequency of CGM readings is sufficient to enable real-time prediction (and possibly prevention) of rapidly evolving BG fluctuations such as hypoglycemic episodes. The one remaining question is to what extent the accuracy of CGM is sufficient to provide actionable information?
Algorithms and Accuracy of CGM
It is important to note that subcutaneous CGM devices measure glucose concentration in a different than blood compartment—the interstitium—and then deduce BG concentration from interstitial glucose (IG) readings. Presumably, IG fluctuations are related to BG via a diffusion process, which results in a well-defined codependence allowing BG changes to be deduced from IG dynamics.19–21 To account for the gradient between BG and IG, CGM glucose is calibrated using capillary glucose measurements to match BG levels. The rather complex conversion of IG concentration into BG measurement suggests that, in addition to sensor chemistry, several algorithms are involved in the signal processing. Eight years ago, we suggested algorithmic processing of CGM data that involved three key components: (1) denoising of the CGM signal; (2) “smart” calibration, and (3) short-term prediction to mitigate the inherent sensor time lag.22,23 More recently, these ideas were further refined, new versions of these methods have been formulated in the “Smart Sensor Concept,” and were applied to improve CGM accuracy in real time.24,25
The article by Peyser et al.1 published in this issue of Diabetes Technology & Therapeutics reports results from the implementation of the Smart Sensor Concept into the Dexcom G4 Platinum CGM system (software 505), which uses algorithmic signal processing to convert the raw electrochemical sensor data into calibrated BG values. The new data expand the previously reported pivotal trial results26 and demonstrate improved accuracy in the hypoglycemic range.
In the pivotal trial26 the reported overall (across the BG range of 40–400 mg/dL) mean absolute relative difference of this system was 9%, and the mean absolute difference when the reference YSI analyzer values were within hypoglycemic range (≤70 mg/dL) was 6.4 mg/dL; in the present study,1 mean absolute difference when the YSI value was below 70 mg/dL was 6 mg/dL. Compared with the original G4 Platinum sensor, the accuracy in the hypoglycemic range was improved significantly, which was particularly notable in the reduction of large sensor deviations (e.g., those farther than 20% or 20 mg/dL from the reference value): from 18% for the original G4 Platinum to 7% for the same sensor equipped with software 505 (Fig. 2 in Peyser et al.1). This improvement resulted in hypoglycemia (BG ≤70 mg/dL) detection of 95% within 10 min when the CGM alert threshold was set at 80 mg/dL.1 Intuitively, better detection should lead to reduction of hypoglycemia through better-informed self-treatment behaviors (barring “alarm fatigue”27) or through better-informed control algorithms in the case of closed-loop implementations.
Accuracy of CGM and Hypoglycemia Reduction
To address the relationship between CGM accuracy and the frequency of hypoglycemic episodes, we have conducted an in silico study combining real data with computer simulation experiments to assess the sensor accuracy needed for nonadjunct use of CGM. The results from this study are reported in a previous publication.28 As discussed in our article,28 a precise assessment of the relationship between CGM accuracy and hypoglycemia is not possible in a clinical trial because the exact same glycemic/treatment conditions cannot be reproduced in vivo at varying degrees of sensor error. Thus, the only option we had to gauge the degree of influence of sensor error on glycemic control was modeling and simulation. Our in silico investigation yielded several findings that are directly relevant to the results now presented by Peyser et al.1:
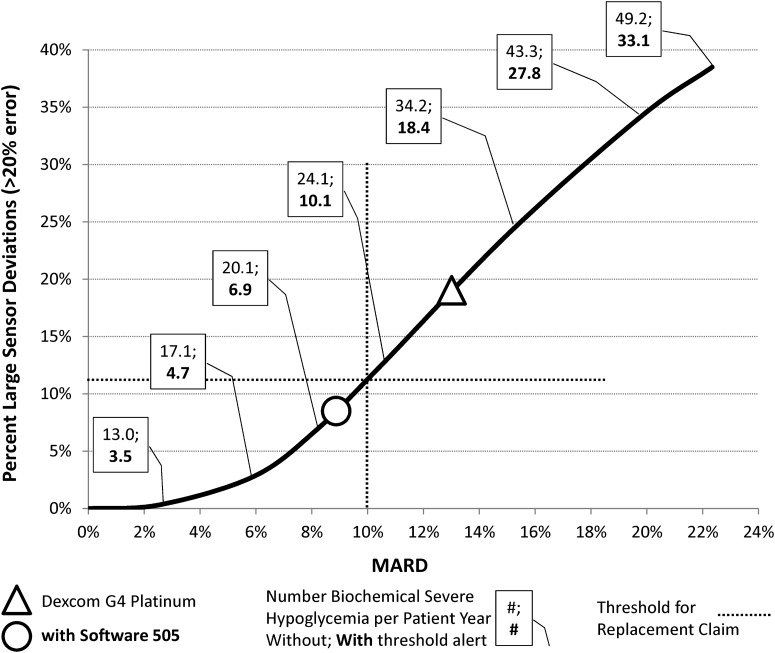
As shown in Figure 4A of our previous article28 and redrawn in Figure 1 here, there is an apparent curvilinear relationship between a sensor mean absolute relative difference and the percentage sensor deviations exceeding 20% from reference. This relationship depends on the distribution of sensor errors and appears to hold across various devices. In particular, the errors reported for both Dexcom G4 Platinum and Dexcom G4 Platinum (software 505) fall on this curve (see Fig. 1).
FIG. 1.
Curvilinear relationship between sensor mean absolute relative difference (MARD) and percentage sensor deviations exceeding 20% from reference blood glucose level as defined by in silico experiments based on real continuous glucose monitoring–use data. Superimposed are (1) the placement of Dexcom G4 Platinum (triangle) and the same sensor with software 505 (circle), (2) the frequency per patient-year of biochemical severe hypoglycemia (defined as reference blood glucose of ≤39 mg/dL) during point-of-care continuous glucose monitoring use as a direct replacement of self-monitoring of blood glucose for insulin dosing and with threshold alerts that are properly attended to 100% of the time, and (3) the thresholds for nonadjunct sensor use as determined in silico.
The number of biochemical severe hypoglycemia (defined as reference BG ≤39 mg/dL) per patient-year as determined by our in silico experiments increases along the curvilinear relationship: a fourfold increase (from 13 to 49.2 episodes) is observed in experiments with direct insulin dosing based on sensor values (point-of-care replacement use), and a 10-fold increase (from 3.5 to 33.1 episodes) is observed in experiments involving a threshold alert that is properly reacted to 100% of the time.28 Based on these estimated frequencies, we can expect that, compared with the original sensor, Dexcom G4 Platinum (software 505) would result in an approximately 25% reduction in biochemical severe hypoglycemia during point-of-care use and in a 45% reduction in biochemical severe hypoglycemia if all hypoglycemia alerts are adequately attended to.
Conclusions
There is a direct relationship between the accuracy of CGM and the reduction of hypoglycemia confirmed by several clinical trials and precisely quantified by our in silico experiments. In addition, as discussed by Polonsky and Hessler,29 “The available data suggest that greater satisfaction with accuracy is linked to better real-time CGM adherence, more confident and aggressive insulin adjustments, improvements in quality of life, reduced reliance on self-monitoring of blood glucose, and—potentially—less alarm fatigue.” Thus, the results presented by Peyser et al.1 mark a positive step for the continued adoption of CGM, particularly in intensive treatments increasing patients' risk for hypoglycemia. We can also conclude that, as determined by our in silico experiments,28 the error levels of Dexcom G4 Platinum with software 505 are below the thresholds that would allow CGM use for insulin dosing decisions (i.e., below the thresholds for “nonadjunct use”).
Acknowledgments
The writing of this commentary and the database used were supported by grant RO1 DK 085623 from the National Institute of Diabetes and Digestive Diseases and the Kidney, National Institutes of Health. The in silico trials referred to were supported by a research grant from Becton, Dickinson, and Company to the University of Virginia. The JDRF Artificial Pancreas Project at the University of Virginia supported the building of our computer simulation environment.
Author Disclosure Statement
B.P.K. served as an advisor to Becton, Dickinson, and Company and Sanofi-Aventis, has received research support from Animas Inc., BD, Dexcom, Insulet, Roche Diagnostics, Sanofi-Aventis, and Tandem Diabetes Care, and owns stock in TypeZero Technologies.
References
- 1.Peyser TA, Nakamura K, Price D, et al. : Hypoglycemic accuracy and improved low glucose alerts of the latest Dexcom G4 Platinum continuous glucose monitoring system. Diabetes Technol Ther 2015:17:548–554 [DOI] [PubMed] [Google Scholar]
- 2.Amiel SA, Sherwin RS, Simonson DC, et al. : Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 1988;37:901–907 [DOI] [PubMed] [Google Scholar]
- 3.Amiel SA, Tamborlane WV, Simonson DC, et al. : Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE, Gerich JE: Glucose counterregulation, hypoglycemia, and intensive therapy of diabetes mellitus. N Engl J Med 1985;313:232–241 [DOI] [PubMed] [Google Scholar]
- 5.White NH, Skor DA, Cryer PE, et al. : Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med 1983;308:485–491 [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group: Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 7.Cryer PE: Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM: a vicious cycle. Diabetes 1992;41:255–260 [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE: Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 9.Weinstock RS, Xing D, Maahs DM, et al. : Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 10.Ly TT, Hewitt J, Davey RJ, et al. : Improving epinephrine responses in hypoglycemia unawareness with real-time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care 2011;34:50–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhary P, Ramasamy S, Green L, et al. : Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care 2013;36:4160–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergenstal RM, Klonoff DC, Garg SK, et al. : Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 13.Buckingham BA, Cameron F, Calhoun P, et al. : Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther 2013;15:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillip M, Battelino T, Atlas E, et al. : Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 15.Hovorka R, Elleri D, Thabit H, et al. : Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2014;37:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovatchev BP, Renard E, Cobelli C, et al. : Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care 2014;37:1789–1796,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ly TT, Breton MD, Keith-Hynes P, et al. : Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care 2014;37:37:2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SA, Kovatchev BP, Breton MD, et al. : Multinight “bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther 2015;17:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebrin K, Steil GM, van Antwerp WP, et al. : Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol Endocrinol Metab 1999;277:E561–E571 [DOI] [PubMed] [Google Scholar]
- 20.Aussedat B, Dupire-Angel M, Gifford R, et al. : Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab 2000;278:E716–E728 [DOI] [PubMed] [Google Scholar]
- 21.Steil GM, Rebrin K, Hariri F, et al. : Interstitial fluid glucose dynamics during insulin-induced hypoglycaemia. Diabetologia 2005;48:1833–1840 [DOI] [PubMed] [Google Scholar]
- 22.King CR, Anderson SM, Breton MD, et al. : Modeling of calibration effectiveness and blood-to-interstitial glucose dynamics as potential confounders of the accuracy of continuous glucose sensors during hyperinsulinemic clamp. J Diabetes Sci Technol 2007;1:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Clarke WL: Peculiarities of the continuous glucose monitoring data stream and their impact on developing closed-loop control technology. J Diabetes Sci Technol 2008;2:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facchinetti A, Sparacino G, Guerra S, et al. : Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care 2013;36:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia A, Rack-Gomer AL, Bhavaraju NC, et al. : Dexcom G4AP: an advanced continuous glucose monitor for the artificial pancreas. J Diabetes Sci Technol 2013;7:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey TS, Chang A, Christiansen M: Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2014;9:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickup JC, Ford Holloway M, Samsi K: Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care 2015;38:544–550 [DOI] [PubMed] [Google Scholar]
- 28.Kovatchev BP, Patek SD, Ortiz EA, et al. : Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther 2015;17:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polonsky WH, Hessler D: Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol 2015;9:339–341 [DOI] [PMC free article] [PubMed] [Google Scholar]