Abstract
Background
Liver resection is used to treat primary and secondary malignancies. Historically, these procedures were associated with significant complications, which may affect cancer-specific outcome. This study analyzes the changes in morbidity and mortality after hepatic resection over time.
Study Design
Records of all patients undergoing liver resection for a malignant diagnosis from 1993 to 2012 at Memorial Sloan Kettering were analyzed. Patients were divided into early (1993-1999), middle (2000-2006), and recent (2007-2012) eras. Major hepatectomy was defined as resection of 3 or more segments. Univariate and multivariate analyses were made with t-tests or Mann-Whitney tests.
Results
3,875 patients underwent 4,152 resections for malignancy. The most common diagnosis was metastatic colorectal cancer (n=2,476, 64% of patients). Over the study period, 90-day mortality rate decreased from 5% to 1.6% (p<0.001). Perioperative morbidity decreased from 53% to 20% (p<0.001). The percentage of major hepatectomies decreased from 66% to 36% (p<0.001). The rate of perioperative transfusion decreased from 51% to 21% (p<0.001). The spectrum of perioperative morbidity changed markedly over time, with abdominal infections (43% of complications) overtaking cardiopulmonary complications (22% of complications). Peak postoperative bilirubin (OR 1.1, p<0.001), blood loss (OR 1.5, p=0.001), major hepatectomy (OR 1.3, p=0.031), and concurrent partial colectomy (OR 2.4, p<0.001) were independent predictors of perioperative morbidity. The mortality associated with trisectionectomy (6%) and right hepatectomy (3%) remained unchanged over time.
Conclusions
Morbidity and mortality rates after partial hepatectomy for cancer have decreased substantially as the major hepatectomy rate dropped. Encouraging parenchymal preservation and preventing abdominal infections are vital for continued improvement of liver resection outcomes.
Keywords: colorectal cancer, liver metastases, hepatectomy
Introduction
Liver resection is the most effective treatment for several malignant diseases and employed worldwide today. Historically, a major limitation of liver surgery has been the high morbidity and mortality related to blood loss and loss of functional liver. Recently, sharp reductions in perioperative mortality have been reported, from 10% in the 1980s to below 4%.(1-3) These improvements have resulted from better patient selection and perioperative management. The focus on intraoperative parenchymal preservation has led to substantial reductions in estimated blood loss (EBL), blood product transfusion, and postoperative liver failure.(4, 5) This transformation in the safety profile of hepatic resection is largely responsible for its emergence as an effective cancer therapy.
The mortality rate for major liver resections, however, such as right trisectionectomy, remains as high as 9%.(3, 6) Furthermore, there are serious complications associated with liver surgery, many of which are procedure-specific, but external factors play an increasingly important role. Co-morbid medical conditions as well as the current trend of extensive preoperative systemic chemotherapy with its impact on perioperative outcome(7) are important examples. Continued efforts to improve perioperative morbidity rates are warranted. Also, there is evidence that perioperative morbidity is a powerful predictor and possible cause of adverse disease-specific survival.(8, 9) In that light, reducing perioperative morbidity assumes even greater importance.
The type of morbidity seen after liver resection varies considerably, ranging from liver-specific, such as liver dysfunction or bile leak, to cardiopulmonary, gastrointestinal, renal, or infectious. Correcting this wide spectrum of complications is a challenge because multiple specific areas must be targeted. The present report is an analysis of 4,152 hepatic resections for malignancy over 19 years at a single center, focusing on trends in perioperative outcome variables and changes in practice.
Methods
The liver resection database at Memorial Sloan Kettering (MSK) was queried to identify patients undergoing hepatectomy for malignancy from 1993 to 2012. The MSK Institutional Review Board provided a waiver from IRB review and HIPAA Authorization. Patients were divided into 3 groups of similar size according to time period [early (1993-1999), middle (2000-2006), and recent (2007-2012)].
The general approach to patients under consideration for liver resection has been previously documented.(10) CT, MR, or PET imaging were used for preoperative radiologic evaluations, and intraoperative ultrasound was used in all cases. Portal inflow occlusion (Pringle maneuver) was used frequently, in 5- to 15-minute intervals. Control of portal, arterial, and biliary in-flow pedicles was performed extrahepatically or intrahepatically, depending on the resection planned, disease location, and surgeon preference. Low central venous pressure (<5 mmHg) was used in all cases when feasible. Parenchymal division was performed via a clamp-crushing technique with sutures or clips to control intrahepatic biliary and vascular structures; more recently, thermal bipolar devices were added. Postoperatively, patients were managed for 12-24 hours in the recovery room and then transferred to the surgical ward, unless clinical factors dictated a monitored setting. Red blood cell transfusions were guided by patient hemodynamic status in combination with Hgb level (<8 mg/dL).
Liver resections were quantified by number of segments resected using the Brisbane 2000 terminology of hepatic anatomy and resection:(11) enucleations (0), wedge resection and formal segmentectomy (1), sectionectomy (left lateral, right anterior or posterior, 2), left hepatectomy (3), right hepatectomy (4), and extended hepatectomy (5). Major hepatectomy was defined as resection of ≥three segments. For our data, “perioperative” referred to the time period that included both operation and recovery room. Complications from 1993 to 2002 were identified retrospectively. From 2002 to 2012 complications were entered prospectively. All complications were graded using a score of 1 to 5.(12)
Complications were analyzed per patient and per resection. Major complications were defined as ≥grade 3. Liver dysfunction was defined by presence of at least one from the following: postoperative prolonged hyperbilirubinemia without obstruction or leak, prolonged coagulopathy, ascites (drainage >500 mL/day), or encephalopathy with hyperbilirubinemia.(5) Steatosis was defined as percentage of hepatocytes containing lipid vesicles divided by total number of hepatocytes. It was graded using the Kleiner-Brunt histologic scoring system, (13) where moderate steatosis was defined as 5 - 33%. Mortality was determined at 90-days post procedure.
Summary statistics are reported as median and interquartile range (IQR) for continuous variables, unless otherwise specified. Categorical variables are reported as percentages. Comparisons are made with t-tests or Mann-Whitney tests depending on type of distribution for continuous variables. Categorical variables are compared with Chi-squared or Fisher's exact test depending on number of observations. Multivariate analyses used logistic regression. All reported p values were two-tailed and those ≤0.05 were considered significant. All analyses were conducted using Stata/IC 12.1 (StataCorp LP, College Station, TX).
Results
Patient demographics and pathology
Four thousand two hundred two patients underwent 4,480 hepatic resections for benign and malignant conditions from 1993 to 2012. Of these, 3,875 (92.2%) patients underwent 4,152 (92.7%) resections for malignancy (Table 1). The most common comorbidities were hypertension (13%), ischemic heart disease (7%), and diabetes mellitus (5%). Two hundred five patients underwent preoperative portal vein embolization (PVE; 11% of patients with available data describing PVE status). The largest tumor per patient was 3.7 cm (IQR 2.2-6.5).
Table 1. Demographics and Comorbidities Associated with 4,152 Hepatic Rresections.
| Patient data | Number |
|---|---|
| Age, y (range) | 61 (51-70) |
| Sex, % male | 53% |
| BMI, kg/m2 (IQR) | 26 (23-30) |
| Comorbidity, n (%) | |
| 1 | 620 (14.9%) |
| 2 | 278 (6.7%) |
| ≥3 | 113 (2.7%) |
| Smoking history | 2671 (64.3%) |
| Chronic hepatitis B or C | 186 (4.5%) |
| ASA score (n=2,270) | |
| 1 | 17 (0.7%) |
| 2 | 1056 (46.5%) |
| 3 | 1197 (52.7%) |
| Preoperative, n (%) | |
| Bilirubin, mg/dL (n=4,043) | |
| 0-1 | 3579 (88.5%) |
| 1.1-2 | 348 (8.6%) |
| 2.1-3 | 44 (1.1%) |
| 3.1-10 | 43 (1.1%) |
| >10 | 29 (0.7%) |
| Albumin, g/dL | |
| <4 | 1020 (24.6%) |
| <3.5 | 249 (6.0%) |
| Platelets | |
| <150,000/uL | 539 (13.0%) |
| <100,000/uL | 76 (1.8%) |
| Hgb | 13 (12-14) |
| Neoadjuvant chemotherapy | 1414 (34.0%) |
| Metastatic colorectal cancer | 2476 (59.6%) |
| Primary liver | 623 (15.0%) |
| Extrahepatic biliary | 334 (8.0%) |
IQR, interquartile range; ASA, American Society of Anesthesiologists; Hgb, hemoglobin.
The median operating room time was 232 minutes (IQR 173-300). Portal inflow occlusion (Pringle maneuver) was used in 77% of patients with median time of 31 minutes (IQR 21-45). Of the 2,494 cases with available perioperative transfusion records, 10% received blood, 8% received fresh frozen plasma, and 2% received platelets. Liver resection was combined with resections of other organs in 976 (25.2%) patients. This included colon (9%), portal lymph nodes (7%), and extrahepatic bile duct (3%). One thousand thirty-nine (39.8%) of 2,610 patients had steatosis; 27% of these patients had moderate-to- marked steatosis. The median length of stay after liver resection was 7 days (range 6-10). Thirty-day mortality was 1%, 60-day mortality was 2%, and 90-day mortality was 3%.
Trends over time
The percentage of major hepatectomies decreased from 65.6% to 35.8%, p<0.001, over the three eras (Table 2). The largest decrease was right trisectionectomies, from 27.8% to 6.7%. The median size of resected tumors decreased from 4.5 cm to 3.5 cm to 2.5 cm in each era (p=0.01). The EBL and complication percentages both significantly decreased over time. The percentage of resections combined with ablation increased from 0.6% (n=8) in the first era, to 4.4% (n=65) in the second era, to 19.2% (n=271) in the recent era, p<0.001. In addition, the percentage of minimally invasive cases increased, from zero (0%) in the first era to 70 (5%) in the third era, p<0.001. Ninety-two percent of the resections were R0 (of 2,840 resections with specific margin reported). Of note, the percentage of tumor-involved margins did not change, as less liver was resected using parenchymal-preserving techniques − 9% in the early and recent eras. One hundred twenty-two patients (3% of patients with available data on reoperation) had a second operation to treat a complication within 30 days of the original procedure. This percentage remained constant over time (2-3%). The overall transfusion percentage of blood products over the duration of the hospital stay decreased from 51% in the early era, to 21% in the recent era (p<0.001). Neoadjuvant chemotherapy use increased from 13% to 36% to 55% over three eras (p<0.001).
Table 2. Surgical Procedures Grouped by Era.
| 1993-1999 n=1275 | 2000-2006 n=1465 | 2007-2012 n=1412 | p Value | |
|---|---|---|---|---|
| Major hepatectomy, ≥3 segments | 65.6% (836) | 53.5% (784) | 35.8% (505) | <0.001 |
| Segments resected, median (IQR) | 4 (2-5) | 3 (2 - 4) | 2 (1-4) | <0.001 |
| Left hepatectomy | 6.9% (88) | 8.6% (126) | 8.4% (119) | |
| Left trisectionectomy | 6.1% (78) | 3.0% (44) | 2.5% (36) | |
| Right hepatectomy | 19.9% (254) | 19.1% (280) | 13.6% (192) | |
| Posterior sectionectomy | 4.8% (61) | 5.9% (87) | 5.0% (71) | |
| Right trisectionectomy | 27.8% (355) | 16.6% (244) | 6.7% (95) | |
| Resection combined with ablation | 0.6% (8) | 4.4% (65) | 19.2% (271) | <0.001 |
| Estimated blood loss, median (IQR) | 650 (310 - 1110) | 400 (200 - 750) | 300 (200 - 565) | 0.003 |
| Complications | 53.2% (679) | 34.3% (502) | 19.9% (281) | <0.001 |
| Major complications | 13.2% (169) | 11.2% (164) | 9.8% (138) | 0.017 |
| Readmission, 30 d | 13.2% (169) | 12.3% (180) | 13.6% (192) | 0.6 |
| ASA score (range) | - | 2 (2-3) | 3 (2-3) | <0.001 |
| 90-d mortality | 5.2% (66) | 2.3% (34) | 1.6% (22) | <0.001 |
For major complications, grading of complications was available for 931, 1,301, and 1,412 patients in each era, respectively.
IQR, interquartile range; ASA, American Society of Anesthesiologists.
Complications
At least one complication occurred in 1,462 resections (35%, Table 3). The most common complication was abdominal infection (wound infections, abdominal abscesses, and bilomas), occurring in 11.6% of all resections, but with a significant decrease over time, from 16.5% to 8.6% (Table 4, p<0.001). The pattern of perioperative morbidity significantly changed over the study period (Figure 1). In the first era, 50% of complications that occurred were pulmonary (30%) or cardiovascular (20%) in nature. This decreased to 22% (9% and 13%, respectively) for the recent period. By contrast, abdominal infections rose from 31% to 43% of all complications in the recent period. Of note, however, the morbidity rate differed by diagnosis. Over the entire study period, major and minor complication rates were similarly high for hilar cholangiocarcinoma (49%) and metastatic colorectal cancer (42%), but were lower for hepatocellular cancer (28%), gallbladder cancer (27%), and intrahepatic cholangiocarcinoma (16%, p<0.001).
Table 3. Postoperative Complications.
| Organ system | Specific complication | n | Percentage (of total resections) |
|---|---|---|---|
| Liver/biliary | 236 | 5.7% | |
| Hepatic insufficiency/failure | 82 | 2.0% | |
| Sterile perihepatic fluid collections | 161 | 3.9% | |
| Biliary stricture | 6 | <1% | |
| Cholangitis | 3 | <1% | |
| Pulmonary | 311 | 7.5% | |
| Pleural effusion | 188 | 4.5% | |
| Atelectasis | 57 | 1.4% | |
| Respiratory insufficiency/failure | 58 | 1.4% | |
| Pulmonary embolism | 40 | 1.0% | |
| Cardiovascular | 239 | 5.8% | |
| Arrhythmia | 129 | 3.1% | |
| DVT | 46 | 1.1% | |
| CHF | 24 | <1% | |
| Angina pectoris/MI | 16 | <1% | |
| Cardiac arrest | 12 | <1% | |
| Stroke/TIA/CNS hemorrhage | 11 | <1% | |
| Pericarditis/pericardial effusion | 2 | <1% | |
| Genitourinary | 99 | 2.4% | |
| Renal insufficiency/failure | 56 | 1.3% | |
| Urinary retention | 48 | 1.2% | |
| Gastrointestinal | 190 | 4.6% | |
| Ileus | 121 | 2.9% | |
| Bowel obstruction | 36 | <1% | |
| GI hemorrhage | 21 | <1% | |
| Colitis (includes C. diff) | 28 | <1% | |
| Bowel perforation | 8 | <1% | |
| Infection | 585 | 14.1% | |
| Abdominal | 483 | 11.6% | |
| Wound infection | 255 | 6.1% | |
| Perihepatic abscess | 214 | 5.2% | |
| Bile leak/biloma | 105 | 2.5% | |
| Non-abdominal | 203 | 4.9% | |
| Sepsis/bacteremia | 61 | 1.5% | |
| UTI | 99 | 2.4% | |
| Pneumonia | 79 | 1.9% | |
| Miscellaneous | 286 | 6.9% | |
| Perioperative hemorrhage | 38 | <1% | |
| Delirium | 26 | <1% | |
| Wound breakdown/fascial dehiscence | 33 | <1% | |
| Ascites | 44 | 1.0% | |
| Pump dysfunction | 27 | <1% | |
| Multisystem organ failure | 18 | <1% | |
| Other | 135 | 3.3% |
Resections with multiple associated complications in the same category were counted only once.
DVT, deep-vein thrombosis; CHF, congestive heart failure; TIA, transient ischemic attack; CNS, central nervous system; GI, gastrointestinal; UTI, urinary tract infection.
Table 4. Summary of Complications after All Resections over 3 Eras, and of Complications after Major Resections over 3 Eras.
| Era | |||||||
|---|---|---|---|---|---|---|---|
| 1993 - 1999 | 2000 - 2006 | 2007 - 2012 | p Value | ||||
| n | % | n | % | n | % | ||
| All resections | |||||||
| Total cases | 1,275 | 1,465 | 1,412 | ||||
| Total cases with complications | 679 | 53.2% | 502 | 34.3% | 281 | 19.9% | p < 0.001 |
| Abdominal infections | 211 | 16.5% | 151 | 10.3% | 121 | 8.6% | p < 0.001 |
| GI | 98 | 7.7% | 52 | 3.5% | 40 | 2.8% | p < 0.001 |
| Non-abdominal infections | 120 | 9.4% | 64 | 4.4% | 19 | 1.3% | p < 0.001 |
| Liver/Biliary | 109 | 8.5% | 97 | 6.6% | 30 | 2.1% | p < 0.001 |
| Pulmonary | 208 | 16.3% | 79 | 5.4% | 24 | 1.7% | p < 0.001 |
| Cardiovascular | 133 | 10.4% | 70 | 4.8% | 36 | 2.5% | p < 0.001 |
| GU | 64 | 5.0% | 22 | 1.5% | 13 | 0.9% | p < 0.001 |
| Major resections | |||||||
| Total | 836 | 65.6% | 784 | 53.5% | 505 | 35.8% | |
| Total with complications | 491 | 58.7% | 312 | 39.8% | 133 | 26.3% | p < 0.001 |
| Abdominal infections | 168 | 20.1% | 106 | 13.5% | 57 | 11.3% | p < 0.001 |
| Gastrointestinal | 66 | 7.9% | 29 | 3.7% | 15 | 3.0% | p < 0.001 |
| Liver/Biliary | 100 | 12.0% | 74 | 9.4% | 22 | 4.4% | p < 0.001 |
| Pulmonary | 156 | 18.7% | 58 | 7.4% | 21 | 4.2% | p < 0.001 |
| Non-abdominal infections | 92 | 11.0% | 39 | 5.0% | 9 | 1.8% | p < 0.001 |
| Genitourinary | 46 | 5.5% | 18 | 2.3% | 8 | 1.6% | p < 0.001 |
| Cardiovascular | 94 | 11.2% | 49 | 6.2% | 16 | 3.2% | p < 0.001 |
GI, gastrointestinal; GU, genitourinary.
Figure 1.
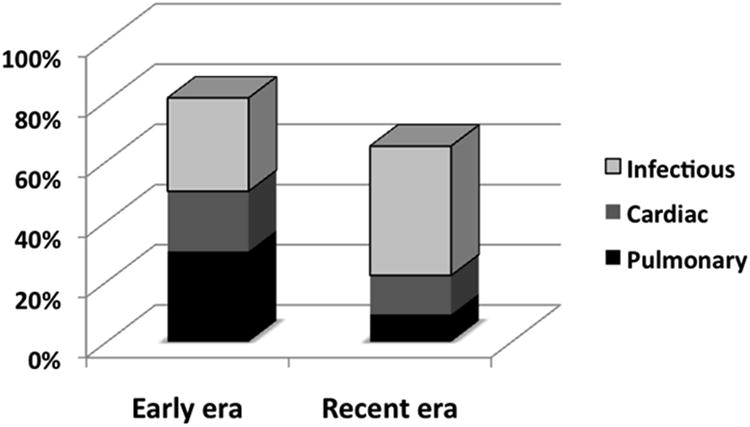
Cause of complication in patients in the early and recent era who had a reported complication (p<0.001).
Factors associated with complications were analyzed in univariate and multivariate analyses. Peak postoperative bilirubin (OR 1.1, p<0.001), EBL (OR 1.5, p=0.001), perioperative transfusion (OR 1.5, p<0.001), major hepatectomy (OR 1.3, p=0.031), and hepatectomy combined with a colorectal resection (OR 2.4, p<0.001) were independent predictors of postoperative morbidity on multivariate analysis. Minimally invasive surgery, use of a drain, a positive margin, and Pringle time greater than 25 minutes all lost significance on multivariate analysis. Since postoperative bilirubin level may be influenced by multiple factors unrelated to liver dysfunction, the analysis was repeated excluding this variable. In this model, perioperative transfusion (OR 2.0), blood loss (OR 1.6), major hepatectomy (OR 1.7), and hepatectomy combined with a colon resection (OR 1.6) remained independent predictors of postoperative complications. Data from synchronous colorectal resections showed almost twice the risk of abdominal infectious complications (21%) when compared to staged procedures (11%, p<0.001). In addition, preoperative jaundice was significantly associated with infectious complications, with an odds ratio of 2.1 with preoperative bilirubin greater than 3 (95% CI 1.27-3.6; p=0.004). Postoperative liver dysfunction decreased in all cases from 3% in the first era to 1% in the recent era (p<0.001). For major resections, postoperative liver dysfunction rates decreased from 5% to 3% (p<0.001). On multivariate analysis, transfusion showed a strong association with the development of liver dysfunction, followed by number of segments resected, number of medical comorbidities, and preoperative abnormalities in bilirubin and albumin.
In parallel with fewer major hepatectomies performed over time, the 90-day mortality rate for all cases decreased from 5.2% in the early era to 1.6% in the recent era, p<0.001. The median time to postoperative death was 36 days (IQR 18-64). The proximate cause of postoperative mortality was unknown in 31 patients; in the remaining patients, liver failure (n=26), multi-system organ failure (n=18), intra-abdominal abscess (n=4), and hemorrhage (n=7) were clearly identified as causative factors. Forty-one percent of patients with an unknown cause of mortality had ≥4 segments resected in the first era and had documented evidence of liver failure. Although these patients did not have a clearly documented cause of death, it is likely that most were liver-related. The majority of perioperative deaths (79%) occurred after major resections. Only 2 of the 31 (6.5%) mortalities after resection of <3 segments were due to liver failure, which was 0.1% of 2,027 patients subjected to a minor resection. In comparison, 23 (24.2%) of the 95 patients that died after a major resection had liver failure (p=0.06), representing 1.1% of all major hepatectomies (n=2125).
The overall mortality rate for major resections was 4.5% (96/2125). Although the overall mortality rate clearly decreased over the three eras, it remained high and unchanged for right and left trisectionectomies (6%) and right hepatectomies (3%) within the recent era (Table 5). As the percentage of major hepatectomies decreased over time, the 90-day mortality rate for all resections decreased from 5.2% in the first era to 1.6% in the recent era (p<0.001; Table 2). Peak postoperative bilirubin ≥7 mg/dL was associated with a mortality rate of 17% (OR 10; 95% CI 6.24-16.1, p<0.001). The mortality rate for peak postoperative bilirubin ≥7 mg/dL in postoperative days 1 to 3 was 14% (n=10), days 4 to 7 was 17% (n=7), and later than day 7 was 35% (n=6, p=0.1). The sensitivity of a bilirubin level ≥7 mg/dL for predicting mortality was 38%, with specificity of 97%.
Table 5. 90-day Mortality for High-Risk Major Hepatectomies.
| Procedure | Era 1 | Era 2 | Era 3 | Total |
|---|---|---|---|---|
| Left trisectionectomy | 10.2% (8) | 0% (0) | 5.6% (2) | 6% (10) |
| Right trisectionectomy | 8.7% (31) | 2.9% (7) | 6.3% (6) | 6% (44) |
| Right hepatectomy | 4.7% (12) | 3.6% (10) | 2.6% (5) | 4% (27) |
| Left hepatectomy | 4.5% (4) | 3.2% (4) | 0.8% (1) | 3% (9) |
| Fewer than 4 segments resected | 2% (11) | 2% (13) | 1% (8) | 1% (32) |
Discussion
Liver surgery for the treatment of primary and secondary malignancy has evolved dramatically over the last twenty years. Notable changes include increased utilization of parenchymal-sparing resections and low intraoperative central venous pressure, better patient selection, use of 2-stage resections in patients with advanced disease, utilization of liver volumetric assessment of future liver remnant, improvements in perioperative management, and increased utilization of preoperative chemotherapy. In concert with these changes, the mortality associated with hepatic resection has significantly decreased.(14, 15) Two large studies reported that despite the higher proportion of older patients with an attendant increase in comorbidities and abnormal livers, both complication and mortality rates have decreased with the recent era.(3, 4)
The present study showed a significant decrease in overall complications from 53.2% to 19.9% over the past two decades. Furthermore, the 90-day mortality rate in this series decreased from 5.2% to 1.6%. Much of the decrease in mortality was likely related to the decreasing number of major resections. Evidence in support of this is the persistently high mortality rates over the three eras for resections associated with substantial parenchymal sacrifice (right and left liver trisectionectomy, and right hepatectomy). Thus, continued efforts to pursue parenchymal-sparing resections will likely translate over time into further reduction in operative mortality.
Despite declining mortality rates, the morbidity associated with liver resection, although also declining, remains substantial. It is important to assess the pattern of complications because the oncologic sequelae of perioperative morbidity can be significant. In our series, abdominal infections had become the most common complication by the recent era. We previously analyzed the oncologic outcomes of 1,067 patients with liver resections at MSK to treat metastatic colorectal cancer.(16) A subgroup analysis of patients with low clinical risk score revealed that complications were associated with a significant reduction in disease-specific survival and disease-free survival. Additionally, in patients with metastatic colorectal cancer enrolled in a randomized trial of intraoperative hemodilution, postoperative complications were associated with significant decreases in recurrence-free survival (69 to 23 months, p<0.001) and overall survival (74 to 28 months, p<0.001).(9) The mechanism that translates perioperative morbidity into reduced cancer-specific survival is unknown, although results from several animal models have suggested that infectious complications increase neutrophil numbers and activation, and may in turn promote liver metastasis.(17, 18)
Given the independent association of the number of segments resected with complications and mortality, hepatic preservation appears to have played a major role in reducing complications. Due to changes in patient selection criteria for resection and adjuvant/neoadjuvant therapies over the last 20 years, it is difficult to determine the contribution that parenchymal-preserving resection has played in long-term outcomes, particularly for the subgroup with colorectal cancer. However, the strong association of complications with adverse survival, and the strong association of hepatic preservation with a decrease in complications make a compelling argument. We found a decrease in major hepatectomies from 65.6% to 35.8% over 19 years (p<0.001), with a corresponding decrease in the median number of segments resected from 4 to 2 (p<0.001). This change in approach to resection appears to be largely responsible for the observed decrease in overall mortality, given that the mortality rate associated with extended liver resections remained constant over the entire study period. In support of this conclusion, a recent series reported a 90-day mortality rate of 9% for major hepatectomies in patients that required PVE.(6) Given the persistently high mortality rate associated with major hepatectomies, a parenchymal-preserving approach, when possible, is important for limiting morbidity and mortality, and in so doing, may improve cancer-related outcomes. Furthermore, data from the present study show that parenchymal-sparing resections do not compromise oncologic integrity.
One of the sequelae of removing less liver is that the transfusion rate decreased from 51% to 21% (p<0.001) and liver dysfunction for all cases decreased. One factor that likely contributed to the increasing use of parenchymal preservation was the expansion of ablation in patients with colorectal metastases. The use of ablation combined with resection has allowed preservation of parenchyma in the future liver remnant, thereby expanding the indications for operative therapy in patients with advanced disease. Ablations were involved in 19.2% of cases in the recent era (increased from 0.6% of the early era). This strategy appears to be oncologically reasonable with small tumors, as the local recurrence rate for tumors <1 cm is 6%, with over 90% of recurrences occurring elsewhere in the liver or systemically. The lower EBL as fewer segments were resected is important, as EBL and transfusion are both associated with perioperative complications. Other factors in this regard included postoperative hyperbilirubinemia, concomitant colon resection, and ≥3 segments resected. Recently, Aloia et al, using the National Surgical Quality Improvement Program (NSQIP) dataset, identified similar factors in 2,313 hepatectomies.(19) Given the limitations inherent to NSQIP, only the 30-day mortality rate could be analyzed. It was 2.5%, along with a 30-day major morbidity rate of 19.6%. In our series the mortality rate tripled from 30 to 90 days.
In addition to hepatic preservation, other preoperative and intraoperative strategies can affect outcomes after liver surgery. Portal vein embolization is a valuable tool to optimize size of future remnant in patients for major liver resection and is now practiced widely (7% of patients in the recent time period). Patients with underlying liver disease, including fibrosis, cirrhosis, and chemotherapy-associated liver injury, appear to benefit most, as demonstrated in a recent randomized trial (20). Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been suggested as an alternative technique to decrease liver failure, but it is likely applicable in only a few percent of cases and is associated with a prohibitively high mortality rate (>10% in-hospital mortality rate).(6) An effective PVE is usually sufficient and has lower morbidity and mortality.(6, 21) Intraoperatively, maintenance of a low central venous pressure assists with minimizing blood loss, and is thus an important consideration for a liver surgery anesthesia team.
Liver failure is the most serious liver-specific complication posthepatectomy. Liver failure has been identified in as many as 10% of patients in some series.(22) One study reported posthepatectomy total bilirubin level greater than 7 mg/dL had a sensitivity of 93% and specificity of 94% for predicting liver-related death.(23) We found a sensitivity of 38% but specificity of 97% for predicting overall survival with this postoperative bilirubin level. We found that the peak postoperative bilirubin greater than 7 mg/dL had an odds ratio of 10 for postoperative mortality with a 17% mortality rate. The immediate postoperative platelet count has also been associated with delayed normalization of liver function. In the present study, only one of 70 patients with postoperative platelet count <100,000 died, so this did not apply.
The limitations of this study are several. First and foremost, much of the data is retrospective and there are missing values for some patients, especially in the earliest era. Complications have been recorded prospectively only since 2002. The value of this large database, however, is that it demonstrates a shift in technique and pattern of complications over the last 19 years, with a focus on parenchymal preservation. This approach to liver surgery should be reinforced as a method of decreasing morbidity and mortality, which in turn may lead to improvements in cancer-specific outcome. Despite the increased safety of these procedures, however, complications are still common. Abdominal infections are the most common complication of modern liver surgery, and should be targeted to further improve liver surgery outcomes.
Acknowledgments
The authors thank Lila J Lande, MPH, for editorial assistance with this manuscript.
Support: This study was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 126th Annual Meeting, Palm Beach, FL, November 30-December 3, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards WH, Jr, Blumgart LH. Liver resection in malignant disease. Sem Surg Oncol. 1987;3(1):1–11. doi: 10.1002/ssu.2980030102. [DOI] [PubMed] [Google Scholar]
- 2.Fortner JG, Kim DK, Maclean BJ, et al. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188(3):363–371. doi: 10.1097/00000658-197809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dokmak S, Fteriche FS, Borscheid R, et al. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 2013 Mar 6; doi: 10.1111/hpb.12069. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240(4):698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion 708-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gönen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217(1):126–133. doi: 10.1016/j.jamcollsurg.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kneuertz PJ, Maithel SK, Staley CA, Kooby DA. Chemotherapy-associated liver injury: impact on surgical management of colorectal cancer liver metastases. Ann Surg Oncol. 2011;18(1):181–190. doi: 10.1245/s10434-010-1201-2. [DOI] [PubMed] [Google Scholar]
- 8.Ito H, Are C, Gönen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247(6):994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]
- 9.Correa-Gallego C, Gönen M, Fischer M, et al. Perioperative complications influence recurrence and survival after resection of hepatic colorectal metastases. Ann Surg Oncol. 2013;20(8):2477–2484. doi: 10.1245/s10434-013-2975-9. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 11.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-339. HPB. 2002;4(2):99. doi: 10.1080/136518202760378489. author reply 99-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin RC, 2nd, Jaques DP, Brennan MF, Karpeh M. Achieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg. 2002;194(5):568–577. doi: 10.1016/s1072-7515(02)01116-x. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Zimmitti G, Roses RE, Andreou A, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg. 2013;17(1):57–64. doi: 10.1007/s11605-012-2000-9. discussion 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadamori H, Yagi T, Shinoura S, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg. 2013;100(1):122–129. doi: 10.1002/bjs.8957. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Are C, Gönen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247(6):994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]
- 17.Spicer JD, McDonald B, Cools-Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- 18.Hsu RY, Chan CH, Spicer JD, et al. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71(5):1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. [DOI] [PubMed] [Google Scholar]
- 19.Aloia TA, Fahy BN, Fischer CP, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11(6):510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(2):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 22.Serra I, Diehl AK. Number and size of stones in patients with asymptomatic and symptomatic gallstones and gallbladder carcinoma. J Gastrointest Surg. 2002;6(2):272–273. doi: 10.1016/s1091-255x(01)00044-0. author reply 273. [DOI] [PubMed] [Google Scholar]
- 23.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204(5):854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862-864. [DOI] [PubMed] [Google Scholar]


