Abstract
Nav1.6 is a major voltage-gated sodium channel in the central and peripheral nervous systems. Within neurons, the channel protein is concentrated at the axon initial segment and nodes of Ranvier, where it functions in initiation and propagation of action potentials. We examined the role of Nav1.6 in general anesthesia using two mouse mutants with reduced activity of Nav1.6, Scn8a medJ/medJ and Scn8a 9J/9J. The mice were exposed to the general anesthetics isoflurane and sevoflurane in step-wise increments; the concentration required to produce loss of righting reflex, a surrogate for anesthetic-induced unconsciousness in rodents, was determined. Mice homozygous for these mutations exhibited increased sensitivity to both isoflurane and sevoflurane. The increased sensitivity was observed during induction of unconsciousness but not during the recovery phase, suggesting that the effect is not attributable to compromised systemic physiology. Electroencephalographic theta power during baseline waking was lower in mutants, suggesting decreased arousal and reduced neuronal excitability. This is the first report linking reduced activity of a specific voltage-gated sodium channel to increased sensitivity to general anesthetics in vivo.
Introduction
Nav1.6 is encoded by the gene Scn8a and is one of the major voltage-gated sodium channels in the central nervous system [1,2]. The Nav1.6 protein is concentrated within neurons at the axonal initial segment, where it regulates the initiation of action potentials [3,4]. The channel is also concentrated at nodes of Ranvier and is present at lower abundance in soma and dendrites [5–7]. Nav1.6 is important for generation of persistent and resurgent currents and is expressed throughout the brain, including in the prefrontal cortex [8], basal ganglia [9], hippocampus [3,10], cerebellum [10–12], and brainstem [13].
Complete loss-of-function mutations of Scn8a in the mouse are associated with hind limb paralysis and juvenile lethality [10,14]. Two hypomorphic mutants with partial loss of channel activity, Scn8a medJ/medJ and Scn8a 9J/9J, have been characterized. Scn8a medJ/medJ has a splice site mutation that does not alter the amino acid sequence but reduces the efficiency of splicing and the level of expression of the Nav1.6 protein to 5–10% of wildtype levels [15,16]. The Scn8a 9J mutation is an in-frame deletion of isoleucine residue 1750 in transmembrane segment DIVS6 that results in a profound reduction of channel activity (http://www.informatics.jax.org/allele/MGI:3838627). Scn8a medJ/medJ and Scn8a 9J/9J homozygotes survive to adulthood and exhibit progressive movement disorders including tremor, ataxia, and dystonia. Reduction of Nav1.6 expression in Scn8a medJ/medJ mice does not result in compensatory up-regulation of the other major sodium channels Nav1.1 and Nav1.2 [16].
The interactions of anesthetic drugs with specific voltage-gated sodium channels have been assessed using heterologous assays [17]. In the Xenopus oocyte expression system, Nav1.6, Nav1.2, and Nav1.4 are inhibited by the volatile anesthetic isoflurane [18]. Sevoflurane, at clinically-relevant concentrations, did not produce a significant inhibition of the transient inward currents of Nav1.8, Nav1.7, and Nav1.4 expressed in the Xenopus oocytes [19]. However, clinically-relevant concentrations of sevoflurane and isoflurane were reported to produce a concentration and voltage dependent inhibition of Nav1.4 channel expressed in a mammalian cell line [20]. In mammalian cell line, isoflurane reduces the peak sodium current of Nav1.2 by a negative shift in the voltage dependence of channel inactivation and a delay in the recovery from inactivation [21]. Inactivation of the bacterial voltage-gated sodium channel by the volatile anesthetics isoflurane and sevoflurane is proposed to occur by a multisite mechanism [22,23].
In vivo studies have also implicated sodium channels in the response to anesthesia, but without specifying the channel subtypes involved. Pretreatment with the sodium channel blockers tetrodotoxin or lidocaine reduced the concentrations of isoflurane and sevoflurane required to produce anesthesia [24,25]. Conversely, administration of the sodium channel activator veratridine decreased the sensitivity to isoflurane [25].
In this study, we investigated the specific role of Nav1.6 by examining the in vivo response to volatile anesthetics in two hypomorphic mutants with reduced channel activity. We report reduced electroencephalographic theta power and increased sensitivity of both mutant mice to isoflurane and sevoflurane, providing evidence for a functional role of Nav1.6 in the maintenance of arousal and anesthetic-induced unconsciousness.
Materials and Methods
Mice
All experimental procedures were approved by the University of Michigan Committee on Use and Care of Animals (Protocol #PRO00002561) and were in compliance with the Guide for the Care and Use of Laboratory Animals (8th Edition, The National Academies Press, Washington D.C.) and the ARRIVE guidelines (S1 File). Scn8a medJ/+ mice have been maintained in our laboratory for 20 years [15]. Homozygous Scn8a medJ mice (N = 7) and littermate controls (N = 7) were studied on a (C57BL/6J X C3HeB/FeJ)F1 strain background to avoid the juvenile lethality of homozygotes on the C57BL/6J background [16,26]. The Scn8a 9J mutation arose spontaneously in strain BALB/cJ at the Jackson Laboratory and has been maintained by backcrossing to strain C57BL/6J. Homozygous mutants (N = 7) and littermate controls (N = 7) were generated by intercrossing heterozygous mice. The Scn8a 9J mice were from backcross generations N6 and N7 to strain C57BL/6J. Mice were maintained specific pathogen free in ventilated cages on a 12h light: 12h dark cycle (lights on at 6:00 am) with ad libitum Picolab Laboratory Rodent Diet 5L0D and water on corn cob bedding. Homozygous mutants were supplemented with soft diet gel 76A from ClearH2O. Homozygous Scn8a medJ mice were maintained with the addition of pine shavings to aid movement in the cage. Mice were studied between 3–6 months of age, when their body weights were between 20–28 grams. Littermate controls included heterozygous mutant mice and homozygous wildtype mice, which did not differ in their responses and were combined for analysis. Mice of both sexes were included.
Surgical procedures
Under surgical levels of isoflurane anesthesia, the mice were implanted with stainless steel screw electrodes over frontal cortex (1.5 mm anterior and 2.0 mm lateral to Bregma) and parietal cortex (1.5 mm posterior and 2.0 mm lateral to Bregma) to record electroencephalogram (EEG). In addition, a pair of insulated wires (Cooner Wires, Inc., Chatsworth, CA), exposed at the tips, were positioned bilaterally into the nuchal muscles to record electromyogram (EMG). A screw electrode over the cerebellum served as the reference electrode for the electroencephalographic recordings. All electrodes were mated with a 6-pin pedestal (Plastics One, Inc., Roanoke, VA) and the entire electrode assembly was affixed to the cranium using dental acrylic (Stoelting Co., IL). Rimadyl (5 mg/kg body wt, s.c.) was administered after surgery for analgesia.
Electrophysiological recordings and power spectrum density analysis
EEG and EMG were recorded using a Grass Model 15LT physiodata amplifier (15A54 Quad) system (Astro-Med, Inc.) interfaced with a BIOPAC MP-150 data acquisition unit and AcqKnowledge (version 4.1.1) software (BIOPAC Systems, Inc.). Bipolar frontal-parietal and parietal-parietal EEG were band pass filtered between 0.1–100Hz and sampled at 250Hz. The EMG was band pass filtered between 1–100Hz and sampled at 250Hz. Monopolar parietal EEG, referenced to the electrode over the cerebellum and band pass filtered between 0.1–300Hz, was sampled at 1kHz and used for the calculation of power spectral density (PSD) in theta band (4–10Hz). An IIR notch filter was applied to remove 60Hz line noise. Absolute PSD between 4–10Hz was calculated with Welch’s PSD estimate method by segmenting the data into eight Hamming windows with 50% overlap, implemented in the MATLAB Signal Processing Toolbox (MathWorks Inc., Natick, MA). Relative power was calculated for each epoch by dividing the mean absolute power for theta frequency band by the total power across the entire frequency range.
Experimental design
All experiments were conducted between 10:00 am—2:00 pm in a custom built temperature-controlled airtight clear cylindrical chamber (5.67L) that allows simultaneous electrophysiological recordings and behavioral assessment. The mice were provided at least 10–14 days of post-surgical recovery, during which they were conditioned to the EEG recording cable and the testing chamber for 2–4h for two consecutive days. On the day of the experiment, baseline EEG was recorded for 30 minutes while holding the behavioral state constant by keeping the mice awake using gentle tapping on the outside of testing chamber. The testing chamber was maintained at 37° Celsius with 100% oxygen inflow at 8L/min. The EEG recording continued uninterrupted for the entire experimental session. The inflow and outflow anesthetic concentration was monitored using vapor analyzers (Datex Medical Instrumentation, Inc., Tewksbury, MA). After 30 minutes of baseline wake EEG recording, anesthetic exposure (isoflurane or sevoflurane) started and mice were allowed to equilibrate to each anesthetic level for 15 minutes, following which the anesthetic concentration was increased in 0.1% increments. At the end of 14 minutes of anesthetic exposure, the chamber was turned in order to flip the mouse on its back and assess the loss of righting reflex (LORR), a surrogate for anesthetic-induced unconsciousness in rodents [27–34]. The ataxia in homozygous mutant mice presented a potential confound in the determination of the unconscious anesthetized state based solely on LORR, which depends on motor activity. Therefore, in addition to the behavioral measurement of LORR, we used EEG recordings to assess brain state transitions. The appearance of high-voltage/low-frequency EEG, a defining neurophysiological feature of non-rapid eye movement sleep and anesthetic-induced unconsciousness [31,35–38], was used in conjunction with the onset of LORR as a secondary confirmation. The mice were deemed to have lost consciousness if righting reflex was suppressed for at least 60 seconds and the EEG transitioned from a low-voltage/high-frequency to high-voltage/low-frequency pattern. After achieving anesthetic-induced unconsciousness (LORR), the anesthetic concentration was increased by one more level for 15 minutes, following which the anesthetic exposure was stopped and time to return of righting reflex (RORR), a surrogate for return of consciousness, was recorded. The inflow of 100% oxygen continued until the mice demonstrated full recovery. We also analyzed changes in EEG theta power, a surrogate index for state of arousal in rodents, to compare the anesthetic depth at the point of LORR between the mutants and littermate controls. EEG theta power has been shown to decrease with cortical depression and increase with cortical activation [32,33], supporting its use as a marker for anesthetic state transitions. EEG theta power in the waking state was also compared between the mutant and littermate controls. Theta power at the point of LORR was computed by PSD analysis of 10 minute EEG period from the epoch corresponding to the anesthetic level at which LORR happened. Similarly, a 10 minute EEG epoch was selected just before the start of anesthetic exposure for the computation of pre-anesthesia baseline waking theta power.
The mutant mice and their respective littermate controls were tested independently for isoflurane and sevoflurane and all mice received both anesthetics in a counterbalanced order with an interval of 4–5 days between experimental sessions. Each experiment was conducted two times to ensure the reproducibility of the data and to reduce the margin of error in the determination of the anesthetic concentration at which LORR and RORR occurs. The starting anesthetic concentration was based on a set of preliminary experiments using Scn8a medJ/medJ and Scn8a 9J/9J mice and littermate controls. Isoflurane exposure started at 0.5% for both Scn8a medJ/medJ and Scn8a 9J/9J mice while the respective littermate controls received a starting concentration of 0.6% and 0.7%, respectively. Sevoflurane exposure started at 1.1% for Scn8a medJ/medJ and 1.5% for the littermate control group, and at 0.6% for Scn8a 9J/9J and 1.5% for the littermate control group.
Statistical analysis
Statistical analysis was conducted in consultation with the Center for Statistical Consultation and Research at the University of Michigan. The number of animals used was based on a similar study from our laboratory [39] and a priori power analysis (nQuery Advisor + nTerim, Statistical Solutions Ltd, MA) to ensure that the study had 80% power at an alpha of 0.05. For each mouse the average dose at which it lost righting reflex over two trials was computed. The proportion of mice that lost the righting reflex at each anesthetic dose was graphed and compared between groups. The LORR data were fit to a sigmoidal dose-response curve with a variable slope. Confidence intervals for the curve were computed by maximum likelihood and the delta method. The best-fit EC50% values—effective concentration at which 50% of the subjects loses the righting reflex—were calculated with 95% confidence intervals. Equality between mutant and littermate control groups was tested using likelihood ratio test. The time to recovery of righting reflex (RORR) for isoflurane and sevoflurane between mutant mice and the respective littermate control groups was statistically compared using an unpaired two-tailed t-test. An unpaired two-tailed t-test was used for the comparison of waking theta power between the mutant mice and the respective littermate control groups. The data on RORR and theta power are reported as mean ± standard deviation (S.D.) with confidence intervals (CI) in parenthesis. Statistical comparisons were performed with Graph Pad Prism 6.05 (Graph Pad Software, Inc., La Jolla, CA) and R 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Mice with reduced Nav1.6 activity exhibit increased sensitivity to induction of anesthesia
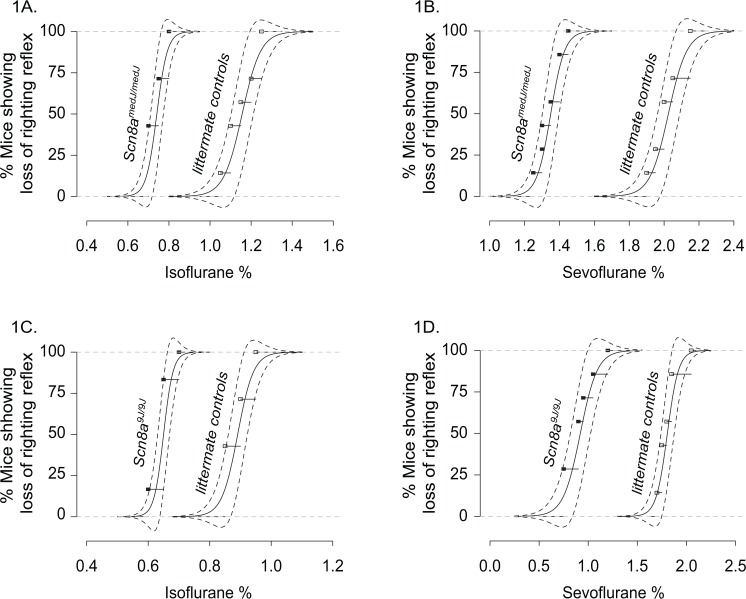
The two mutants tested are associated with different abnormalities of Nav1.6 leading to reduced channel activity in vivo. Scn8a medJ/medJ mice have a reduction in the amount of Nav1.6 protein to 10% of wildtype level but no change in the amino acid sequence of the protein. Scn8a 9J/9J mice have an amino acid deletion in the last transmembrane segment that alters the conformation of the channel pore resulting in loss of channel activity without loss of channel protein. Homozygous Scn8a medJ and Scn8a 9J mice, and their respective littermate controls, were exposed to increasing concentrations of the volatile anesthetics isoflurane and sevoflurane during successive 15 minute periods and the righting reflex was tested at the end of each period. There was a significant shift to the left in the best-fit curves and a decrease in EC50% value for isoflurane and sevoflurane for both Scn8a medJ/medJ [Isoflurane: EC50% (95% CI) = 0.74(0.71–0.78) for Scn8a medJ/medJ vs 1.2(1.1–1.2) for littermate control group, p<0.0001, chi-square(df) = 38.5(2); Sevoflurane: 1.3(1.3–1.4) for Scn8a medJ/medJ vs 2.0(1.9–2.1) for littermate control group, p<0.0001, chi-square(df) = 43.3(2)] (Fig 1A and 1B) and Scn8a 9J/9J [Isoflurane: EC50% (95% CI) = 0.65(0.63–0.67) for Scn8a 9J/9J vs 0.89(0.86–0.93) for littermate control group, p<0.0001, chi-square(df) = 34.4(2); Sevoflurane: 0.92(0.81–1.0) for Scn8a 9J/9J vs 1.8(1.7–1.8) for littermate control group, p<0.0001, chi-square(df) = 38.4(2)] mice (Fig 1C and 1D).
Fig 1. Increased anesthetic sensitivity in mice with Scn8a medJ and Scn8a 9Jmutations.
Best-fit dose response curves along with 95% confidence band (dashed lines) show the behavioral response (loss of righting reflex) of Scn8a medJ/medJ (A and B) and Scn8a 9J/9J (C and D) mice and their respective littermate control groups to increasing concentration of isoflurane and sevoflurane anesthesia. Seven mice were used for each group. Loss of righting reflex was used as a surrogate for unconsciousness. Filled (mutant mice) and open (littermate controls) squares represents the percentage of mice that lost the righting reflex in response to isoflurane (A and C) and sevoflurane (B and D). Data were combined from two trials for each anesthetic and for each mouse. EC50% shows a statistically significant (p<0.0001) leftward shift in the mutant mice, indicating hypersensitivity to anesthesia.
As noted in Materials and Methods, real-time neurophysiological features were the criteria used to confirm unconsciousness in addition to the observed LORR. However, in order to demonstrate quantitatively that the more potent anesthetic effects on the righting reflex in Scn8a medJ/medJ and Scn8a 9J/9J mice were not due to the ataxic phenotype, we compared theta power, an independent measure of electroencephalographic and behavioral arousal, between the mutants and the littermate controls at the point of LORR. The Scn8a 9J/9J mice and littermate controls showed an equivalent decrease in theta power at LORR produced by isoflurane (64% for Scn8a 9J/9J vs 58% for littermates) and sevoflurane (12% for Scn8a 9J/9J vs 17% for littermates), which indicates that the observed sensitivity is due to anesthetic effects on arousal rather than the movement abnormalities. Similarly, the Scn8a medJ/medJ mice and littermate controls showed a comparable decrease in theta power produced by isoflurane (46% for Scn8a medJ/medJ vs 32% for littermates). The decrease in theta power was 3-fold greater for Scn8a medJ/medJ mice compared to littermate controls for sevoflurane-induced LORR (11% for Scn8a medJ/medJ vs 35% for littermates).
Influence of reduced Nav1.6 activity on the recovery time from anesthesia
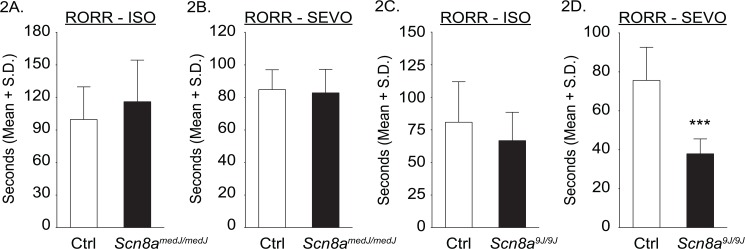
The time required for emergence from anesthesia (recovery of righting reflex, RORR) was not significantly different between Scn8a medJ/medJ mice and littermate controls [Isoflurane: Mean ± S.D. (95% CI) = 116s ± 38 (81–152) for Scn8a medJ/medJ vs 100s ± 30 (72–127) for littermate control group, t = 0.89, df = 12, p = 0.39; Sevoflurane 83s ± 14 (69–96) for Scn8a medJ/medJ vs 85s ± 12 (74–96) for littermate control group, t = 0.28, df = 12, p = 0.78] (Fig 2A and 2B). The time required for emergence from anesthesia for the Scn8a 9J/9J mice showed an anesthetic dependent effect. There was no significant difference between Scn8a 9J/9J mice and littermate controls for isoflurane [67s ± 22 (47–87) for Scn8a 9J/9J vs 81s ± 31 (52–110) for littermate control mice, t = 0.98, df = 12, p = 0.35)], whereas the Scn8a 9J/9J mice emerged faster from sevoflurane anesthesia [38s ± 7.7 (31–45)] as compared to the control mice [76s ± 17 (60–91)] (t = 5.4, df = 12, p = 0.0002; 95% CI for the difference between means = 22–53) (Fig 2C and 2D).
Fig 2. Effect of Scn8a medJ and Scn8a 9Jmutations on the time to emergence from isoflurane and sevoflurane anesthesia.
Compared to the littermate control mice, there was no significant difference in the time to emergence—return of righting reflex (RORR)—from isoflurane anesthesia (ISO) for either Scn8a medJ/medJ (A) or Scn8a 9J/9J (C) mice, or from sevoflurane anesthesia (SEVO) for Scn8a medJ/medJ (B) mice. The mice with Scn8a 9J mutation (D) emerged significantly faster from sevoflurane anesthesia. Seven mice were used for each group. Data were combined from two trials for each anesthetic and for each mouse. ***p = 0.0002 compared to littermate control mice (Ctrl). S.D.—standard deviation.
Reduced Nav1.6 activity depresses EEG theta power during the baseline wake state
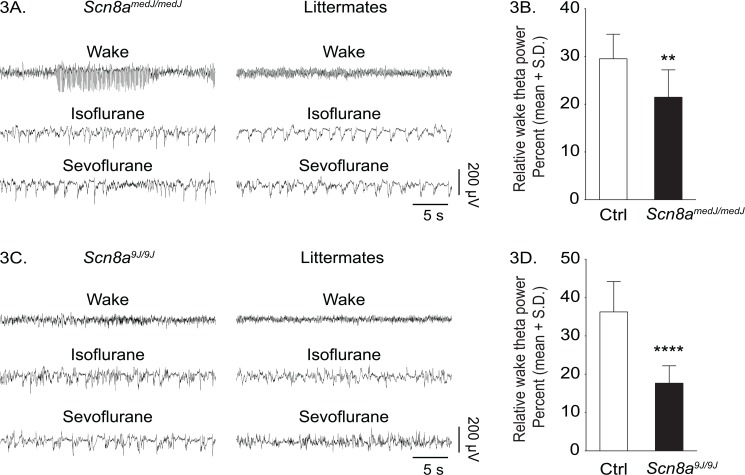
Since alterations of sleep homeostasis can affect sensitivity to inhaled anesthetics [27,31], we assessed signs of sleep deprivation in the mutant mice during pre-anesthesia baseline wake state. Increased theta power during wake state is a reliable marker for rodents with increased propensity to sleep [40]. Therefore, we analyzed the raw EEG and compared the EEG theta power during pre-anesthesia baseline waking state between the mutant mice and the respective littermate controls. Scn8a medJ/medJ mice showed bursts of spike-wave discharge activity during waking, which is indicative of epileptiform EEG, although no overt seizures were observed (Fig 3A). These spike-wave discharges occurred exclusively in association with low muscle tone, as determined by electromyography. The EEG transitioned from high-frequency/low-amplitude pattern during waking to low-frequency/high-amplitude EEG after exposure to anesthesia. In contrast, Scn8a 9J/9J mice showed a normal wake-related high-frequency/low-amplitude pattern, which transitioned to low-frequency/high-amplitude EEG after exposure to anesthesia (Fig 3C). Spectral analysis showed that the theta power in Scn8a medJ/medJ mice was significantly lower than the littermate controls during baseline waking state [Mean ± S.D. (95% CI) = 21% ± 5.7 (18–25) for Scn8a medJ/medJ vs 30% ± 5.1 (26–33) for littermate group, t = 3.7, df = 24, p = 0.001; 95% CI for the difference between means = 3.6–13] (Fig 3B). A similar reduction in the theta power was observed for Scn8a 9J/9J mice [18% ± 4.6 (15–20) for Scn8a 9J/9J vs 36% ± 7.9 (32–41) for littermate control group, t = 7.6, df = 26, p<0.0001; 95% CI for the difference between means = 14–24] (Fig 3D). Reduced theta power in the waking state excludes sleep deprivation as the cause of increased anesthetic sensitivity in the mutant mice.
Fig 3. Effect of Scn8a medJ and Scn8a 9Jmutations on qualitative and quantitative changes in EEG.
Representative EEG traces (frontal-parietal derivation) during wake, isoflurane and sevoflurane anesthesia from Scn8a medJ/medJmice and the littermate control group (A), and Scn8a 9J/9J mice and the littermate control group (C). Both Scn8a medJ/medJ (B) and Scn8a 9J/9J (D) mice show significantly lower theta (4–10Hz) power during waking. **p = 0.001, ****p<0.0001 compared to littermate control group (Ctrl). S.D.—standard deviation.
Discussion
We demonstrate that decreased Nav1.6 activity, either by reduction of Nav1.6 protein in Scn8a medJ/medJ mice or by an amino acid deletion in Scn8a 9J/9J mice, increases sensitivity to volatile anesthetics in vivo. Based on our finding of reduced theta activity in the waking state, this sensitivity may result from a decrease in neuronal excitability associated with reduced Nav1.6 activity (reviewed in [10]). Theta power during the waking state is a surrogate for arousal [32,33] and has been shown to increase with increased cortical excitability [41]. The observed decrease in theta power during waking in the Scn8a medJ/medJ and Scn8a 9J/9J mice is consistent with previous evidence that reduction in Nav1.6 activity due to hypomorphic mutations results in decreased excitability in many types of neurons (see Table 1 in [10]).
We also observed frequent bursts of spike-wave discharges in the EEG of Scn8a medJ/medJ mice without any evidence of behavioral seizures. These spike-wave discharges disappeared during the isoflurane- or sevoflurane-induced unconscious state. Similar spike-wave discharges associated with immobility and indicative of absence epilepsy have been reported for other Scn8a alleles (Scn8a 8J, Scn8a med, and Scn8a med-jo) [42,43]. Further studies are needed to confirm the presence of absence epilepsy and the underlying neurobiology for the occurrence of spike-wave discharges in Scn8a medJ/medJ mice. These discharges are likely to be influenced by genetic modifiers contributed by strain C3H to the Scn8a medJ/medJ F1 background [42,43].
Hysteresis is observed in the general anesthetic concentrations at which an organism loses and regains consciousness, with higher doses required to induce unconsciousness than to maintain it. There is increasing evidence that this asymmetry is not mediated by simple pharmacokinetic mechanisms, but rather by distinct neural systems that control the induction of and emergence from general anesthesia [29,30]. While the Nav1.6 mutations increased sensitivity to induction of anesthesia, they did not affect the time to emergence from anesthesia. This asymmetry has two-fold significance. First, it suggests that the effects of the mutation play a specific role in anesthetic state transitions and that the sensitivity does not merely reflect compromised systemic physiology. Physiological compromise would be expected to increase sensitivity during both the induction and recovery phases of anesthesia. Of note, Nav1.6 is expressed at a very low level in the cardiovascular system, which further precludes the possibility of a non-specific systemic effect on the arousal state of these mice [44]. Second, it supports the emerging evidence for a distinction between the neurobiology of anesthetic induction and emergence [30,45]. Further work testing the effects of stepwise anesthetic down-titration during emergence will be required to confirm the observation in this model.
There are a number of limitations to this study. First, the mutant mice exhibit ataxia and dystonia which are potential confounds in the determination of anesthetic-induced unconsciousness based on righting reflex. We addressed this problem by conducting simultaneous EEG recording and used the appearance of high-voltage/low-frequency EEG, consistent with an unconscious state [31,35–38], to confirm our assessment of the onset of LORR. Our approach was validated by high reproducibility of LORR data across trials (separated by 4–5 days). Second, although both isoflurane and sevoflurane caused a well-documented reduction in theta activity at the point of LORR [32,33], the extent of theta power depression showed variability across genotypes and anesthetics. Both mutants differed from their genetically matched littermate controls in response to isoflurane and sevoflurane, demonstrating that these differences are not specific to strain background and instead reflect differences in effects of the sodium channel mutations on anesthetic pharmacodynamics. Furthermore, strain background did not influence the identification of unconscious states because determination of anesthetic-induced unconsciousness was made in real time while theta power was calculated off-line after the experiment was completed. Third, we observed a large difference in EC50% for littermate controls on different strain backgrounds. The strain background was C57BL/6J for the Scn8a 9J mutation and (C57BL/6J X C3H)F1 for the Scn8a medJ mutation, which is lethal at 3 weeks on the C57BL/6J background due to a variant in a splicing factor in strain C57BL/6J [14,26,46]. The difference in EC50% between littermate controls from these strains may be related to genetic polymorphisms as previously described for minimum alveolar concentration [47,48]. In our study the genotype of the littermates is identical to the genotype of the mutants. All F1 mice have identical genotypes, with one chromosome inherited from each inbred parent. The C57BL/6J background is likewise identical for the 9J mutants and littermate controls.
Conclusion
We investigated mice with mutations in Scn8a to determine the unique contribution of the sodium channel Nav1.6 to the in vivo response to general anesthetics. The large effects of these mutations on both anesthetic drug sensitivity and baseline theta power suggest that Nav1.6 plays a significant role in maintaining arousal. This effect on drug sensitivity may be relevant to clinical management of patients with early infantile epileptic encephalopathy due to mutations in SCN8A [49]. Analysis of mice with reduced activity of Nav1.1 and Nav1.2 would be valuable for defining the contributions of each of the major neuronal sodium channels to arousal and the response to volatile anesthetics.
Supporting Information
(PDF)
Acknowledgments
The authors would like to thank Brian H. Silverstein of the University of Michigan for assistance with the power spectral density analysis, and Chris Andrews of the University of Michigan Center for Statistical Consultation and Research for help with statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by NIH R01 NS34509 (M.M.), NIH RO1 GM098578 (G.A.M.) and the Department of Anesthesiology (G.A.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schaller KL, Krzemien DM, Yarowsky PJ, Krueger BK, Caldwell JH. A novel, abundant sodium channel expressed in neurons and glia. J Neurosci. 1995;15(5 Pt 1):3231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tzoumaka E, Tischler AC, Sangameswaran L, Eglen RM, Hunter JC, Novakovic SD. Differential distribution of the tetrodotoxin-sensitive rPN4/NaCh6/Scn8a sodium channel in the nervous system. J Neurosci Res. 2000;60(1):37–44. [DOI] [PubMed] [Google Scholar]
- 3. Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol. 2008;100(4):2361–80. 10.1152/jn.90332.2008 [DOI] [PubMed] [Google Scholar]
- 4. Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12(8):996–1002. 10.1038/nn.2359 [DOI] [PubMed] [Google Scholar]
- 5. Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97(10):5616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28(53):14329–40. 10.1523/JNEUROSCI.4833-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328(5980):906–9. 10.1126/science.1187958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21(7):2268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci. 2007;27(49):13552–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Brien JE, Meisler MH. Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front Genet. 2013;4:213 10.3389/fgene.2013.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19(4):881–91. [DOI] [PubMed] [Google Scholar]
- 12. Osorio N, Cathala L, Meisler MH, Crest M, Magistretti J, Delmas P. Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells. J Physiol. 2010;588(Pt 4):651–70. 10.1113/jphysiol.2010.183798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enomoto A, Han JM, Hsiao CF, Chandler SH. Sodium currents in mesencephalic trigeminal neurons from Nav1.6 null mice. J Neurophysiol. 2007;98(2):710–9. [DOI] [PubMed] [Google Scholar]
- 14. Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, et al. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant 'motor endplate disease'. Nat Genet. 1995;10(4):461–5. [DOI] [PubMed] [Google Scholar]
- 15. Kohrman DC, Harris JB, Meisler MH. Mutation detection in the med and medJ alleles of the sodium channel Scn8a. Unusual splicing due to a minor class AT-AC intron. J Biol Chem. 1996;271(29):17576–81. [DOI] [PubMed] [Google Scholar]
- 16. Kearney JA, Buchner DA, De Haan G, Adamska M, Levin SI, Furay AR, et al. Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6). Hum Mol Genet. 2002;11(22):2765–75. [DOI] [PubMed] [Google Scholar]
- 17. Herold KF, Hemmings HC Jr. Sodium channels as targets for volatile anesthetics. Front Pharmacol. 2012;3:50 10.3389/fphar.2012.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiraishi M, Harris RA. Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. J Pharmacol Exp Ther. 2004;309(3):987–94. [DOI] [PubMed] [Google Scholar]
- 19. Yokoyama T, Minami K, Sudo Y, Horishita T, Ogata J, Yanagita T, et al. Effects of sevoflurane on voltage-gated sodium channel Na(v)1.8, Na(v)1.7, and Na(v)1.4 expressed in Xenopus oocytes. J Anesth. 2011;25(4):609–13. 10.1007/s00540-011-1167-7 [DOI] [PubMed] [Google Scholar]
- 20. Ouyang W, Herold KF, Hemmings HC, Jr. Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110(3):582–90. 10.1097/ALN.0b013e318197941e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rehberg B, Xiao YH, Duch DS. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 1996;84(5):1223–33; discussion 27A. [DOI] [PubMed] [Google Scholar]
- 22. Raju SG, Barber AF, LeBard DN, Klein ML, Carnevale V. Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLoS Comput Biol. 2013;9(6):e1003090 10.1371/journal.pcbi.1003090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barber AF, Carnevale V, Klein ML, Eckenhoff RG, Covarrubias M. Modulation of a voltage-gated Na+ channel by sevoflurane involves multiple sites and distinct mechanisms. Proc Natl Acad Sci U S A. 2014;111(18):6726–31. 10.1073/pnas.1405768111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodgson PS, Liu SS, Gras TW. Does epidural anesthesia have general anesthetic effects? A prospective, randomized, double-blind, placebo-controlled trial. Anesthesiology. 1999;91(6):1687–92. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Guzinski M, Eger EI 2nd, Laster MJ, Sharma M, Harris RA, et al. Bidirectional modulation of isoflurane potency by intrathecal tetrodotoxin and veratridine in rats. Br J Pharmacol. 2010;159(4):872–8. 10.1111/j.1476-5381.2009.00583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buchner DA, Trudeau M, Meisler MH. SCNM1, a putative RNA splicing factor that modifies disease severity in mice. Science. 2003;301(5635):967–9. [DOI] [PubMed] [Google Scholar]
- 27. Tung A, Szafran MJ, Bluhm B, Mendelson WB. Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by propofol and isoflurane. Anesthesiology. 2002;97(4):906–11. [DOI] [PubMed] [Google Scholar]
- 28. Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–86. 10.1038/nrn2372 [DOI] [PubMed] [Google Scholar]
- 29. Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105(4):1309–14. 10.1073/pnas.0707146105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One. 2010;5(7):e11903 10.1371/journal.pone.0011903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pal D, Lipinski WJ, Walker AJ, Turner AM, Mashour GA. State-specific effects of sevoflurane anesthesia on sleep homeostasis: selective recovery of slow wave but not rapid eye movement sleep. Anesthesiology. 2011;114(2):302–10. 10.1097/ALN.0b013e318204e064 [DOI] [PubMed] [Google Scholar]
- 32. Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115(4):791–803. 10.1097/ALN.0b013e31822e92e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A. 2014;111(10):3859–64. 10.1073/pnas.1310025111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vanini G, Nemanis K, Baghdoyan HA, Lydic R. GABAergic transmission in rat pontine reticular formation regulates the induction phase of anesthesia and modulates hyperalgesia caused by sleep deprivation. Eur J Neurosci. 2014;40(1):2264–73. 10.1111/ejn.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638–50. 10.1056/NEJMra0808281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mashour GA, Pal D. Interfaces of sleep and anesthesia. Anesthesiol Clin. 2012;30(2):385–98. 10.1016/j.anclin.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 37. Mhuircheartaigh RN, Warnaby C, Rogers R, Jbabdi S, Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5(208):208ra148 10.1126/scitranslmed.3006007 [DOI] [PubMed] [Google Scholar]
- 38. Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110(12):E1142–51. 10.1073/pnas.1221180110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pal D, Walton ME, Lipinski WJ, Koch LG, Lydic R, Britton SL, et al. Determination of minimum alveolar concentration for isoflurane and sevoflurane in a rodent model of human metabolic syndrome. Anesth Analg. 2012;114(2):297–302. 10.1213/ANE.0b013e31823ede22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050(1–2):64–71. [DOI] [PubMed] [Google Scholar]
- 41. Mangia AL, Pirini M, Cappello A. Transcranial direct current stimulation and power spectral parameters: a tDCS/EEG co-registration study. Front Hum Neurosci. 2014;8:601 10.3389/fnhum.2014.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papale LA, Beyer B, Jones JM, Sharkey LM, Tufik S, Epstein M, et al. Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Hum Mol Genet. 2009;18(9):1633–41. 10.1093/hmg/ddp081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oliva MK, McGarr TC, Beyer BJ, Gazina E, Kaplan DI, Cordeiro L, et al. Physiological and genetic analysis of multiple sodium channel variants in a model of genetic absence epilepsy. Neurobiol Dis. 2014;67:180–90. 10.1016/j.nbd.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noujaim SF, Kaur K, Milstein M, Jones JM, Furspan P, Jiang D, et al. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J. 2012;26(1):63–72. 10.1096/fj.10-179770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joiner WJ, Friedman EB, Hung HT, Koh K, Sowcik M, Sehgal A, et al. Genetic and anatomical basis of the barrier separating wakefulness and anesthetic-induced unresponsiveness. PLoS Genet. 2013;9(9):e1003605 10.1371/journal.pgen.1003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howell VM, Jones JM, Bergren SK, Li L, Billi AC, Avenarius MR, et al. Evidence for a direct role of the disease modifier SCNM1 in splicing. Hum Mol Genet. 2007;16(20):2506–16. [DOI] [PubMed] [Google Scholar]
- 47. Sonner JM, Gong D, Li J, Eger EI 2nd, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg. 1999;89(4):1030–4. [DOI] [PubMed] [Google Scholar]
- 48. Sonner JM, Gong D, Eger EI 2nd. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91(3):720–6. [DOI] [PubMed] [Google Scholar]
- 49. Wagnon JL, Meisler MH. Recurrent and non-recurrent mutations of SCN8A in epileptic encephalopathy. Front Neurol. 2015;6:104 10.3389/fneur.2015.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.