Abstract
Over the past few years new insights have been added to the study of stem cells in the adult lung. The exploration of the endogenous lung progenitors as well as the study of exogenously delivered stem cell populations holds promise for advancing our understanding of the biology of lung repair mechanisms. Moreover, it opens new possibilities for the use of stem cell therapy for the development of regenerative medicine approaches for the treatment of lung disease. Here, we discuss the main types of lung epithelial progenitor populations; the potential of endothelial progenitors, mesenchymal stem cells and embryonic stem cells for lung therapy; as well as summarize the cellular mechanisms involved. The de it provides novel insights for the development of regenerative medicine approaches for the treatment of lung disease.
1. Introduction
Lung disease is one of the leading causes of death in the world. Current treatments are focused on improving the quality of life of lung disease patients by reducing inflammation or pharmacologically inhibiting disease specific pathways [1]. Regenerative medicine treatments that attempt to reverse structural damage to the lungs are scant at best. Focused on harnessing the power of stem cells, regenerative medicine attempts to utilize the body's inherent regenerative capacities to restore function to damaged cells, tissues and organs.
Here, we provide a concise summary of the current knowledge and challenges regarding the main lung progenitor populations (Figure 1), the mechanisms regulating their behavior and their potential to initiate or augment lung repair.
Figure 1.
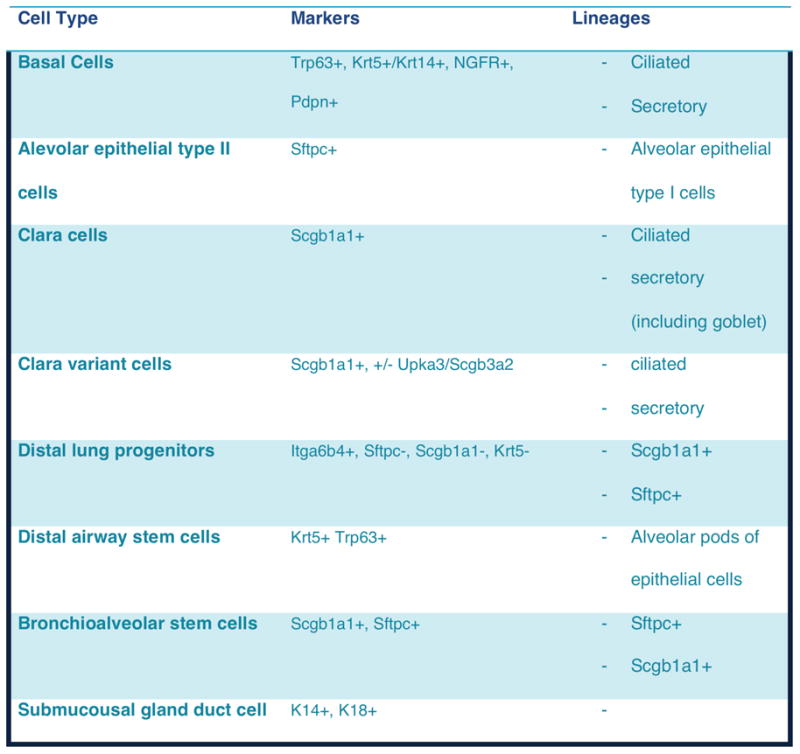
Summary of resident stem and progenitor cell types in the lung. Table modified from [69].
2. Endogenous Lung Stem and Progenitor Cells
Rapidly renewing tissues contain rare populations of tissue specific adult stem cells that have the capacity to proliferate and give rise to transit amplifying cells which in turn can give rise to differentiated cells. In some tissues, fully differentiated cells can also be stimulated to proliferate upon homeostatic pressure or injury. These cells, usually termed facultative progenitor cells, a) show highly infrequent proliferation, but, following injury, they can undergo transition to a continuous proliferation state and b) possess the ability to transition from a differentiated state to an undifferentiated state and vice-versa between normal and injury/repair conditions [2].
Although cells with both stem cell and facultative progenitor cell characteristics have been identified in the lung, their classification has been challenging and, it is still questionable whether adult lung stem cells exist. Studies in mice have shown that, under normal conditions, these progenitor cells are sufficient to maintain the epithelium [3]. However, evidence for their capacity to regenerate the lung following acute injury is still lacking. Nevertheless, several studies have identified airway epithelial cells that have the ability to enter the cell cycle after injury to the lungs and thus be considered as facultative progenitor cells: basal, Clara-like, Clara, pulmonary neuroendocrine, and alveolar type 2 cells [4]. These cells show high regional specialization of functions [5]. The lung microenvironment, containing a number of different cell types, diverse extracellular matrix proteins and other growth factors, constitutes a “stem cell niche”, which is essential in determining the progenitor cells' function and differential potency [5]. As a result, resident lung progenitor cell populations can further be classified by their location in the lung: intralobar airways, tracheobronchial region, bronchiole-alveolar duct junctions, and the alveoli.
2.1 Intralobar Airways
The columnar epithelium lining the distal intralobar airways of the mouse lung is mainly composed of multiciliated and secretory cells, lacking basal cells. Early experiments have shown that mature ciliated cells are postmitotic and thus do not contribute to the maintenance of the airway epithelium under steady-state conditions or in response to injury [8]. In contrast, several studies have shown that, following injury to the mouse bronchioles, Clara like cells can both self-renew and give rise to new ciliated cells [6-8]. For instance, it has been shown that a special subset of Clara cells known as variant Clara cells, which are resistant to naphthaelene injury, have the potential to self-renew and generate ciliated cells, making them candidate stem cells of the intralobar airway epithelium [9,10]. However, it is uncertain whether these cells are actually naphthalene-resistant secretory cells or simply immature secretory cells that lack enzymes for naphthalene metabolism [3]. It is hypothesized that the niche for these variant Clara cells are the neuroepithelial bodies that contain clusters of neuroendocrine cells [11]. However, the precise peptides and growth factors secreted by neuroepithelial bodies that act on adjacent secretory cells are still largely unknown though [10]. In addition, naphthalene resistant Clara cells have been identified at the bronchioalveolar duct junction (BADJ) [2,10]. These cells co-express Scgb1a1 (Secretoglobin 1a1), a marker of Clara cells, and an alveolar type 2 cell marker Surfactant protein C (SftpC) and proliferate upon lung injury. Based on their in vitro differentiation potential, it has been proposed that they are bronchioalveolar stem cells (BASCs) that give rise to both bronchiolar and alveolar cells in vivo [12] [13]. However, further studies using lineage tracing demonstrated that there was no contribution of lineage tagged Clara cells to alveolar epithelia during either steady state homeostasis or after hyperoxic injury [12]. Other identified stem cell populations in the intralobar mouse airways include EpCAMhi/CD104+/CD24- cells [14].
2.2 Tracheobronchial region
The tracheobronchial region is also known as the lung airways and extends from the trachea to the terminal bronchioles. Basal cells being positive for protein markers such as Trp63, cytokeratin 5 (ck5), cytokeratin 14 (ck14) and nerve growth factor receptor (Ngfr) constitute multipotent progenitor cells of the tracheobronchial region [15-17]. Indeed these cells not only show capacity for self-renewal and clonal expansion, but they are also capable of giving rise to basal, ciliated and secretory lineages, both during steady state and epithelium post-damage repair conditions [15-17]. Differences between the various basal cells subpopulation, however, do exist and the differentiation time points, differentiation mechanisms and niches of all these basal cells remain a topic of much study [18,19]. As an example, it has been shown that asymmetric division of differentiating basal cells to generate multi-potent Trp63– Krt8+ luminal early progenitors is a Notch-dependent process [20]. It is hypothesized that these early progenitors have limited capacity for proliferation and in response to a second Notch signaling event will generate terminally differentiated ciliated or secretory cells [20]. Indeed, sustained high levels of Notch signals drive differentiation of basal cells into Scgb1a1+ and Spdef+ Muc5AC+ secretory cells instead of ciliated cells [20].
In the trachea, the majority of Scgb1a1+ Clara cells behave as transit-amplifying cells derived from an unknown progenitor [12]. However, it cannot be excluded that there is a small population of long-term self-renewing Clara cells in the trachea as the lineage label is maintained in a small percentage of tracheal Clara cells for up to 1 year [12].
2.3 Alveoli
The alveolar epithelium consists of type I and type II alveolar epithelial cells (AEC1 and AEC2). Cell turnover in the alveolar region of the adult mouse lung is normally 28–35 days [21], which is fairly slow. Pulse-labeling experiments have demonstrated that AEC2 cells actually give rise to AEC1 cells during development and in response to injury [22]. It is hypothesized that damage to alveolar capillary endothelial cells leads to the release of local cytokines and signaling factors that promote the proliferation and differentiation of Type II AECs [23]. It is also possible that death of Type I AECs leads to denudation of the alveolar basal lamina, triggering the proliferation and expansion of Type II AECs as well as their differentiation to Type I cells [23].
Interestingly, recent studies have identified novel populations that hold the potential of distal lung progenitors. Among those Chapman and colleagues have reported that a subset of AECs expressing the laminin receptor α 6β 4, but little or no pro–surfactant C (pro-SPC) which holds regenerative capacity [24]. Indeed, these cells showed capacity for both aiway and alveolar differentiation in vitro and appeared to proliferate in response to lung bleomycin injury in vivo [24].
In addition, using a model of H1N1 influenza virus induced airway and alveolar region damage, Kumar et al., have identified a progenitor population of Trp63+ Krt5+ basal cells in the peribronchiolar region [25]. These cells proliferated after injury and were shown to give rise to several epithelial ‘pods’ in the distal lung. The epithelial ‘pods’ in turn continue to proliferate and give rise to functional alveoli [25]. The precise origin of the ectopic basal cells is currently unknown. It has been postulated that they arise from rare Trp63+ cells in the smallest bronchioles through reprogramming of resident cells or that they may be dislodged from more proximal regions of the lung [19,26].
3. Circulating and exogenously administered stem/progenitor cells
3.1 Endothelial progenitor cells
Endothelial progenitor cells (EPCs) were originally described as circulating bone marrow-derived cells that possess the potential to proliferate and differentiate into mature endothelial cells [27,28]. EPCs can therefore provide a circulating pool of reservoir cells that could potentially integrate into the site of lung injury and replace damaged endothelium. In addition, isolation and exogenous manipulation of EPCs can constitute a cell source for cell therapeutic approaches for lung injury.
However, no clear consensus regarding the identity of EPC cells exist and the term is used to describe a number of cell types with putative roles in vascular homeostasis and disease [29,30]. Nevertheless, there is some agreement that two main types of EPCs can be isolated from human peripheral blood and cultured in vitro: early EPCs and late outgrowth EPCs. Early EPCs are CD34+ CD31+ CD14+, have a spindle shape morphology, grow early in vitro, cannot form tubes in a matrigel tube forming assay, and secrete high levels of growth factor and cytokines. Late outgrowth EPCs appear after 2 weeks of culture, are CD31+ CD144+ CD146+ CD105+ CD45- CD14- CD115-, have cobblestone-like shape and possess the unique ability to spontaneously form human blood vessels in certain environments. In the context of the lungs, it is postulated that early EPCs can act as paracrine cells while late EPCs can function to restore vasculature structures [4]. Indeed, when infused together the two seem to play a synergistic role in restoring vascular structure and function [31]. Interestingly, resident microvascular endothelial progenitor cells have been identified in the pulmonary microvascular endothelium of mouse, rat and human lung [32]. These cells seem to possess rapid vasculogenic capacity compared to EPCs derived from other sources [32].
In vivo, circulating EPCs can be recruited from the periphery and potentially integrate in sites of injury and promote repair by enhancing neovascularization. This can happen by either their direct differentiation to more mature vascular cells or by the release of p paracrine factors that promote cell growth and angiogenesis [33]. Disease state, age and lifestyle choices, all have been shown to reduce EPC counts and their potential reparative capacity (reviewed in [34] and references therein). Recent evidence suggests that hypoxia might play an important role in EPC mobilization, growth and differentiation, however, the precise mechanisms are unclear. Still, the numbers of circulating EPCs do seem to correlate with lung disease states. For example, increased circulating EPC numbers generally correlate with better survival and less residual lung [35-37]. However, there are cases such as non–small cell lung cancer where increased numbers of circulating EPCs correlates with worse survival [38,39].
3.2 Mesenchymal Stem cells
Perhaps the best researched of non-resident stem cells of the lung are bone marrow derived mesenchymal stem cells or mesenchymal stromal cells (MSCs). It is important to note that MSCs represent a “heterogeneous” population expressing different levels of a panel of cell surface markers which, although not specific, in combination are associated with defining MSCs. MSCs are further defined by cell attachment, self-renewal, clonogenicity, and the ability to differentiate towards multiple lineages [40,41]. The minimal criteria for defining human MSCs as recommended from the according to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy as of 2006, is as follows: a) plastic adherence in standard tissue culture conditions, b) expression of CD73, CD90, and CD105 c) no expression for CD 11bCD14, CD34, CD45, CD79α/CD19, and HLA-DR-, and d) differentiation in vitro to osteoblasts, adipocytes, and chondroblasts [42]. Interestingly, MSC-like cells have been isolated from adult mouse [43-45] and human lungs [46-48]. However, the physiologic importance of these cell populations is still under investigation.
Transplantation of MSCs offers many advantages for regenerative therapy as MSCs are immunologically well tolerated, which allows for administration of allogenic MSCs without significant host responses[49]. Furthermore, they can be expanded ex vivo and easily manipulated genetically to enhance their survival and engraftment as well as the delivery desired molecules [50]. While a major effort in MSC research has been focused on their regenerative capacity, several studies have reported that MSCs can play a role in tissue repair by the secretion of paracrine factors that activate cytoprotection, cell growth, neovascularization and immunomodulation [40,51,52].
In regard to lung therapy, MSCs can be induced in vitro to express markers of either airway or alveolar epithelial cells [53]. However, since it is a rare event [54] emphasis has been shifted to their paracrine properties (Figure 2). For example, soluble mediators released by MSCs such as IL-1 receptor antagonist, IL-10, keratinocyte growth factor, hepatocyte growth factor, angiopoietin 1, and TGFβ appear to play a significant role in the repair of acute and fibrotic injuries ([55-57]; also summarized in [4]), however the actual mechanisms by which they do this are not yet fully understood. Of note, several studies support a role of MSC in lung inflammation and injury by modulation of the immune system. Indeed, MSCs secrete anti-inflammatory agents such interleukin 1 receptor antagonist interleukin-6, interleukin-10, and prostaglandin E2 [58] while in parallel can suppress pro-inflammatory cytokine expression (i.e. TNF- α stimulated gene/protein 6) [54]. In addition, MSCs can also secrete anti-microbial peptides such as LL-37[59] and tumour-necrosis-factor-alpha-induced-protein-6 [60,61]. Indeed, in vivo delivery of mesenchymal stem cells increased bacterial clearance and enhanced host cell phagocytosis in septic mice [61,62].
Figure 2.
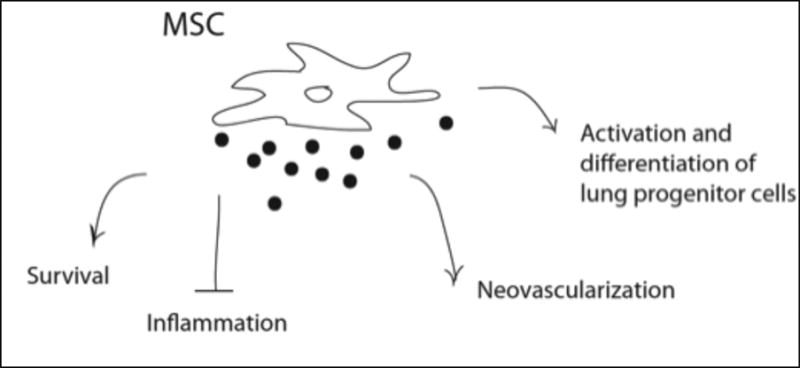
Overview of MSC paracrine effects in the lung.
3.3. Embryonic Stem Cells, Induced Pluripotent Stem Cells
Embryonic stem cells (ESCs) are derived from the ICM of the blastocyst. They are undifferentiated, pluripotent cells. In well-defined culture, they can be maintained indefinitely in this state, or they can be directed towards a specific cell fate [63]. Recently it was shown the stem cells could be generated from somatic cells by cellular reprogramming [64]. These induced pluripotent cells (iPS), although are not as robust or pluripotent as human embryonic stem cells, they do appear very similar morphologically and do possess the same ability to differentiate into cell types from all three germ layers [64].
This raises the possibility that epithelial cells could be created from somatic cells, which would allow for patient-specific iPS stem cells or the generation of gene-corrected iPS cells for individual with genetic diseases such as cystic fibrosis [65], alpha-1-antitrypsin deficiency [65].
Several studies over the past few years have shown that both mouse and human ESCs can be induced in culture to generate cells with phenotypic characteristics of type 2 alveolar epithelial cells. These include expression of surfactant proteins and lamellar bodies, formation of pseudoglandular structures [66]. Moreover, it has been shown that intratracheal administration of the AECII cells derived from hESCs in a bleomycin-induced lung injury mouse model resulted in the engraftment and hESCs -derived SPS expressing cells. Importantly, mice receiving the AECII cells derived from hESCs showed significant reduction in lung injury compared to controls [67]. Using a similar model of lung injury, Soh and colleagues have shown recently that transplantation of hESCs and iPS derived CD166+ lung epithelial progenitor cells resulted in enhanced survivability of mice and improved lung pulmonary functions [68]. Despite the progress, generation of lung epithelia from ES or iPS and their therapeutic application remain challenging. These challenges include the complexity and lack of knowledge of the lung epithelia cell phenotype, the need for improved protocols and the tumorogenicity of the implanted due to the presence of undifferentiated pluripotent stem cells.
4. Challenges and Future directions
Despite the progress, uncovering the mechanisms by which progenitor/stem cell populations in the lung maintain homeostasis has been prove to be very challenging. One of the most significant challenges is the identification of appropriate/specific marker for endogenous lung stem/progenitor cells population in both rodent and humans. Progress has been additionally slowed by the complex 3D structure of the lung, which has made it difficult to perform live monitoring of progenitor cells or of any microcellular interactions during development and repair. The development of better genetic tools (cell lineage tracing methods) as well advancements in the area of tissue engineering (to allow 3D studies ex vivo) can significantly contribute to the further development of the field. In addition, significant gaps remain in our knowledge regarding the development of lung cell therapy protocols. Indeed optimal methods for cell administration, dosage regimens and efficacy still need to be addressed. Future studies hold promise for providing new insights into the biology of lung stem cells as well for the translation of pre-clinical studies to new therapeutic modalities for lung disease therapy.
References
- 1.Kiley JP, Senior RM. Pulmonary research in 2013 and beyond: a National Heart, Lung, and Blood Institute perspective. American journal of physiology Lung cellular and molecular physiology. 2012 Nov 1;303:L729–32. doi: 10.1152/ajplung.00305.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proceedings of the American Thoracic Society. 2008 Aug 15;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock JR, Hogan BLM. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011 Nov 10;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 4.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, et al. Stem cells and cell therapies in lung biology and lung diseases. Proceedings of the American Thoracic Society. 2011 Jun;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004 May 19;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 1986 Apr;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000 Jan;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007 Jan 9;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. American journal of respiratory cell and molecular biology. 2001 Jun;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 10.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002 Jul;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linnoila RI. Functional facets of the pulmonary neuroendocrine system. Lab Invest. 2006 May;86:425–444. doi: 10.1038/labinvest.3700412. [DOI] [PubMed] [Google Scholar]
- 12.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009 Jun 5;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005 Jun 17;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 14.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. PNAS. 2010 Jan 26;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. PNAS. 2009 Aug 4;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. American journal of physiology Lung cellular and molecular physiology. 2004 Apr;286:L643–9. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 17.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal Basal cells: a facultative progenitor cell pool. Am J Pathol. 2010 Jul;177:362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Disease models & mechanisms. 2010 Sep-Oct;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock J, Konigshoff M. Endogenous lung regeneration: Potential and limitations. American journal of respiratory and critical care medicine. 2012 Sep 20; doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 20.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011 Jun 3;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauffman SL. Cell proliferation in the mammalian lung. International review of experimental pathology. 1980;22:131–191. [PubMed] [Google Scholar]
- 22.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Experimental and molecular pathology. 1975 Feb;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 23.Wansleeben C, Barkauskas CE, Rock JR, Hogan BLM. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. WIREs Dev Biol. 2012 May 3; doi: 10.1002/wdev.58. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 24.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011 Jul;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011 Oct 28;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotton DN. Next-generation regeneration: the hope and hype of lung stem cell research. American journal of respiratory and critical care medicine. 2012 Jun 15;185:1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb 14;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 28.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003 Jul 1;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 29.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007 Mar 1;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008 Sep;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005 Sep 13;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 32.Yoder MC. Progenitor cells in the pulmonary circulation. Proceedings of the American Thoracic Society. 2011 Nov;8:466–470. doi: 10.1513/pats.201101-006MW. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T, Suzuki S, Kubo H, Yamaya M, Kurosawa S, Kato M. Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology. 2011 May;16:680–687. doi: 10.1111/j.1440-1843.2011.01959.x. [DOI] [PubMed] [Google Scholar]
- 34.Mao M, Xu X, Zhang Y, Zhang B, Fu ZH. Endothelial progenitor cells: the promise of cell-based therapies for acute lung injury. Inflamm Res. 2012 Nov 9; doi: 10.1007/s00011-012-0570-3. [DOI] [PubMed] [Google Scholar]
- 35.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. American journal of respiratory and critical care medicine. 2005 Oct 1;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 36.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005 May;60:410–413. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LSG, Marchesan D, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2009 Oct 15;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J, et al. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin Cancer Res. 2009 Mar 1;15:1741–1746. doi: 10.1158/1078-0432.CCR-08-1372. [DOI] [PubMed] [Google Scholar]
- 39.Nowak K, Rafat N, Belle S, Weiss C, Hanusch C, Hohenberger P, et al. Circulating endothelial progenitor cells are increased in human lung cancer and correlate with stage of disease. Eur J Cardiothorac Surg. 2010 Apr;37:758–763. doi: 10.1016/j.ejcts.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Keating A. Mesenchymal Stromal Cells: New Directions. Stem Cell. 2012 Jun 14;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011 Feb 1;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 43.Gadepalli VS, Vaughan C, Rao RR. Isolation and characterization of murine multipotent lung stem cells. Methods Mol Biol. 2013;962:183–191. doi: 10.1007/978-1-62703-236-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Summer R, Fitzsimmons K, Dwyer D. Isolation of an adult mouse lung mesenchymal progenitor cell population. … of respiratory cell and …. 2007 doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, et al. Evidence for human lung stem cells. N Engl J Med. 2011 May 12;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Lama VN, Smith L, Badri L, Flint A. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. Journal of Clinical …. 2007 doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ, et al. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev. 2011 Nov;20:1995–2007. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Covas DT, Panepucci RA, Fontes AM, Silva WA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ … Experimental …. 2008 doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 49.DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators of inflammation. 2010 doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010 Nov;21:1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011 Feb;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JW, Fang X, Krasnodembskaya A, Howard JP. Concise Review: Mesenchymal Stem Cells for Acute Lung Injury: Role of Paracrine Soluble Factors. Stem Cells. 2011 May 23;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricciardi M, Malpeli G, Bifari F, Bassi G, Pacelli L, Nwabo Kamdje AH, et al. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS ONE. 2012;7:e35639. doi: 10.1371/journal.pone.0035639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Molecular Therapy. 2009 Jun;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akram KM, Samad S, Spiteri M, Forsyth NR. Mesenchymal Stem Cell Therapy and Lung Diseases. 2012:1–25. doi: 10.1007/10_2012_140. [DOI] [PubMed] [Google Scholar]
- 56.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS medicine. 2007 Sep;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. American journal of respiratory and critical care medicine. 2009 Dec 1;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009 Jan;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010 Dec;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayes M, Curley G, Laffey JG. Mesenchymal stem cells - a promising therapy for Acute Respiratory Distress Syndrome. F1000 Med Rep. 2012;4:2. doi: 10.3410/M4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mei SHJ, Haitsma JJ, Santos Dos CC, Deng Y, Lai PFH, Slutsky AS, et al. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. American journal of respiratory and critical care medicine. 2010 Oct 14;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 63.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010 Oct;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wetsel RA, Wang D, Calame DG. Therapeutic potential of lung epithelial progenitor cells derived from embryonic and induced pluripotent stem cells. Annu Rev Med. 2011;62:95–105. doi: 10.1146/annurev-med-052009-172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Molecular Therapy. 2010 Mar;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soh BS, Zheng D, Li Yeo JS, Yang HH, Ng SY, Wong LH, et al. CD166(pos) Subpopulation From Differentiated Human ES and iPS Cells Support Repair of Acute Lung Injury. Molecular Therapy. 2012 Sep 11; doi: 10.1038/mt.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rock J, Königshoff M. Endogenous lung regeneration: Potential and limitations. American journal of respiratory and critical care medicine. 2012 Sep 20; doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]


