Abstract
The larvae of the common green bottle fly Lucilia sericata (Diptera: Calliphoridae) have been used for centuries to promote wound healing, but the molecular basis of their antimicrobial, debridement and healing functions remains largely unknown. The analysis of differential gene expression in specific larval tissues before and after immune challenge could be used to identify key molecular factors, but the most sensitive and reproducible method qRT-PCR requires validated reference genes. We therefore selected 10 candidate reference genes encoding products from different functional classes (18S rRNA, 28S rRNA, actin, β-tubulin, RPS3, RPLP0, EF1α, PKA, GAPDH and GST1). Two widely applied algorithms (GeNorm and Normfinder) were used to analyze reference gene candidates in different larval tissues associated with secretion, digestion, and antimicrobial activity (midgut, hindgut, salivary glands, crop and fat body). The Gram-negative bacterium Pseudomonas aeruginosa was then used to boost the larval immune system and the stability of reference gene expression was tested in comparison to three immune genes (lucimycin, defensin-1 and attacin-2), which target different pathogen classes. We observed no differential expression of the antifungal peptide lucimycin, whereas the representative targeting Gram-positive bacteria (defensin-1) was upregulated in salivary glands, crop, nerve ganglion and reached its maximum in fat body (up to 300-fold). The strongest upregulation in all immune challenged tissues (over 50,000-fold induction in the fat body) was monitored for attacin-2, the representative targeting Gram-negative bacteria. Here we identified and validated a set of reference genes that allows the accurate normalization of gene expression in specific tissues of L. sericata after immune challenge.
Introduction
Maggot debridement therapy (MDT) was established at the beginning of the 20th century and became a popular treatment for chronic and recalcitrant wounds. The advent of antibiotics made MDT largely obsolete [1], but intractable wounds still present a challenge for modern medicine and MDT has re-emerged as an alternative therapeutic approach [2,3]. One hallmark of the renewed popularity of MDT is its approval as a medical device by the United States Food and Drug Administration in 2004 (FDA case number K033391), and sterile larvae of the common green bottle fly Lucilia sericata (Diptera: Calliphoridae) have therefore become the exclusive species used for MDT.
Sterile maggots are applied to a wound bed where they debride the necrotic tissue, disinfect the wound and promote wound healing [4,5]. Debridement is accomplished by the secretion of enzymes such as collagenases [6], proteases [7] and nucleases [8], which liquefy the tissue and allow the uptake of nutrients by the maggots. Some of these enzymes can also inhibit or degrade biofilms [8,9,10].
Disinfection is facilitated by the uptake and digestion of microorganisms [11], and by the secretion of antimicrobial metabolites [12,13,14] (e.g. Seraticin) and a variety of antimicrobial peptides (AMPs) [15,16,17,18]. These molecules have the potential to address the emergence and spread of antibiotic resistant pathogens [19]. Indeed, maggot secretions have shown promising activities against clinically relevant strains of drug-resistant pathogens and provide a source of lead structures for new antibiotics [13,14,17]. The antimicrobial activity of maggot secretions has a dose-dependent relationship according to the number and nature of bacteria the larvae encounter [20,21,22]. The secretions of sterile larvae do not possess antimicrobial activity whereas larvae confronted with Staphylococcus aureus or Pseudomonas aeruginosa (the most prominent Gram-positive and Gram-negative bacteria in chronic wounds, respectively [23,24,25,26,27,28]) produce distinct antimicrobial secretions that are not mutually antagonistic [20]. Both bacteria are well known to form biofilms, which protect them against external cues and considerably hamper the treatment [29,30,31].
MDT can also stimulate cell growth and wound healing but this is poorly understood. Maggot secretions can suppress pro-inflammatory responses [32,33], induce blood clotting [34], encourage fibroblasts to spread through the wound [35,36] and regulate the activation of human complement system [37]. Medical maggots also secrete allantoin and urea, which are thought to promote wound healing and are included in a number of medical products [38,39].
Although the molecular basis of MDT has been investigated, little is known about the key molecules responsible for these processes. Next-generation sequencing provides a rapid approach for the generation of genome and transcriptome datasets from non-model organisms such as L. sericata. These datasets allow the identification of medically and industrially relevant genes. To precisely characterize these genes based on their differing expression profiles in specific tissues or under specific conditions other approaches are necessary. The quantitative reverse transcription real-time polymerase chain reaction (qRT-PCR) is a powerful and sensitive method for the quantitation of gene expression [40,41] but is highly dependent on appropriate normalization to correct systemic variations [42,43,44]. The most reliable normalization is achieved by comparing gene expression profiles to stably-expressed reference genes [43,45,46]. A universal reference gene that remains stable under all conditions is unlikely to exist, so panels of candidate reference genes must be validated for certain experimental settings, such as stability during an immune challenge experiment. Here we investigated a panel of 10 candidate L. sericata reference genes in different tissues to allow the normalization of gene expression data in immune challenge experiments, to validate the identification of genes involved in maggot therapy.
Materials and Methods
Maggot rearing
L. sericata larvae were obtained from BioMonde GmbH (Barsbüttel, Germany). First instar larvae were reared on Columbia Agar with Sheep Blood PLUS (Thermo Scientific Oxoid) for 72 hours (h) at 28°C in the dark. The fed larvae were cleaned using sterile water before immune challenge or dissection.
Immune challenge and zone of inhibition assays
Larvae were placed in Petri dishes on ice to reduce motility and facilitate injection of larvae with Pseudomonas aeruginosa. The dorsal posterior was pricked with a sterile needle (wounded) or with a needle dipped in P. aeruginosa (DSM 50071) suspension (OD600 = 60) in phosphate buffered saline (immune-challenged). Treated larvae were supplied with fresh blood agar and incubated for additional 24 h at 28°C in the dark followed by the dissection of individual tissues. For the zone of inhibition assays, 7 ml of 1% LB agar per plate was cooled to 42°C, supplemented with 7 μl of fresh E. coli D31 [47] culture (OD600 = 0.5) and poured into a Petri dish, before 3-mm wells were stamped into the agar using a sterile hole puncher. After 24 h equal volumes (3 μl) of hemolymph from naïve, wounded and immune-challenged larvae were collected and immediately applied to the agar wells. The plates were incubated at 37°C for 24 h and 3 μl of 100 mg/ml ampicillin were used as a positive control.
Tissue dissection, RNA isolation and cDNA synthesis
Larvae were cooled on ice and dissected by ripping the dorso-anterior cuticle in sterile DEPC-treated PBS under a binocular microscope. The midgut, hindgut, salivary glands, crop, fat body and nerve ganglion were harvested following Freeman and Bracegirdle [48] and equivalent amounts of tissue-specific RNA were collected by preparing three pools of midgut (n = 5), hindgut (n = 10), salivary glands, crop, fat body and nerve ganglion (each n = 25) in RA1 buffer (Macherey-Nagel) before RNA isolation.
Total RNA from naïve and immune-challenged larvae (n = 5) subsequently pooled in equimolar RNA amounts, as well as the dissected larval tissues listed above, were extracted using the NucleoSpin RNA kit (Macherey-Nagel) including a 15-min DNase I on-column digest. The concentration, purity and quality of the RNA were determined by spectrophotometry (Take3, BioTek) and agarose gel electrophoresis (S1 Fig). Only samples with A260/A280 and A260/A230 > 1.8 and at least one sharp band representing 18S rRNA were used for cDNA synthesis. RNA samples that did not meet these criteria were cleaned and concentrated by sodium acetate precipitation [49].
Complementary DNA was synthesized using 1.5 μg of total RNA, oligo(dT)18 primers and the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to manufacturer’s recommendations. The resulting cDNA was diluted to a working concentration of 400 pg/μl, divided into aliquots and stored at –80°C.
Candidate gene sequence assembly
L. sericata reference gene candidates were selected based on known reference genes from the closely-related species Lucilia cuprina [50] and other arthropods [51,52]. The peptide sequences of each gene (or the nucleotide sequences of 18S and 28S rRNA) were queried against the L. sericata transcriptome database [15] using the BLAST algorithm. BLASTn was used to find all reads matching the search results and the pool of these reads was expanded to include further paired-end read partners. The final group of reads was assembled using Trinity [53] and this step was repeated until the complete coding sequences were acquired. Finally, Gap5 [54] was used to verify the finished sequences with mapped reads assembled by Bowtie [55].
Primer design and evaluation
Gene-specific primers were designed using Oligo Explorer v1.1.2 (http://www.softpedia.com/get/Science-CAD/Oligo-Explorer.shtml) to yield primers 19–23 nucleotides in length with amplification products of 50–210 bp and Tm values of ~60°C. All primers were subsequently tested in a standard curve assay including melt curve analysis using the StepOnePlus Real-Time PCR System (Applied Biosystems). The cDNA concentration ranging from 50 ng to 3.2 pg of total RNA was used in 5-fold dilutions with the cycling conditions recommended by the manufacturer. Briefly, hot-start PCR with denaturation at 95°C for 10 min was followed by 40 cycles of 95°C for 15 s and 60°C for 60 s, and finally the melt curve analysis with a temperature increase from 60°C to 95°C in 0.5°C steps. Reaction efficiency was calculated using StepOne Software v2.3 and only primers with an efficiency of 90–110%, R2 ≥ 0.99 and a single sharp melt curve peak were used for reference gene evaluation.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was carried out on a StepOnePlus system (Applied Biosystems) using optical 96-well plates (Applied Biosystems). The total reaction volume of 10 μl contained 5 μl Power SYBR Green PCR Master Mix (Applied Biosystems), 1 μl (400 pg) cDNA and 300 nM of each primer except for β-tubulin (150 nM), EF1α (150 nM) and actin (450 nM forward primer and 150 nM reverse primer). All reactions were carried out in three technical replicates under the reaction conditions stated above. Baseline correction was performed automatically by StepOne Software v2.3 and the quantification cycle (Cq) was always determined at an intensity of 0.15. All cDNA samples and corresponding RNA without reverse transcription (no-RT control) were tested with the 18S rRNA primers to estimate the remnants of genomic DNA [56] and only samples with a ΔCq ≥ 10 were accepted.
Normfinder and GeNorm analysis
The Normfinder and GeNorm algorithms were used for reference gene assessment. Normfinder [57] was used as an add-in for Microsoft Excel (http://moma.dk/normfinder-software), and GeNorm [46] was used as part of the R package NormqPCR with R version 3.1.1 [58]. In both cases, raw Cq values were used and log transfer was performed by the software.
Analysis of gene expression following immune challenge
Gene-specific primers were designed for the coding sequence of three L. sericata immune genes lucimycin, defensin-1 and attacin-2, which are differentially expressed when the larval immune system is challenged [15]. Genes were chosen based on their activity spectrum, with lucimycin targeting fungi [16], defensin-1 targeting Gram-positive [59] and attacin-2 targeting Gram-negative bacteria [59], respectively. Gene expression profiles upon immune challenge were determined by qRT-PCR using the ΔΔCq method [60] in Rest2009 (http://www.gene-quantification.de/rest-2009.html). The data were normalized using three different combinations of genes from the reference gene assessment: (a) the two best reference genes for every sample (GeNorm); (b) the three overall best reference genes (GeNorm and Normfinder); and (c) the six overall best reference genes (GeNorm). Efficiencies greater than 100% were set to 100% because Rest2009 does not allow higher values.
Results
Selection of candidate reference genes
We monitored the expression level of 10 L. sericata candidate reference genes before and after challenging the larvae with P. aeruginosa (Table 1). Our goal was to identify reference genes appropriate for different larval tissues, with the key criterion that gene expression is not affected by immune challenge. The tissues were selected based on their role in secretion and/or digestion (salivary glands, crop, midgut and hindgut) and supplemented by additional tissues (nerve ganglion and fat body). Candidates were chosen based on earlier studies in arthropods using orthologs of these genes [50,52]. To minimize the risk of co-regulation, we selected genes representing different functional classes of proteins. The L. sericata transcriptome database was explored to identify orthologs and the coding sequences of the 10 selected candidate reference genes were submitted to GenBank (Table 1).
Table 1. Genes and qRT-PCR primers evaluated in this study.
| Gene name | Abbreviation | Accession no. | Primer sequences (5'-3') | L (bp) a | E (%) b | R2 c |
|---|---|---|---|---|---|---|
| 18S ribosomal RNA | 18S rRNA | KR133393* | Fwd 5' AGCAGTTTGGGGGCATTAG 3' | 171 | 94 | 0.996 |
| Rev 5' GCTGGCATCGTTTATGGTTAG 3' | ||||||
| 28S ribosomal RNA | 28S rRNA | KR133394* | Fwd 5' CCAAAGAGTCGTGTTGCTTG 3' | 180 | 91 | 0.997 |
| Rev 5' ATTCAGGTTCATCGGGCTTA 3' | ||||||
| 40S ribosomal protein S3 | RPS3 | KR133395* | Fwd 5' TCAAGGTGTTTTGGGTATCAAGG 3' | 156 | 90 | 0.999 |
| Rev 5' GCGGGCATTTTGTATTCTGTTTC 3' | ||||||
| Elongation factor 1-alpha 1 | EF1α | KR133396* | Fwd 5' TGTCGGTGTCAACAAGATGG 3' | 137 | 93 | 0.998 |
| Rev 5' GAGATGGGAACGAAGGCAAC 3' | ||||||
| 60S acidic ribosomal protein P0 | RPLP0 | KR133397* | Fwd 5' GGTGCTGATAATGTTGGTTC 3' | 78 | 101 | 0.995 |
| Rev 5' ACCCATAAGGACGACACC 3' | ||||||
| actin | actin | KR133398* | Fwd 5' TGCCGATCGTATGCAAAA 3' | 90 | 100 | 0.998 |
| Rev 5' ACGGAGTATTTGCGTTCTGG 3' | ||||||
| Beta-1-tubulin | ß-tubulin | KR133399* | Fwd 5' AAACTAACCACACCCACATACGG 3' | 173 | 90 | 0.999 |
| Rev 5' AGAGGAGCAAAACCAGGCAT 3' | ||||||
| cAMP-dependent protein kinase | PKA | KR133400* | Fwd 5' CAACACAAGCCGACAAAAGAC 3' | 145 | 106 | 0.995 |
| Rev 5' GATAGCGTAGGGAAACCAAGAA 3' | ||||||
| Glyceraldehyde-3-phosphate dehydrogenase 1 | GAPDH | KR133401* | Fwd 5' GAACGGCAAACTCACTGGTATG 3' | 182 | 104 | 0.997 |
| Rev 5' CGGTGGAAACGACTTCTTCATC 3' | ||||||
| Glutathione S-transferases 1–1 | GST1 | KR133402* | Fwd 5' GCCAGTGTCAGCACCTTCG 3' | 120 | 92 | 0.999 |
| Rev 5' GCAACCTTCCCAGTTTTCATC 3' | ||||||
| attacin-2 | atta2 | KR920003* | Fwd 5' GCACCTTAGCCTACAATAACAATGG 3' | 92 | 103 | 0.999 |
| Rev 5' ACTGATGCTCTTGGTCAAAGTATCG 3' | ||||||
| defensin-1 | def1 | KT149727* | Fwd 5' CGGAGTTACATGGTCGTTACAAGAG 3' | 164 | 109 | 0.993 |
| Rev 5' CGGTGTCCAATCAACAAACAGTG 3' | ||||||
| lucimycin | afp | KJ413251 | Fwd 5' TCGCTTTAATCGCCGTTGTT 3' | 103 | 96 | 0.999 |
| Rev 5' ATGATGCCCAGCCTGTTGTTC 3' |
*Indicates GenBank submission of sequence obtained in the present study.
aLength of amplicon.
bQuantitative RT-PCR efficiency.
cCoefficient of determination.
Quantitative RT-PCR
The efficiencies of each qRT-PCR primer pair were generally high. Based on the standard curve slopes determined using StepOne software, the efficiencies ranged from 90% to 109% with R2 values > 0.99 for all pairs (Table 1). Linear behavior was observed over a concentration range spanning four orders of magnitude (50 ng to 3.2 pg of cDNA). The absence of primer dimers was confirmed by a melting curve analysis, which resulted in only one sharp peak for each amplicon under our experimental conditions (S2 Fig). All 10 reference genes were expressed in all naïve and immune-challenged samples. Median expression values ranged from 19 Cq (18S rRNA) to 26 Cq (PKA) and standard deviations ranged from 0.75 Cq (RPS3) to 2.1 Cq (actin) as shown in Fig 1.
Fig 1. Distribution of quantification cycle (Cq) values for all L. sericata candidate genes obtained by qRT-PCR.
Data for all naïve and immune-challenged samples were pooled (n = 42). Boxplots show first to third quartile of values in the box, the center line indicates the median, vertical dotted bars extend to the highest and lowest value.
Zone of inhibition assays and initial analysis of attacin-2 expression
To monitor the changes in larval reference gene expression triggered by interaction with bacteria, we chose the opportunistic pathogen P. aeruginosa and direct infection by pricking the L. sericata larvae with a bacteria-coated needle [61]. P. aeruginosa has previously been used to induce an immune response [15] and is also a common pathogen found in chronic wounds. Two independent tests were used to monitor the success of the immune challenge.
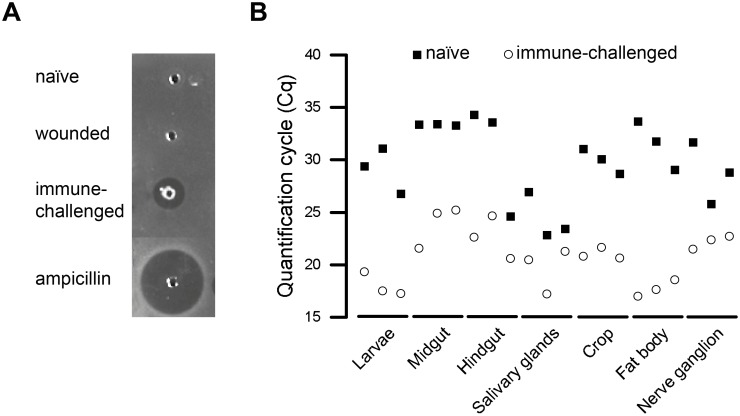
The first was a zone of inhibition assay with E. coli strain D31 [47]. Hemolymph from naïve, wounded and immune-challenged larvae were applied to the agar wells and the plates were incubated at 37°C for 24 h. Only the hemolymph from immune-challenged larvae produced distinct inhibition zones (Fig 2a).
Fig 2. Validation of Pseudomonas aeruginosa immune challenge.
A: Samples of hemolymph from naïve, wounded and immune-challenged larvae were tested in the E. coli zone of inhibition assay, with 100 mg/ml ampicillin as a positive control. Only immune-challenged larvae generated a zone of inhibition. B: Distribution of quantification cycle (Cq) values for immune gene attacin-2 in all naïve and immune-challenged samples. Raw values for all three biological replicates of the six tissue and the larvae samples are displayed separately.
As a second test, we carried out an initial analysis of gene expression profiles to monitor changes at the RNA level triggered by immune challenge, using attacin-2 mRNA as a marker because this AMP is known to be upregulated under similar conditions [15]. The raw Cq values for attacin-2 mRNA from two distinct populations, i.e. the naïve samples (Cq ~30) and the immune-challenged (Cq ~20), are shown in (Fig 2b). No naïve sample showed a lower value than the corresponding immune-challenged sample. These raw data supported our initial hypothesis that L. sericata attacin-2 mRNA is induced by immune challenge with a Gram-negative bacterium, but can only be validated by normalizing against the expression levels of reliable reference gene(s).
Validation of candidate reference genes using Normfinder
The model-based Normfinder approach provides a direct estimation of variance in so-called stability values, with lower numbers equating a more stable expression. The program does not sequentially eliminate unsuitable genes so the pre-exclusion of genes with large variances is necessary to achieve reliable results.
Normfinder analysis of the 10 candidate reference genes (Table 2) ranked RPS3, RPLP0 and EF1α as the three most stable genes considering immune challenge. The worst stability values (approximately 2-fold higher across all samples) were observed for 18S rRNA, actin and 28S rRNA. The overall ranking was not transferable to all specific tissues or to whole larvae because the stability of the candidates differed according to the sample type. Intragroup variation was generally low with only 28S rRNA showing variation (S1 Table). Intergroup variation was generally much higher, with the highest scores generated by 18S rRNA, 28S rRNA and actin (S2 Table).
Table 2. Normfinder ranking of the stability values of candidate reference genes.
| Rank | Larvae | Midgut | Hindgut | Salivary glands | Crop | Fat body | Nerve ganglion | Overall |
|---|---|---|---|---|---|---|---|---|
| 1 | RPLP0 | EF1α | RPLP0 | PKA | β-tubulin | RPLP0 | PKA | RPLP0 |
| (0.010) | (0.010) | (0.006) | (0.005) | (0.007) | (0.006) | (0.010) | (0.027) | |
| 2 | actin | actin | EF1α | RPS3 | actin | 18S rRNA | GST1 | RPS3 |
| (0.017) | (0.010) | (0.012) | (0.005) | (0.011) | (0.007) | (0.011) | (0.029) | |
| 3 | GAPDH | GST1 | RPS3 | GAPDH | PKA | β-tubulin | actin | EF1α |
| (0.020) | (0.012) | (0.015) | (0.014) | (0.016) | (0.009) | (0.015) | (0.029) | |
| 4 | RPS3 | RPLP0 | GAPDH | GST1 | RPS3 | RPS3 | 18S rRNA | PKA |
| (0.021) | (0.014) | (0.018) | (0.016) | (0.019) | (0.013) | (0.017) | (0.037) | |
| 5 | GST1 | PKA | actin | actin | GAPDH | PKA | RPLP0 | GST1 |
| (0.022) | (0.019) | (0.020) | (0.024) | (0.026) | (0.019) | (0.022) | (0.039) | |
| 6 | EF1α | 18S rRNA | PKA | β-tubulin | GST1 | EF1α | GAPDH | GAPDH |
| (0.025) | (0.020) | (0.022) | (0.027) | (0.027) | (0.036) | (0.029) | (0.041) | |
| 7 | PKA | β-tubulin | β-tubulin | EF1α | 18S rRNA | GST1 | β-tubulin | β-tubulin |
| (0.026) | (0.020) | (0.028) | (0.039) | (0.030) | (0.042) | (0.033) | (0.052) | |
| 8 | 18S rRNA | GAPDH | 18S rRNA | RPLP0 | EF1α | GAPDH | RPS3 | 18S rRNA |
| (0.036) | (0.021) | (0.033) | (0.041) | (0.030) | (0.044) | (0.034) | (0.061) | |
| 9 | β-tubulin | RPS3 | GST1 | 18S rRNA | RPLP0 | actin | EF1α | actin |
| (0.037) | (0.031) | (0.034) | (0.057) | (0.030) | (0.056) | (0.037) | (0.068) | |
| 10 | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA |
| (0.051) | (0.074) | (0.056) | (0.073) | (0.036) | (0.131) | (0.078) | (0.072) |
Validation of candidate reference genes using GeNorm
The GeNorm algorithm calculates the pairwise variation among all tested genes and assigns stability measures (M). In every cycle, the gene with the highest stability measure (i.e. the least stable) is excluded until only the two best genes remain. GeNorm analysis of our 10 candidate reference genes (Table 3) yielded similar results to Normfinder. GeNorm ranked RPS3, RPLP0 and EF1α as the three best genes and 18S rRNA, actin and 28S rRNA as the three worst, with M values of 2 to 4-fold higher than the best candidates across all samples. As for the Normfinder data, the overall GeNorm ranking list was not transferable to all individual tissues or whole larvae.
Table 3. GeNorm ranking of stability measures for candidate reference genes and attacin-2.
| Rank | Larvae | Midgut | Hindgut | Salivary glands | Crop | Fat body | Nerve ganglion | Overall |
|---|---|---|---|---|---|---|---|---|
| 1 | RPLP0 | β-tubulin | actin | PKA | β-tubulin | RPLP0 | EF1α | EF1α |
| (0.341) | (0.270) | (0.128) | (0.209) | (0.252) | (0.357) | (0.241) | (0.557) | |
| 1 | actin | EF1α | RPS3 | RPS3 | RPS3 | RPS3 | RPLP0 | RPLP0 |
| (0.341) | (0.270) | (0.128) | (0.209) | (0.252) | (0.357) | (0.241) | (0.557) | |
| 3 | EF1α | PKA | PKA | GAPDH | actin | β-tubulin | GAPDH | RPS3 |
| (0.403) | (0.313) | (0.366) | (0.514) | (0.308) | (0.489) | (0.418) | (0.841) | |
| 4 | GAPDH | RPS3 | RPLP0 | β-tubulin | GST1 | 18S rRNA | actin | GST1 |
| (0.512) | (0.356) | (0.424) | (0.522) | (0.384) | (0.636) | (0.452) | (1.164) | |
| 5 | GST1 | GAPDH | EF1α | actin | GAPDH | PKA | PKA | GAPDH |
| (0.586) | (0.427) | (0.408) | (0.536) | (0.410) | (0.778) | (0.672) | (1.154) | |
| 6 | 18S rRNA | actin | GST1 | GST1 | PKA | GAPDH | GST1 | PKA |
| (0.588) | (0.557) | (0.522) | (0.620) | (0.540) | (0.947) | (0.646) | (1.219) | |
| 7 | RPS3 | RPLP0 | β-tubulin | EF1α | 18S rRNA | EF1α | 18S rRNA | β-tubulin |
| (0.712) | (0.550) | (0.608) | (0.685) | (0.729) | (0.983) | (0.656) | (1.266) | |
| 8 | PKA | GST1 | GAPDH | RPLP0 | EF1α | GST1 | β-tubulin | 18S rRNA |
| (0.694) | (0.547) | (0.691) | (0.730) | (0.825) | (1.015) | (0.698) | (1.637) | |
| 9 | β-tubulin | 18S rRNA | 18S rRNA | 18S rRNA | RPLP0 | actin | RPS3 | actin |
| (0.817) | (0.660) | (0.838) | (1.339) | (0.859) | (1.207) | (0.820) | (1.836) | |
| 10 | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA | 28S rRNA |
| (1.092) | (1.490) | (1.229) | (1.528) | (0.888) | (2.645) | (1.658) | (2.095) | |
| atta2 | atta2 | atta2 | atta2 | atta2 | atta2 | atta2 | atta2 | atta2 |
| (6.201) | (5.662) | (5.874) | (3.501) | (5.550) | (8.538) | (4.569) | (5.888) |
To illustrate the differences in stability measures we also included attacin-2, which is strongly induced by immune-challenge and therefore expected to be unstable. As anticipated, the M value for attacin-2 was ~20-fold higher than the most stable candidate genes in each sample (Table 3).
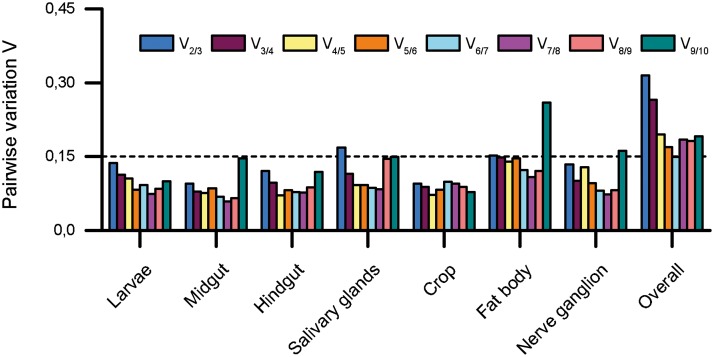
Because neither algorithm selected a single reference gene suitable for all samples, we used GeNorm pairwise variation comparison and select the most appropriate number of reference genes for normalization, which is an appropriate strategy when single reference genes are unsuitable [46]. This comparison (Vn/n+1) examines the normalization factors NFn and NFn+1 in each analysis cycle. If the calculated value falls below a set threshold of 0.15 [46], the addition of further reference genes does not improve the quality of normalization. As shown in Fig 3, we found that most tissues reached the recommended threshold of 0.15 with just two reference genes, whereas three reference genes were required for the normalization of expression levels in the salivary glands (V2/3 = 0.17). When comparing overall variation, the 0.15 threshold is reached at V6/7 which means that six reference genes would be necessary for appropriate simultaneous normalization within all tested samples.
Fig 3. Determination of the optimal number of control genes for normalization.
Pairwise variation (Vn/n+1) analysis between the normalization factors NFn and NFn+1 to determine the number of reference genes required for accurate normalization in every individual sample group. The dashed line at 0.15 represents the set threshold below which the number of reference genes is optimal.
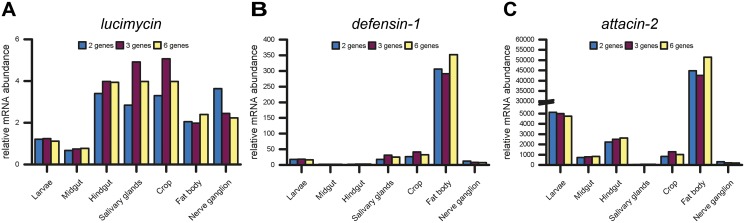
The Cq values for lucimycin, defensin-1 and attacin-2 mRNA were therefore normalized using three combinations of candidate reference genes: (a) the two best candidate reference genes for every sample defined by GeNorm analysis; (b) the overall best three reference genes defined by both algorithms (RPS3, RPLP0 and EF1α); and (c) the overall best six reference genes defined by GeNorm pairwise variation comparison. Our results show that the antifungal peptide lucimycin is not differentially expressed (Fig 4a), whereas defensin-1, which is specifically targeting Gram-positive bacteria, shows different upregulation in salivary glands, crop, nerve ganglion and fat body, ranging from a 8-fold induction in the nerve ganglion to a 290-fold induction in the fat body (Fig 4b). The strongest upregulation was monitored for attacin-2 that targets primarily Gram-negative bacteria. Attacin-2 was strongly induced by immune challenge in all tested tissues, ranging from a 37-fold induction in the salivary glands to a 51,463-fold induction in the fat body (Fig 4c). As shown in Fig 4, all three reference gene combinations lead to similar expression results with slight variations in relative values.
Fig 4. Quantitative RT-PCR analysis of lucimycin, defensin-1 and attacin-2 upon immune challenge.
The mRNA expression of lucimycin (A), defensin-1 (B) and attacin-2 (C) was determined in different L. sericata tissues. Relative mRNA expression levels of individual immune genes were compared in between immune-challenged and naïve samples and normalized with two, three or six reference genes.
Discussion
MDT has undergone a renaissance since the end of the 20th century, but although recent studies have addressed the underlying mechanisms many of the key proteins responsible for the beneficial effects of medical maggots remain unknown [4,5]. The falling cost of next generation sequencing means that the sequencing of genome and transcriptome datasets from non-model organisms can now be used as a first step to screen for medically and industrially relevant sequences. In a second step qRT-PCR can validate these candidates, but only if appropriate reference genes are available for the normalization of expression data [43,45,46]. This approach is ideal for the identification of L. sericata genes with important roles in wound healing. Thus such genes are likely to show different expression profiles in specific tissues or are modulated in the presence of wound pathogens.
We investigated the tissue specific expression stability of 10 candidate reference genes and 3 immune genes (lucimycin, defensin-1 and attacin-2), which target different pathogen classes. We monitored the expression of these immune genes in different L. sericata tissues before and after the immune challenge with the Gram-negative bacterium P. aeruginosa, which was introduced into third-instar L. sericata larvae by pricking with a contaminated needle. We chose this invasive strategy to ensure that the comparator underwent a strong change in expression allowing the stability of the candidate reference genes to be tested robustly, even though this far exceeds the intensity of the bacterial challenge that maggots would encounter in human wounds. As expected, we monitor the strong expression of attacin-2 (targeting Gram-negative bacteria [59]) in all larval tissues reaching its maximum in fat body (over 50,000-fold). We also did not observe any differential expression of the antifungal peptide lucimycin, which is known to have no effect against Gram-negative bacteria [16]. Interestingly, defensin-1 as a representative targeting Gram-positive bacteria [59], was differentially upregulated in salivary glands, crop, nerve ganglion and fat body. This unexpected result can be easily explained by the wounding procedure itself, which has been shown to induce insect immune response previously [62]. Our results demonstrate that even under such harsh conditions, which heavily perturbed the system and led to the strong induction of attacin-2, the expression of most of the candidate reference genes remained stable. This success rate reflects our choice of genes that have been used as references in other insects, allowing the knowledge-based pre-selection of candidates that are likely to be suitable as reference genes in L. sericata [50,63,64,65,66,67].
The Normfinder and GeNorm algorithms were unable to identify a single optimal reference gene or an ideal combination of two to three reference genes that worked consistently across tested samples. RPLP0, EF1α and RPS3 were identified as the most stable reference genes across all samples even though each of them ranked low for stability in at least one sample. They are all involved in protein expression [68,69,70] and co-regulation cannot be ruled out as explanation for their high ranking. However, comparison with the other candidate genes does not indicate generally co-regulated expression. Although it may be necessary to use specific candidates for particular tissues to achieve the most accurate data normalization, the use of these overall three best reference genes should be more than adequate for most experimental scenarios, as illustrated by our expression analysis of three immune genes (Fig 4).
Among the remaining candidates, some (e.g. GST1 and GAPDH) occupy mid-ranking positions in many tissues and have poor scores in others but never rank as the most suitable reference gene, whereas others (e.g. PKA and β-tubulin) also occupy mid-raking position in many tissues but are the most stable reference genes in others, i.e. the salivary glands and the nerve ganglion (PKA) or the crop (β-tubulin). Their overall mid-to-low ranking reflects their tendency to show good scores in a few tissues but poor scores in most others.
Remarkably, actin has a low overall score but good scores in several tissues (second most stable reference in three tissues and third in another). However, the standard deviation of 2.1 Cq is the highest among all the tested candidate genes, reflecting high intergroup variation (S1 Table) rather than changes in expression induced by the immune challenge, which would lead to higher intragroup variations. It is not surprising that a fundamental structural protein like actin would be expressed at different levels in diverse tissues such as the gut and the fat body. Therefore, despite its low overall ranking, actin would be useful as a reference gene in most individual tissues but not in more diverse collections of samples.
Compared to the other candidates, the genes for 18S and 28S rRNA were the least stable and therefore the least suitable for normalization, which contrasts with the findings in other arthropods [50,71]. This may be a species-dependent phenomenon, but may also reflect the technical approach, e.g. the method used for RNA isolation and cDNA synthesis. For example, we used oligo(dT)18 primers which only partly reverse transcribe the rRNA genes due to the lack of a canonical polyadenylate tail.
While dissecting the fat body from immune-challenged larvae we observed the remodeling and/or degradation of this tissue. The physical appearance of the fat body changed from clusters of round cells to loosely-associated amorphous cells which yielded lower amounts of total RNA with poorer quality compared to the other tissues. After immune challenge, fat body total RNA from all biological replicates had to be precipitated and concentrated to meet the required criteria for cDNA synthesis. The insect fat body is responsible for AMP production [72] and two defense pathways have been described in Drosophila melanogaster, i.e. the Toll pathway against Gram-positive species and the immune deficiency (IMD) pathway against Gram-negative species [73]. Constitutive triggering of the IMD pathway has been shown to induce apoptosis [74]. Our results show that although attacin-2 is expressed ubiquitously and globally upregulated by immune challenge, the strongest induction (over 50,000-fold) occurs in the fat body. We used P. aeruginosa, which as a Gram-negative species is targeted by attacins [59] and we hypothesize that the IMD pathway plays a major role in this immune response. The induction of attacin-2 may therefore be part of this IMD pathway response, ultimately resulting in apoptosis which may explain the physical changes in the fat body we observed.
There is probably no universally ideal reference gene in any experimental system so it is important to distinguish between the best gene and combination of genes suitable for different experimental settings. Despite the diverse expression profiles of the 10 candidate reference genes in our dissected tissue samples, several combinations of these genes gave identical results when used for the normalization of immune genes expression during an immune challenge, suggesting that the analysis of six reference genes per sample or different reference genes in each tissue is unnecessary. From our own experience, the reliable normalization of L. sericata expression data among all dissected tissues (Fig 4) is also achieved using the three best reference genes based on our overall rankings (RPLP0, EF1α and RPS3), which balances accuracy with convenience and the cost of additional experiments. The primer pairs and reference gene candidates evaluated in this study provided a valuable tool for the normalization of gene expression data in medical maggots, which will facilitate the identification and functional analysis of genes which are responsible for the beneficial effects of maggot therapy.
Supporting Information
RNA from all L. sericata tissues from naïve (top) and immune-challenged (bottom) larvae was analyzed using agarose gel with exception of one “crop” sample and three “fat body” samples in immune-challenged larvae. These samples were sodium acetate precipitated after the RNA isolation and limited in sample amount.
(TIF)
(PDF)
(DOCX)
(DOCX)
Acknowledgments
We thank Dr Richard M Twyman for editing the manuscript.
Data Availability
Sequences of all reference genes (KR133393 - KR133402) and lucimycin (KJ413251) are available from the NCBI database. The sequences of attacin-2 (KR920003) and defensin-1 (KT149727) have been submitted to the NCBI database.
Funding Statement
This study was funded by the Hessen State Ministry of Higher Education, Research and the Arts (HMWK) via a generous grant for the LOEWE Center for “Insect Biotechnology and Bioresources”. This work was also supported by the Alexander von Humboldt foundation.
References
- 1. Sherman RA, Hall MJ, Thomas S (2000) Medicinal maggots: an ancient remedy for some contemporary afflictions. Annual review of entomology 45: 55–81. [DOI] [PubMed] [Google Scholar]
- 2. Cazander G, Pritchard DI, Nigam Y, Jung W, Nibbering PH (2013) Multiple actions of Lucilia sericata larvae in hard-to-heal wounds: larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection. BioEssays: news and reviews in molecular, cellular and developmental biology 35: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 3. Vilcinskas A (2011) From Traditional Maggot Therapy to Modern Biosurgery In: Vilcinskas A, editor. Insect Biotechnology: Springer; Netherlands: pp. 67–75. [Google Scholar]
- 4. Sherman RA (2014) Mechanisms of maggot-induced wound healing: what do we know, and where do we go from here? Evidence-based complementary and alternative medicine: eCAM 2014: 592419 10.1155/2014/592419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davydov L (2011) Maggot therapy in wound management in modern era and a review of published literature. Journal of pharmacy practice 24: 89–93. [DOI] [PubMed] [Google Scholar]
- 6. Hobson RP (1931) On an enzyme from blow-fly larvae [Lucilia sericata] which digests collagen in alkaline solution. The Biochemical journal 25: 1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidtchen A, Wolff H, Rydengard V, Hansson C (2003) Detection of serine proteases secreted by Lucilia sericata in vitro and during treatment of a chronic leg ulcer. Acta dermato-venereologica 83: 310–311. [DOI] [PubMed] [Google Scholar]
- 8. Brown A, Horobin A, Blount DG, Hill PJ, English J, Rich A, et al. (2012) Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Medical and veterinary entomology 26: 432–439. 10.1111/j.1365-2915.2012.01029.x [DOI] [PubMed] [Google Scholar]
- 9. van der Plas MJ, Jukema GN, Wai SW, Dogterom-Ballering HC, Lagendijk EL, van Gulpen C, et al. (2008) Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. The Journal of antimicrobial chemotherapy 61: 117–122. [DOI] [PubMed] [Google Scholar]
- 10. Harris LG, Nigam Y, Sawyer J, Mack D, Pritchard DI (2013) Lucilia sericata chymotrypsin disrupts protein adhesin-mediated staphylococcal biofilm formation. Applied and environmental microbiology 79: 1393–1395. 10.1128/AEM.03689-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mumcuoglu KY, Miller J, Mumcuoglu M, Friger M, Tarshis M (2001) Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae). Journal of medical entomology 38: 161–166. [DOI] [PubMed] [Google Scholar]
- 12. Huberman L, Gollop N, Mumcuoglu KY, Breuer E, Bhusare SR, Shai Y, et al. (2007) Antibacterial substances of low molecular weight isolated from the blowfly, Lucilia sericata. Medical and veterinary entomology 21: 127–131. [DOI] [PubMed] [Google Scholar]
- 13. Huberman L, Gollop N, Mumcuoglu KY, Block C, Galun R (2007) Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. Journal of wound care 16: 123–127. [DOI] [PubMed] [Google Scholar]
- 14. Bexfield A, Bond AE, Roberts EC, Dudley E, Nigam Y, Thomas S, et al. (2008) The antibacterial activity against MRSA strains and other bacteria of a <500Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae). Microbes and infection / Institut Pasteur 10: 325–333. 10.1016/j.micinf.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 15. Poppel AK, Vogel H, Wiesner J, Vilcinskas A (2015) Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrobial agents and chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poppel AK, Koch A, Kogel KH, Vogel H, Kollewe C, Wiesner J, et al. (2014) Lucimycin, an antifungal peptide from the therapeutic maggot of the common green bottle fly Lucilia sericata. Biological chemistry 395: 649–656. 10.1515/hsz-2013-0263 [DOI] [PubMed] [Google Scholar]
- 17. Cerovsky V, Bem R (2014) Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals 7: 251–264. 10.3390/ph7030251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Altincicek B, Vilcinskas A (2009) Septic injury-inducible genes in medicinal maggots of the green blow fly Lucilia sericata. Insect molecular biology 18: 119–125. 10.1111/j.1365-2583.2008.00856.x [DOI] [PubMed] [Google Scholar]
- 19. Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiology and molecular biology reviews: MMBR 74: 417–433. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawabata T, Mitsui H, Yokota K, Ishino K, Oguma K, Sano S (2010) Induction of antibacterial activity in larvae of the blowfly Lucilia sericata by an infected environment. Medical and veterinary entomology 24: 375–381. 10.1111/j.1365-2915.2010.00902.x [DOI] [PubMed] [Google Scholar]
- 21. Barnes KM, Dixon RA, Gennard DE (2010) The antibacterial potency of the medicinal maggot, Lucilia sericata (Meigen): variation in laboratory evaluation. Journal of microbiological methods 82: 234–237. 10.1016/j.mimet.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 22. Cazander G, van Veen KE, Bernards AT, Jukema GN (2009) Do maggots have an influence on bacterial growth? A study on the susceptibility of strains of six different bacterial species to maggots of Lucilia sericata and their excretions/secretions. Journal of tissue viability 18: 80–87. 10.1016/j.jtv.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 23. Jaklic D, Lapanje A, Zupancic K, Smrke D, Gunde-Cimerman N (2008) Selective antimicrobial activity of maggots against pathogenic bacteria. Journal of medical microbiology 57: 617–625. 10.1099/jmm.0.47515-0 [DOI] [PubMed] [Google Scholar]
- 24. Mihai MM, Holban AM, Giurcaneanu C, Popa LG, Buzea M, Filipov M, et al. (2014) Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie 55: 1401–1408. [PubMed] [Google Scholar]
- 25. Tantawi TI, Gohar YM, Kotb MM, Beshara FM, El-Naggar MM (2007) Clinical and microbiological efficacy of MDT in the treatment of diabetic foot ulcers. Journal of wound care 16: 379–383. [DOI] [PubMed] [Google Scholar]
- 26. Wu M, Ruan H, Huang Y, Liu C, Ni P, Ye J, et al. (2015) Bacteriological Investigation of Chronic Wounds in a Specialized Wound Healing Department: A Retrospective Analysis of 107 Cases. The international journal of lower extremity wounds. [DOI] [PubMed] [Google Scholar]
- 27. Fazli M, Bjarnsholt T, Kirketerp-Moller K, Jorgensen B, Andersen AS, Krogfelt KA, et al. (2009) Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. Journal of clinical microbiology 47: 4084–4089. 10.1128/JCM.01395-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies CE, Hill KE, Wilson MJ, Stephens P, Hill CM, Harding KG, et al. (2004) Use of 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis for analysis of the microfloras of healing and nonhealing chronic venous leg ulcers. Journal of clinical microbiology 42: 3549–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCarthy H, Rudkin JK, Black NS, Gallagher L, O'Neill E, O'Gara JP (2015) Methicillin resistance and the biofilm phenotype in. Frontiers in cellular and infection microbiology 5: 1 10.3389/fcimb.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tolker-Nielsen T (2014) Pseudomonas aeruginosa biofilm infections: from molecular biofilm biology to new treatment possibilities. APMIS Supplementum: 1–51. [DOI] [PubMed] [Google Scholar]
- 31. Gokcen A, Vilcinskas A, Wiesner J (2013) Methods to identify enzymes that degrade the main extracellular polysaccharide component of Staphylococcus epidermidis biofilms. Virulence 4: 260–270. 10.4161/viru.23560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Plas MJ, van der Does AM, Baldry M, Dogterom-Ballering HC, van Gulpen C, van Dissel JT, et al. (2007) Maggot excretions/secretions inhibit multiple neutrophil pro-inflammatory responses. Microbes and infection / Institut Pasteur 9: 507–514. [DOI] [PubMed] [Google Scholar]
- 33. van der Plas MJ, van Dissel JT, Nibbering PH (2009) Maggot secretions skew monocyte-macrophage differentiation away from a pro-inflammatory to a pro-angiogenic type. PloS one 4: e8071 10.1371/journal.pone.0008071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kahl M, Gokcen A, Fischer S, Baumer M, Wiesner J, Lochnit G, et al. (2015) Maggot excretion products from the blowfly Lucilia sericata contain contact phase/intrinsic pathway-like proteases with procoagulant functions. Thrombosis and haemostasis 114. [DOI] [PubMed] [Google Scholar]
- 35. Prete PE (1997) Growth effects of Phaenicia sericata larval extracts on fibroblasts: mechanism for wound healing by maggot therapy. Life sciences 60: 505–510. [DOI] [PubMed] [Google Scholar]
- 36. Horobin AJ, Shakesheff KM, Pritchard DI (2005) Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon the migration of human dermal fibroblasts over a fibronectin-coated surface. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society 13: 422–433. [DOI] [PubMed] [Google Scholar]
- 37. Cazander G, Schreurs MW, Renwarin L, Dorresteijn C, Hamann D, Jukema GN (2012) Maggot excretions affect the human complement system. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society 20: 879–886. [DOI] [PubMed] [Google Scholar]
- 38. Robinson W (1935) Allantoin, a Constituent of Maggot Excretions, Stimulates Healing of Chronic Discharging Wounds. The Journal of parasitology 21: 354–358. [Google Scholar]
- 39. Araujo LU, Grabe-Guimaraes A, Mosqueira VC, Carneiro CM, Silva-Barcellos NM (2010) Profile of wound healing process induced by allantoin. Acta cirurgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia 25: 460–466. [DOI] [PubMed] [Google Scholar]
- 40. Nolan T, Hands RE, Bustin SA (2006) Quantification of mRNA using real-time RT-PCR. Nature protocols 1: 1559–1582. [DOI] [PubMed] [Google Scholar]
- 41. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical chemistry 55: 611–622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 42. Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. Journal of molecular endocrinology 25: 169–193. [DOI] [PubMed] [Google Scholar]
- 43. Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic acids research 30: e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Willems E, Leyns L, Vandesompele J (2008) Standardization of real-time PCR gene expression data from independent biological replicates. Analytical biochemistry 379: 127–129. 10.1016/j.ab.2008.04.036 [DOI] [PubMed] [Google Scholar]
- 45. Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre JF, Louvet R, et al. (2008) The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant biotechnology journal 6: 609–618. 10.1111/j.1467-7652.2008.00346.x [DOI] [PubMed] [Google Scholar]
- 46. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology 3: RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monner DA, Jonsson S, Boman HG (1971) Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. Journal of bacteriology 107: 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freeman WH, Bracegirdle B (1971) An atlas of invertebrate structure. ISBN 0-435-60315-9: Heinemann Educational Books; London. [Google Scholar]
- 49. Walker SE, Lorsch J (2013) RNA purification—precipitation methods. Methods in enzymology 530: 337–343. 10.1016/B978-0-12-420037-1.00019-1 [DOI] [PubMed] [Google Scholar]
- 50. Bagnall NH, Kotze AC (2010) Evaluation of reference genes for real-time PCR quantification of gene expression in the Australian sheep blowfly, Lucilia cuprina. Medical and veterinary entomology 24: 176–181. 10.1111/j.1365-2915.2010.00866.x [DOI] [PubMed] [Google Scholar]
- 51. Beckert A, Wiesner J, Baumann A, Poppel AK, Vogel H, Vilcinskas A (2015) Two c-type lysozymes boost the innate immune system of the invasive ladybird Harmonia axyridis. Developmental and comparative immunology 49: 303–312. 10.1016/j.dci.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 52. Nijhof AM, Balk JA, Postigo M, Jongejan F (2009) Selection of reference genes for quantitative RT-PCR studies in Rhipicephalus (Boophilus) microplus and Rhipicephalus appendiculatus ticks and determination of the expression profile of Bm86. BMC molecular biology 10: 112 10.1186/1471-2199-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature biotechnology 29: 644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonfield JK, Whitwham A (2010) Gap5—editing the billion fragment sequence assembly. Bioinformatics 26: 1699–1703. 10.1093/bioinformatics/btq268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology 10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. Journal of molecular endocrinology 29: 23–39. [DOI] [PubMed] [Google Scholar]
- 57. Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer research 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 58. Perkins JR, Dawes JM, McMahon SB, Bennett DL, Orengo C, Kohl M (2012) ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC genomics 13: 296 10.1186/1471-2164-13-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Imler JL, Bulet P (2005) Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chemical immunology and allergy 86: 1–21. [DOI] [PubMed] [Google Scholar]
- 60. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mulcahy LR, Isabella VM, Lewis K (2014) Pseudomonas aeruginosa biofilms in disease. Microbial ecology 68: 1–12. 10.1007/s00248-013-0297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han YS, Chun J, Schwartz A, Nelson S, Paskewitz SM (1999) Induction of mosquito hemolymph proteins in response to immune challenge and wounding. Developmental and comparative immunology 23: 553–562. [DOI] [PubMed] [Google Scholar]
- 63. Toutges MJ, Hartzer K, Lord J, Oppert B (2010) Evaluation of reference genes for quantitative polymerase chain reaction across life cycle stages and tissue types of Tribolium castaneum. Journal of agricultural and food chemistry 58: 8948–8951. 10.1021/jf101603j [DOI] [PubMed] [Google Scholar]
- 64. Yuan M, Lu Y, Zhu X, Wan H, Shakeel M, Zhan S, et al. (2014) Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PloS one 9: e86503 10.1371/journal.pone.0086503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ponton F, Chapuis MP, Pernice M, Sword GA, Simpson SJ (2011) Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. Journal of insect physiology 57: 840–850. 10.1016/j.jinsphys.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 66. Scharlaken B, de Graaf DC, Goossens K, Brunain M, Peelman LJ, Jacobs FJ (2008) Reference Gene Selection for Insect Expression Studies Using Quantitative Real-Time PCR: The Head of the Honeybee, Apis mellifera, After a Bacterial Challenge. Journal of insect science 8: 1–10. [Google Scholar]
- 67. Sun M, Lu MX, Tang XT, Du YZ (2015) Exploring valid reference genes for quantitative real-time PCR analysis in Sesamia inferens (Lepidoptera: Noctuidae). PloS one 10: e0115979 10.1371/journal.pone.0115979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meriin AB, Zaarur N, Sherman MY (2012) Association of translation factor eEF1A with defective ribosomal products generates a signal for aggresome formation. Journal of cell science 125: 2665–2674. 10.1242/jcs.098954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rich BE, Steitz JA (1987) Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Molecular and cellular biology 7: 4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Polakiewicz RD, Munroe DJ, Sait SN, Tycowski KT, Nowak NJ, Shows TB, et al. (1995) Mapping of ribosomal protein S3 and internally nested snoRNA U15A gene to human chromosome 11q13.3-q13.5. Genomics 25: 577–580. [DOI] [PubMed] [Google Scholar]
- 71. Majerowicz D, Alves-Bezerra M, Logullo R, Fonseca-de-Souza AL, Meyer-Fernandes JR, Braz GR, et al. (2011) Looking for reference genes for real-time quantitative PCR experiments in Rhodnius prolixus (Hemiptera: Reduviidae). Insect molecular biology 20: 713–722. 10.1111/j.1365-2583.2011.01101.x [DOI] [PubMed] [Google Scholar]
- 72. Hetru C, Troxler L, Hoffmann JA (2003) Drosophila melanogaster antimicrobial defense. The Journal of infectious diseases 187 Suppl 2: S327–334. [DOI] [PubMed] [Google Scholar]
- 73. Hoffmann JA, Reichhart JM (2002) Drosophila innate immunity: an evolutionary perspective. Nature immunology 3: 121–126. [DOI] [PubMed] [Google Scholar]
- 74. Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, et al. (2001) Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Developmental cell 1: 503–514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA from all L. sericata tissues from naïve (top) and immune-challenged (bottom) larvae was analyzed using agarose gel with exception of one “crop” sample and three “fat body” samples in immune-challenged larvae. These samples were sodium acetate precipitated after the RNA isolation and limited in sample amount.
(TIF)
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
Sequences of all reference genes (KR133393 - KR133402) and lucimycin (KJ413251) are available from the NCBI database. The sequences of attacin-2 (KR920003) and defensin-1 (KT149727) have been submitted to the NCBI database.