Abstract
Telomeres are nucleoprotein complexes protecting the physical ends of linear eukaryotic chromosomes and therefore helping to ensure their stability and integrity. Additionally, telomeric sequences can be localized in non-terminal regions of chromosomes, forming so-called interstitial telomeric sequences (ITSs). ITSs are traditionally considered to be relics of chromosomal rearrangements and thus very informative in the reconstruction of the evolutionary history of karyotype formation. We examined the distribution of the telomeric motifs (TTAGGG)n using fluorescence in situ hybridization (FISH) in 30 species, representing 17 families of squamate reptiles, and compared them with the collected data from another 38 species from literature. Out of the 68 squamate species analyzed, 35 possess ITSs in pericentromeric regions, centromeric regions and/or within chromosome arms. We conclude that the occurrence of ITSs is rather common in squamates, despite their generally conserved karyotypes, suggesting frequent and independent cryptic chromosomal rearrangements in this vertebrate group.
Introduction
Telomeres are nucleoprotein complexes that protect the physical ends of linear eukaryotic chromosomes, playing a crucial role in maintaining chromosome stability and integrity [1]. In all vertebrates the DNA component of telomeres consists of the non-coding (TTAGGG)n motif [2], which produce long tandem repetitions varying greatly in size between species, individuals and even cell types [3]. The telomeric motif demonstrates a remarkable evolutionary conservation across vertebrate species [4,5]. The telomere-specific complex associated with the telomeric sequence has been described as "shelterin" [6]. Shelterin is composed of three proteins (TRF1, TRF2 and POT1) that directly recognize the (TTAGGG)n motif, and are interconnected by three additional proteins (TIN2, TPP1 and Rap1) to form a duplex structure [7] (for a review see [3]). The telomeric motif is synthesized by telomerase, a reverse transcriptase-like enzyme, which contains an RNA subunit and a catalytic protein subunit called telomerase reverse transcriptase [8]. Telomerase uses the RNA template to add additional sequences directly to the telomeres [9]. In humans telomerase is expressed in embryonic tissues and specific germline cells whilst in adults, the enzyme can be detected mainly in the testis, and is absent in most normal somatic cells, in non-dividing oocytes and mature spermatozoa [10,11].
The main role of telomeres is to protect the edges of the linear chromosomes from degradation, recombination or fusion, preventing the chromosomal ends from being recognized as double-strand brakes by DNA repair machinery [3]. Furthermore, the DNA replication machinery cannot completely replicate the ends of linear chromosomes as there would not be any template strands to guide its synthesis ("end replication problem") [12]. In each cell division 50–200 bp are erased from the edges of the chromosomes decreasing the chromosome length and eventually affecting the inner genetic loci. Telomerase preserves the edge of the chromosomes by adding "expendable" telomeric motifs de novo [3]. However, in several cell types, such as human somatic cells, telomeres become shorter after subsequent replications [13], resulting in a minimum amount of telomeric sequence, leading to replicative senescence and ultimately cell death [14]. This phenomenon has been described as the "telomere hypothesis of cellular aging", a theory that proposes that telomeres serve as a "mitotic clock" controlling lifespan [15].
An additional role of telomeres is the maintenance of the chromosome topology in the nucleus matrix and the correct alignment of chromosomes for recombination during the first meiotic prophase [16–18]. Another important function of telomeres is the silencing of adjacent genes, a phenomenon known as "telomere position effect" [19,20].
As well as the crucial role of telomeres at the edges of chromosomes, non-terminal telomeric motifs known as interstitial telomeric sequences (ITSs) [21] or interstitial telomeric repeats (ITRs) [3], have been observed in many species. The pioneer publication by [5] provided the first cytogenetic evidence of this, reporting that 55 out of the 100 studied species of vertebrates had ITSs. Many more cases were described in the following years in vertebrates, including amphibians [22], fish [23], birds [24], rodents [25–27], marsupials [28–30] and primates [31].
Based on sequence organization and genomic location, Ruiz-Herrera et al. [21] identified two different types of ITSs: short ITSs (s-ITSs) and heterochromatic ITSs (het-ITSs). Other authors have classified ITSs in more detailed categories as short ITSs, long subtelomeric ITSs, fusion ITSs and pericentromeric ITSs [32]. S-ITSs are short sized telomeric repetitions located in internal sites of chromosomes, present in all completely sequenced mammalian genomes (at least 83 in human, 79 in chimpanzee, 244 in mouse and 250 in rat), but often not detectable by cytogenetic techniques such as fluorescent in situ hybridization (FISH) [21]. It was initially thought that s-ITSs were derived from the telomeric fusion of ancestral chromosomes [33]. However, recent studies concluded that s-ITSs are not in fact associated with chromosomal rearrangements [34] but instead were probably inserted by telomerase during the repair of DNA double-strand breaks [21,35,36]. This hypothesis is supported by the frequent association of transposable elements such as SINEs and LINEs with s-ITSs [37].
Het-ITSs are large stretches of telomeric sequences (up to hundreds of kb) localized mainly in heterochromatic chromosomal regions such as in centromeric or pericentromeric areas or within the chromosome arms. In contrast to s-ITSs, het-ITSs are only present in a limited number of species and it is widely believed that they correspond to the remnants of ancestral chromosomal rearrangements which occurred during karyotype evolution [38,39].
As far as we know, ITSs have been described in only 22 lizard species and never in snakes [5,40–53]. In general, squamate reptiles are often considered as a group with evolutionary conserved karyotypes. This view has been supported by classical cytogenetics techniques such as conventional staining and C-banding (e.g. [54–56]) as well as by chromosome painting [57–60], gene mapping [45,61,62], and qPCR mapping of genes linked to sex chromosomes [63–66].
Considering that particular types of ITSs represent relics of chromosome rearrangements, the conservation of karyotypes in squamates suggests that ITSs should be relatively rare in this group. In order to test this hypothesis, we reviewed published data on the occurrence of ITSs and supplemented it with our novel description of ITSs distribution in 13 species of squamates based on FISH experiments.
Material and Methods
Specimens and chromosomal preparations
The distribution pattern of telomeric motifs was studied in the karyotypes of 30 species of squamate reptiles (28 lizards and 2 snakes), belonging to 17 families (Fig 1) from our collection of metaphase chromosome spreads. The specimens originated from pet trade (the companies Animalfarm CZ, Zoopet Sandy, Happy Reptiles, B.A.R. and Zoo Shop Želvička) and were maintained in the reptile breeding laboratory of the Faculty of Science, Charles University in Prague, Czech Republic (accreditation No. 24773/2008-10001). Blood samples were taken from caudal or brachial vessels. The animal procedures were carried out under the supervision and with the approval of the Ethics Committee of the Faculty of Science, Charles University in Prague followed by the Committee for Animal Welfare of the Ministry of Agriculture of the Czech Republic (permission No. 29555/2006-30). Metaphase chromosome spreads were prepared from whole blood cell cultures following the previously described protocol [67] with slight modifications. Briefly, the small amount (approx. 40 μl) of the peripheral blood was cultured for a week at 30°C in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich), enriched with 10% fetal bovine serum (Baria), 0.5% penicillin/streptomycin solution (Gibco), 1% L-glutamine (Sigma-Aldrich), 3% phytohaemagglutinin (Gibco), and 1% lipopolysaccharide (Sigma-Aldrich). Chromosome preparations were made following standard procedures including a 3.5 hours colcemid treatment, hypotonization, and fixation in 3: 1 methanol:acetic acid.
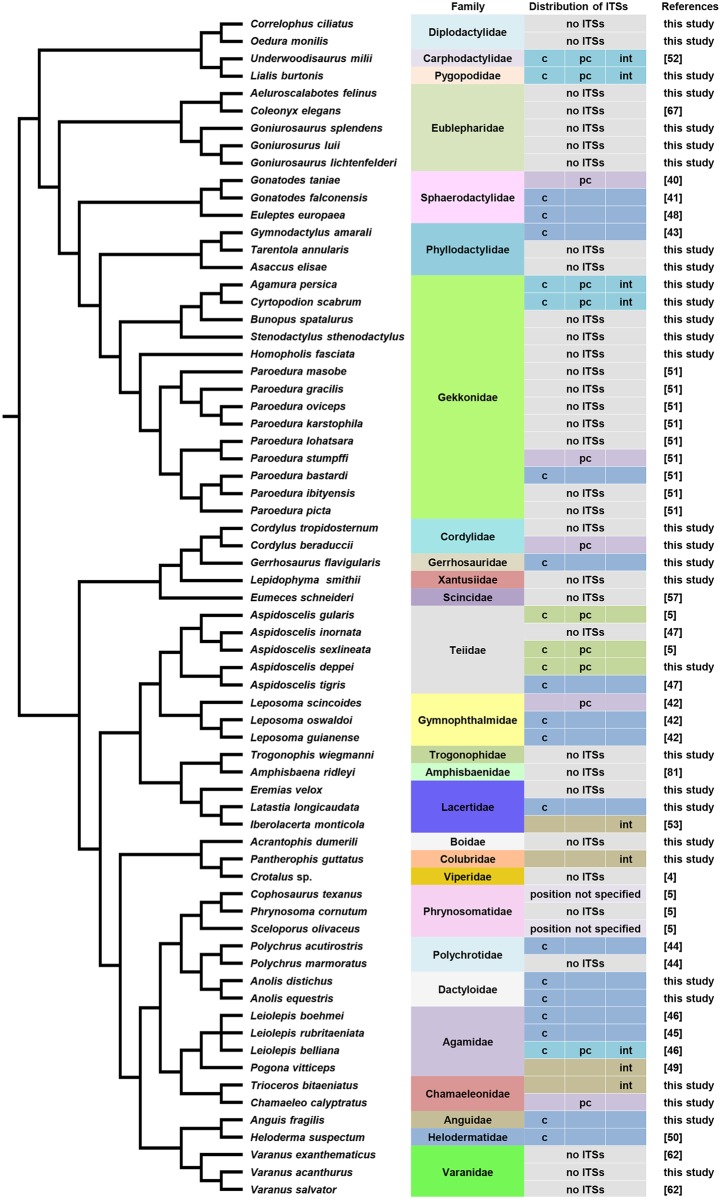
Fig 1. The position of telomeric sequences in 68 species of lizards and snakes.
The telomeric motif (TTAGGG)n was detected in the normal, terminal position of all chromosomes. In addition to the terminal topology, interstitial telomeric repeats (ITSs) were detected in several species within centromeric (c), pericentromeric (pc) and/or intermediate (int) chromosomal regions.
Fluorescent in situ hybridization (FISH)
A specific probe for the telomeric motif (TTAGGG)n was produced and labelled with dUTP-biotin by PCR, using the primers (TTAGGG)5 and (CCCTAA)5, without a DNA template, according to the methodology of Ijdo et al. [68]. Briefly, a PCR reaction was performed in 50 μl final volume, including 0.4 μl of each primer (5 pmol/μl), 5 μl of 10× PCR buffer (Bioline), 2.5 μl MgCl2 (50mM), 1 μl dATP, dCTP, dGTP (10 mM each), 0.7 μl dTTP (10 mM), 1 μl dUTP-biotin (1 mM) and 1 μl BioTaq DNA polymerase (5 U/μl, Bioline). The PCR cycling conditions were as follows: 5 min at 94°C, 10 cycles of 1 min at 94°C, 30 sec at 55°C and 1 min at 72°C, followed by 30 cycles of 1 min at 94°C, 30 sec at 60°C and 30 sec at 72°C, with a final step of 5 min at 72°C. The PCR product was precipitated and re-suspended in 300 μl of hybridization buffer (50% formamide/2× SSC).
Prior to in situ hybridization, 10 μl of the telomeric probe per slide was denatured at 75°C for 10 min and then chilled on ice for 10 min. In parallel, the metaphase slides were subsequently treated with RNase, pepsin, fixed with 4% formaldehyde, dehydrated through a series of 70%, 85% and 100% ethanol washes, denatured in 70% formamide/2× SSC at 75°C for 4 min, dehydrated again and air dried. Afterwards, the probe was applied to each slide and incubated at 37°C for 16–24 hours.
Post-hybridization washes were subsequently carried out in 50% formamide/2× SSC at 42°C (3 × 5 min) and in 2× SSC (2 × 5 min). The slides were incubated in 100 μl of 4× SSC/5% blocking reagent (RocheAρχήφόρμαςTέλοςφόρμας) at 37°C for 45 min. The telomeric signal was detected using a modified avidin-FITC/biotinylated anti-avidin protocol for FITC signal amplification. In detail, we prepared two different solutions: a primary antibody solution with 300 μl of 4× SSC/5% blocking reagent, including 0.3 μl avidin-FITC per slide (Vector laboratories) and a secondary antibody solution with 200 μl of 4× SSC/5% blocking reagent, including 2μl biotinylated anti-avidin per slide (Vector laboratories). The FITC signal was enhanced by five subsequent applications of the primary (three times) and the secondary (two times) antibody solutions at 37°C for 30 min each, using 100 μl of each antibody solution per slide, with intermediate washes in 4× SSC/0.05% Tween20 (3 × 5 min). Afterwards, the slides were dehydrated through an ethanol series, air dried, counterstained with 4',6-diamidino-2-phenylindole (DAPI) and mounted with Vectashield anti-fade medium (Vector Laboratories).
Microscopy and image analyses
An Olympus Provis AX70 fluorescence microscope with a DP30BW digital camera was used to take grayscale images that were processed with DP manager imaging software (Olympus) to record the pattern of the telomeric repeats within the chromosomal metaphases.
Phylogenetic distribution
The phylogenetic distribution of the presence/absence of ITSs across squamate reptiles was visualized using Mesquite v.2.75 [69], based on the phylogenetic tree topology of Pyron et al. [70].
Results
FISH with telomeric probe proved to be a valuable tool in revealing the topology of the telomeric motif (TTAGGG)n in the karyotypes of squamate reptiles. Based on the distribution and the putative origin of the telomeric sequences within the chromosomes we distinguished the following topologies in the karyotypes:
Karyotypes with only terminal distribution of telomeres
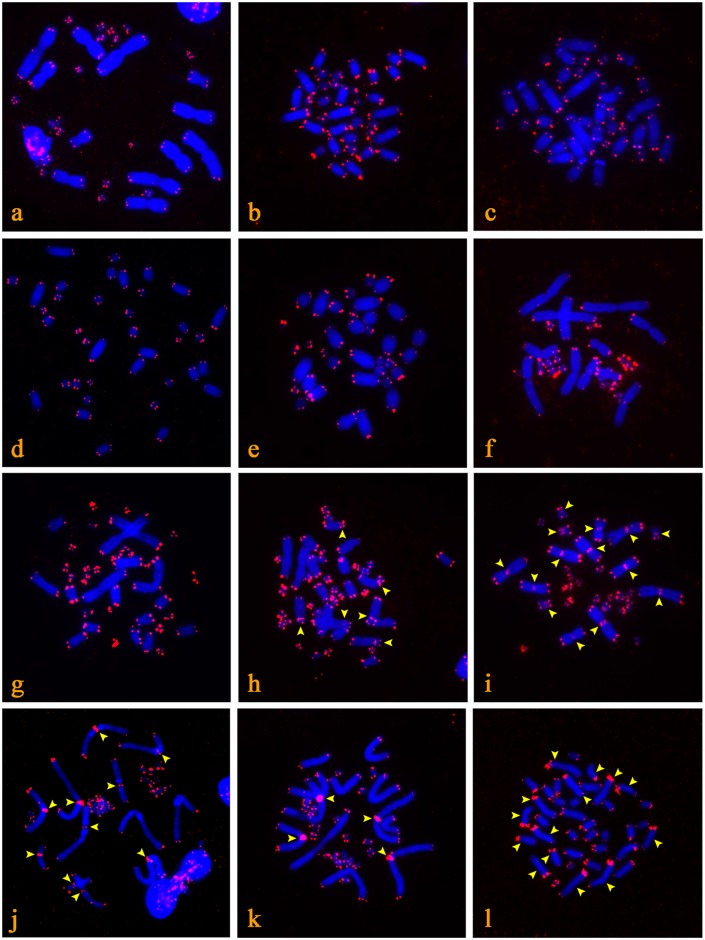
In 17 species we observed telomeric sequences only at the expected terminal positions at the ends of the chromosomes. Specifically, this group includes the species Acrantophis dumerili (Boidae), Cordylus tropidosternum (Fig 2a) (Cordylidae), Correlophus ciliatus (Fig 2b), Oedura monilis (Fig 2c) (Diplodactylidae), Aeluroscalabotes felinus, Goniurosaurus lichtenfelderi, G. luii (Fig 2d), G. splendens (Eublepharidae), Bunopus spatalurus, Homopholis fasciata (Fig 2e), Stenodactylus sthenodactylus (Gekkonidae), Eremias velox (Lacertidae), Asaccus elisae, Tarentola annularis (Phyllodactylidae), Trogonophis wiegmanni (Fig 2f) (Trogonophidae), Varanus acanthurus (Fig 2g) (Varanidae) and Lepidophyma smithii (Xantusiidae).
Fig 2. The position of the telomeric sequences in the chromosomal spreads, as revealed by FISH.
Exclusively terminal distribution: Cordylus tropidosternum (a), Correlophus ciliatus (b), Oedura monilis (c), Goniurosaurus luii (d), Homopholis fasciata (e), Trogonophis wiegmanni (f), Varanus acanthurus (g). ITSs in centromeric regions: Anguis fragilis (h), Anolis distichus (i), Anolis equestris (j), Gerrhosaurus flavigularis (k), Latastia longicaudata (l). Chromosomes are stained with DAPI (blue), while the FITC signal from the telomeric probe is red. Chromosomes with ITSs are indicated by arrows.
Karyotypes with ITSs in centromeric regions
Five species had karyotypes with telomeric motifs at the terminal positions of all chromosomes, and additional ITSs in centromeric regions of one or more chromosomal pairs. In detail, ITSs were detected at the centromeres of three submetacentric pairs in Anguis fragilis (Fig 2h) (Anguidae), seven chromosomal pairs in Anolis distichus (Fig 2i), five chromosomal pairs in Anolis equestris (Fig 2j) (Dactyloidae), two large metacentric pairs in Gerrhosaurus flavigularis (Fig 2k) (Gerrhosauridae) and five chromosomal pairs in Latastia longicaudata (Fig 2l) (Lacertidae).
Karyotypes with ITSs in pericentromeric regions
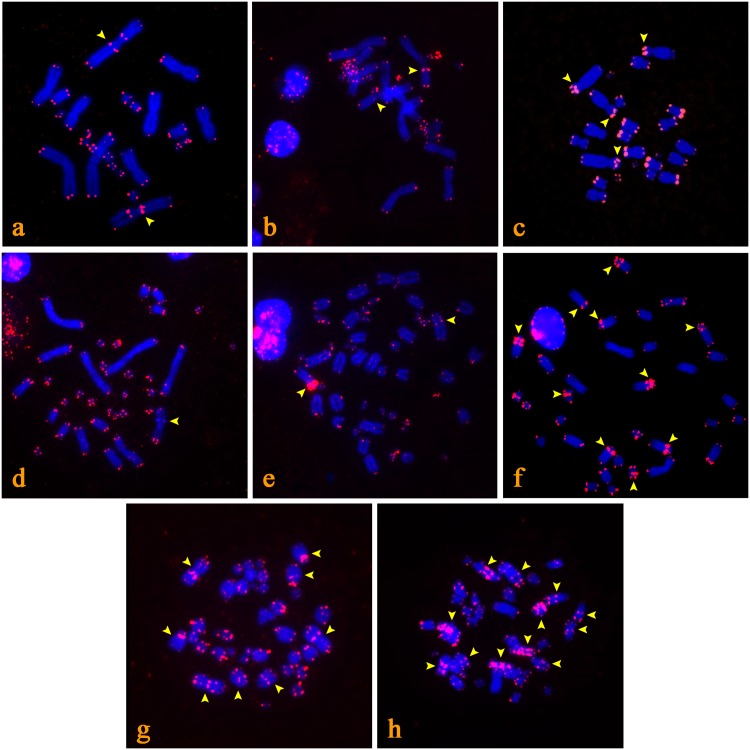
Two lizard species, Chamaeleo calyptratus (Fig 3a) (Chamaeleonidae) and Cordylus beraduccii (Fig 3b) (Cordylidae), had telomeric motifs at the terminal positions of all chromosomes, and additional ITSs in pericentromeric regions of the largest metacentric chromosome pair.
Fig 3. The position of the telomeric sequences in the chromosomal spreads, as revealed by FISH.
ITSs in pericentromeric regions: Chamaeleo calyptratus (a), Cordylus beraduccii (b). ITSs in intermediate positions: Trioceros bitaeniatus (c), Pantherophis guttatus (d). ITSs in numerous positions: Aspidoscelis deppei (e), Lialis burtonis (f), Cyrtopodion scabrum (g), Agamura persica (h). Chromosomes are stained with DAPI (blue), while the FITC signal from the telomeric probe is red. Chromosomes with ITSs are indicated by arrows.
Karyotypes with ITSs within chromosome arms
Two species exhibit ITSs signals between terminal telomeres and the centromeric/pericentromeric regions. In Trioceros bitaeniatus (Fig 3c) (Chamaeleonidae), ITSs are present at intermediate positions on six chromosomal pairs, and in Pantherophis guttatus (Fig 3d) (Colubridae) there is an interstitial telomeric band on a medium sized metacentric chromosome.
Karyotypes with ITSs in numerous positions
Finally, in four species we observed ITSs in numerous positions on chromosomes including all of the above mentioned categories (centromeric, pericentromeric and within the chromosome arms). This extensive accumulation was observed in Aspidoscelis deppei (Fig 3e) (Teiidae), Lialis burtonis (Fig 3f) (Pygopodidae), Cyrtopodion scabrum (Fig 3g) and Agamura persica (Fig 3h) (Gekkonidae).
The phylogenetic distribution of ITSs (Fig 1) suggests that in general ITS emergence/loss is evolutionary dynamic across squamates. A high incidence of ITSs is present in the sister families Teiidae and Gymnophthalmidae, while Iguania also possess the tendency to accumulate ITSs in their genomes, yet ITSs appear to have a rather random distribution across the other squamate lineages.
Discussion
ITSs are present in members of all major lineages of squamates (Fig 1). Taking into account previous publications and our results, only 48.5% of squamates (n = 33 species) demonstrate the normal, expected distribution of telomeres at the edges of all chromosomes. Surprisingly, around half of the studied squamate species (51.5%, n = 35) show ITSs in centromeric regions, pericentromeric regions and/or within chromosomal arms. It therefore appears that the existence of ITSs in squamate genomes is not an exception, but rather a common event (Fig 1). The species with karyotypes demonstrating ITSs seem to be more or less randomly distributed across the phylogeny of squamates, with several families including species with both normal terminal telomeres and ITSs, while a higher incidence of ITSs typifies sister families Teiidae and Gymnophthalmidae and the lineage Iguania (Fig 1). Nevertheless, it should be noted that although our sampling includes members of most major lineages of Squamata, the total number of species tested for the presence of ITSs is still quite small and somewhat patchy, which precludes detailed statistical analyses of ITSs correlations and phylogenetic distribution.
ITSs are commonly observed in centromeric regions of both bi-armed and acrocentric chromosomes. ITSs in the centromeres of bi-armed chromosomes might have originated from the remains of “old” terminal telomeres after Robertsonian fusion (e.g. in the gecko Gymnodactylus amarali; [43]), while the ITSs on the centromeres of acrocentrics may be the result of extensive amplification due to their proximity to satellite sequences. It is well-documented that telomeres can be part of centromere repetitive elements [26,71]. In a recent study [71], it was demonstrated that all centromeres of a vole species exhibit co-localization of ITSs with three other satellite sequences. These ITSs were cloned and sequenced, demonstrating 87% to 94% similarity to the terminal telomeric motif (TTAGGG)n [26]. The extensive amplification of ITSs in the centromeres of acrocentric chromosomes covering a large part of the centromeric region can be observed in several squamates, such as Latastia longicaudata (Fig 2l). However, further studies are needed to reveal the type of repetitive elements co-localized with ITSs in squamates and to show if species with ITSs share a similar content of satellite sequences within their centromeres.
Extended ITSs in intermediate positions in several lizard species may reflect remnants of past intrachromosomal rearrangements. Although sauropsids possess a relatively low rate of interchromosomal rearrangements, it has been shown in birds that intrachromosomal rearrangements occur rather frequently [72–74]. In fact, whereas no interchromosomal rearrangements have been documented in the microchromosomes of the chicken, turkey or zebra finch, there have been numerous intrachromosomal rearrangements recorded in these species [74]. In the same context, several pericentromeric inversions have been discovered in the chromosomal pairs 1–4 of Anolis carolinensis using in situ hybridization with BACs [75]. Furthermore, the comparison between the homologous part of chromosome 15 of the chicken and chromosome X of Anolis carolinensis revealed extensive synteny of the gene content, and numerous intrachromosomal, but few interchromosomal rearrangements in the studied chromosomal region [66]. Evidence for numerous intrachromosomal, but rare interchromosomal rearrangements based on interspecific chromosome painting was recently presented in geckos [76].
In some cases telomeric-like sequences appear to accumulate at the heterochromatic part of sex chromosomes. The exact role of the accumulation of ITSs and satellite sequences (for a review see [77]) on the highly heterochromatic W (e.g. in the gecko Underwoodisaurus milii; [52]) or Y chromosomes remains unclear. It has been speculated that the accumulation of repetitive sequences on one pair facilitates the suppression of recombination between sex chromosome homologues, enabling the accumulation of sexually beneficial mutations on respective sex chromosomes. Some authors however suggest that the repetitive sequences may accumulate near the sex determining locus as a result of the suppression of recombination rather than inducing it ([77] and references within).
Finally, closely related species with similar chromosome morphology seem to possess different patterns of ITSs distribution, e.g. species of the genus Anolis, Cordylus, Paroedura [51] and Leiolepis [45,46] (Fig 1). Such differences could be explained either by the dynamic nature of ITSs (e.g. as part of satellite DNA or transposable elements) or cryptic rearrangements. Many reptile linages, with the exception of their avian clade, show persistent telomerase activity in the somatic tissues of adults which might not only explain their extensive tissue regeneration potential [78], but also the existence of ITSs accumulation at numerous positions in their genome (Fig 1). In fact, telomerase appears to be active in all of the tissues of adult Aspidoscelis sexlineata [78], a species with ITSs accumulation (Fig 1; [5]). Furthermore, skin fibroblasts from the blue racer snake (Coluber constrictor) show increased telomerase activity after a high number of generations in vitro [79]. Moreover, telomere length does not decrease with age in the water python (Liasis fuscus), but instead increases from approximately 7 kb at hatching to 28 kb at adult age [80], providing another exception to the hypothesis of “cellular aging”.
In summary, we detected ITSs for the first time in the genomes of 13 species of squamate reptiles and documented that ITSs were observed in approximately half (35 out of 68) of the species of lizards, snakes and amphisbaenians, e.g. [81], studied so far. Therefore we can conclude that the occurrence of ITSs is surprisingly high in this group of vertebrates which has otherwise stable and conserved karyotypes. This discrepancy suggests that, similar to birds, squamate reptiles may have a rather high rate of intrachromosomal rearrangement and a low rate of interchromosomal rearrangement. The origin of ITSs in some species of squamate reptiles may however be attributed to other factors such as high telomerase activity and/or the repair mechanisms of double-strand breaks, e.g. triggered by the activity of transposable elements. Future studies should be devoted to increasing the taxonomic scope of the testing of ITSs distribution across squamates and to address questions regarding the functional importance of these unusually frequent elements in squamate genomes.
Acknowledgments
The authors would like to express their gratitude to Jan Červenka for the assistance with animal handling, to Petr Ráb for his continuous support and to Ettore Olmo, Marta Svartman and the anonymous reviewer for helpful comments. Christopher Johnson provided valuable language guidance.
Data Availability
All relevant data are within the paper.
Funding Statement
This project was supported by the Czech Science Foundation (506/10/0718) and the Grant Agency of Charles University (GAUK 591712). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blackburn EH, Greider CW (1995) Telomeres. Cold Spring Harbor Laboratory Press. [Google Scholar]
- 2. Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, et al. (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A 85:6622–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolzán AD, Bianchi MS (2006) Telomeres, interstitial telomeric repeat sequences, and chromosomal aberrations. Mutat Res 612:189–214. [DOI] [PubMed] [Google Scholar]
- 4. Meyne J, Ratliff RL, Moyzis RK (1989) Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A 86:7049–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OG, et al. (1990) Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 99:3–10. [DOI] [PubMed] [Google Scholar]
- 6. de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110. [DOI] [PubMed] [Google Scholar]
- 7. Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, et al. (1999) Mammalian telomeres end in a large duplex loop. Cell 97:503–514. [DOI] [PubMed] [Google Scholar]
- 8. Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413. [DOI] [PubMed] [Google Scholar]
- 9. Blackburn EH (2005) Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579:859–862. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. (1997) Telomerase catalytic subunit homologs from fission yeast and humans. Science 277:955–959. [DOI] [PubMed] [Google Scholar]
- 11. Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, et al. (2007) Telomere lengthening early in development. Nat Cell Biol 9:1436–1441. [DOI] [PubMed] [Google Scholar]
- 12. Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41:181–190. [DOI] [PubMed] [Google Scholar]
- 13. Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460. [DOI] [PubMed] [Google Scholar]
- 14. Greider CW (1996) Telomere length regulation. Ann Rev Biochem 65:337–365. [DOI] [PubMed] [Google Scholar]
- 15. Harley CB, Vaziri H, Counter CM, Allsopp RC (1992) The telomere hypothesis of cellular aging. Exp Gerontol 27:375–382. [DOI] [PubMed] [Google Scholar]
- 16. de Lange T (1992) Human telomeres are attached to the nuclear matrix. EMBO J 11:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludérus MEE, van Steensel B, Chong L, Sibon OC, Cremers FF, de Lange T (1996) Structure, subnuclear distribution, and nuclear matrix association of the mammalian telomeric complex. J Cell Biol 135:867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amrichová J, Lukášová E, Kozubek S, Kozubek M (2003) Nuclear and territorial topography of chromosome telomeres in human lymphocytes. Exp Cell Res 289:11–26. [DOI] [PubMed] [Google Scholar]
- 19. Nimmo ER, Cranston G, Allshire RC (1994) Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J 13:3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baur JA, Zou Y, Shay JW, Wright WE (2001) Telomere position effect in human cells. Science 292:2075–2077. [DOI] [PubMed] [Google Scholar]
- 21. Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E (2008) Telomeric repeats far from the ends: mechanisms of origin and role in evolution. Cytogenet Genome Res 122:219–228. 10.1159/000167807 [DOI] [PubMed] [Google Scholar]
- 22. Wiley JE, Meyne J, Little ML, Stout JC (1992) Interstitial hybridization sites of the (TTAGGG)n telomeric sequence on the chromosomes of some North American hylid frogs. Cytogenet Cell Genet 61:55–57. [DOI] [PubMed] [Google Scholar]
- 23. Ocalewicz K (2013) Telomeres in fishes. Cytogenet Genome Res 141:114–125. 10.1159/000354278 [DOI] [PubMed] [Google Scholar]
- 24. Nanda I, Schrama D, Feichtinger W, Haaf T, Schartl M, Schmid M (2002) Distribution of telomeric (TTAGGG)n sequences in avian chromosomes. Chromosoma 111:215–227. [DOI] [PubMed] [Google Scholar]
- 25. Ventura K, Silva MJ, Fagundes V, Christoff AU, Yonenaga-Yassuda Y (2006) Non-telomeric sites as evidence of chromosomal rearrangement and repetitive (TTAGGG)n arrays in heterochromatic and euchromatic regions in four species of Akodon (Rodentia, Muridae). Cytogenet Genome Res 115:169–175. [DOI] [PubMed] [Google Scholar]
- 26. Rovatsos MT, Marchal JA, Romero-Fernández I, Fernández FJ, Giagia-Athanosopoulou EB, Sánchez A (2011) Rapid, independent, and extensive amplification of telomeric repeats in pericentromeric regions in karyotypes of arvicoline rodents. Chromosome Res 19:869–882. 10.1007/s10577-011-9242-3 [DOI] [PubMed] [Google Scholar]
- 27. Nagamachi CY, Pieczarka JC, O'Brien PC, Pinto JA, Malcher SM, Pereira AL, et al. (2013) FISH with whole chromosome and telomeric probes demonstrates huge karyotypic reorganization with ITS between two species of Oryzomyini (Sigmodontinae, Rodentia): Hylaeamys megacephalus probes on Cerradomys langguthi karyotype. Chromosome Res 21:107–119. 10.1007/s10577-013-9341-4 [DOI] [PubMed] [Google Scholar]
- 28. Metcalfe CJ, Eldridge MD, Toder R, Johnston PG (1998) Mapping the distribution of the telomeric sequence (T2AG3)n in the Macropodoidea (Marsupialia), by fluorescence in situ hybridization. I. The swamp wallaby, Wallabia bicolor . Chromosome Res 6:603–610. [DOI] [PubMed] [Google Scholar]
- 29. Metcalfe CJ, Eldridge MD, Johnston PG (2002) Mapping the distribution of the telomeric sequence (T2AG3)n in rock wallabies, Petrogale (Marsupialia: Macropodidae), by fluorescence in situ hybridization. II. The lateralis complex. Cytogenet Genome Res 96:169–175. [DOI] [PubMed] [Google Scholar]
- 30. Metcalfe CJ, Eldridge MD, Johnston PG (2007) Mapping the distribution of the telomeric sequence (T2AG3)n in the Macropodoidea (Marsupialia) by fluorescence in situ hybridization. II. The ancestral 2n = 22 macropodid karyotype. Cytogenet Genome Res 116:212–217. [DOI] [PubMed] [Google Scholar]
- 31. Garagna S, Ronchetti E, Mascheretti S, Crovella S, Formenti D, Rumpler Y, et al. (1997) Non-telomeric chromosome localization of (TTAGGG)n repeats in the genus Eulemur . Chromosome Res 5:487–491. [DOI] [PubMed] [Google Scholar]
- 32. Lin KW, Yan J (2008) Endings in the middle: current knowledge of interstitial telomeric sequences. Mutat Res 658:95–110. [DOI] [PubMed] [Google Scholar]
- 33. Hastie ND, Allshire RC (1989) Human telomeres: fusion and interstitial sites. Trends Genet 5:326–331. [DOI] [PubMed] [Google Scholar]
- 34. Farré M, Ponsà M, Bosch M (2009) Interstitial telomeric sequences (ITSs) are not located at the exact evolutionary breakpoints in primates. Cytogenet Genome Res 124:128–131. 10.1159/000207517 [DOI] [PubMed] [Google Scholar]
- 35. Nergadze SG, Rocchi M, Azzalin CM, Mondello C, Giulotto E (2004) Insertion of telomeric repeats at intrachromosomal break sites during primate evolution. Genome Res 14:1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nergadze SG, Santagostino MA, Salzano A, Mondello C, Giulotto E (2007) Contribution of telomerase RNA retrotranscription to DNA double-strand break repair during mammalian genome evolution. Genome Biol 8:R260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azzalin CM, Nergadze SG, Giulotto E (2001) Human intrachromosomal telomeric-like repeats: sequence organization and mechanisms of origin. Chromosoma 110:75–82. [DOI] [PubMed] [Google Scholar]
- 38. Lee C, Sasi R, Lin CC (1993) Interstitial localization of telomeric DNA sequences in the Indian muntjac chromosomes: further evidence for tandem chromosome fusions in the karyotypic evolution of the Asian muntjacs. Cytogenet Cell Genet 63:156–159. [DOI] [PubMed] [Google Scholar]
- 39. Slijepcevic P (1998) Telomeres and mechanisms of Robertsonian fusion. Chromosoma 107:136–140. [DOI] [PubMed] [Google Scholar]
- 40. Schmid M, Feichtinger W, Nanda I, Schakowski R, Visbal Garcia R, Manzanilla Puppo J, et al. (1994) An extraordinarily low diploid chromosome number in the reptile Gonatodes taniae (Squamata, Gekkonidae). J Hered 85:255–260. [DOI] [PubMed] [Google Scholar]
- 41. Schmid M, Steinlein C, Feichtinger W, Haaf T, Mijares-Urrutia A, Schargel WE, et al. (2014) Cytogenetic studies on Gonatodes (Reptilia, Squamata, Sphaerodactylidae). Cytogenet Genome Res 21:47–61. [DOI] [PubMed] [Google Scholar]
- 42. Pellegrino KCM, Rodrigues MT, Yonenaga-Yassuda Y (1999) Chromosomal evolution in the Brazilian lizards of genus Leposoma (Squamata, Gymnophthalmidae) from Amazon and Atlantic rain forests: banding patterns and FISH of telomeric sequences. Hereditas 131:15–21. [DOI] [PubMed] [Google Scholar]
- 43. Pellegrino KCM, dos Santos RML, Rodrigues MT, Laguna MM, Amaro RC, Yonenaga-Yassuda (2009) Chromosomal evolution in the Brazilian geckos of the genus Gymnodactylus (Squamata, Phyllodactylidae) from the biomes of Cerrado, Caatinga and Atlantic rain forest: evidence of Robertsonian fusion events and supernumerary chromosomes. Cytogenet Genome Res 127:191–203. 10.1159/000295175 [DOI] [PubMed] [Google Scholar]
- 44. Bertolotto CEV, Rodrigues MT, Yonenaga-Yassuda Y (2001) Banding patterns, multiple sex chromosome system and localization of telomeric (TTAGGG)n sequences by FISH on two species of Polychrus (Squamata, Polychrotidae). Caryologia 54:217–226. [Google Scholar]
- 45. Srikulnath K, Matsubara K, Uno Y, Thongpan A, Suputtitada S, Apisitwanich S, et al. (2009) Karyological characterization of the butterfly lizard (Leiolepis reevesii rubritaeniata, Agamidae, Squamata) by molecular cytogenetic approach. Cytogenet Genome Res 125:213–223. 10.1159/000230005 [DOI] [PubMed] [Google Scholar]
- 46. Srikulnath K, Uno Y, Matsubara K, Thongpan A, Suputtitada S, Apisitwanich S, et al. (2011) Chromosomal localization of the 18S-28S and 5S rRNA genes and (TTAGGG)n sequences of butterfly lizards (Leiolepis belliana belliana and Leiolepis boehmei, Agamidae, Squamata). Genet Mol Biol 586:582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lutes AA, Neaves WB, Baumann DP, Wiegraebe W, Baumann P (2010) Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards. Nature 464:283–286. 10.1038/nature08818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gornung E, Mosconi F, Annesi F, Castiglia R (2013) The first cytogenetic description of Euleptes europaea (Gené, 1839) from Northern Sardinia reveals the highest diploid chromosome number among sphaerodactylid geckos (Sphaerodactylidae, Squamata). Comp Cytogenet 7:153–161. 10.3897/CompCytogen.v7i2.4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young MJ, O'Meally D, Sarre SD, Georges A, Ezaz T (2013) Molecular cytogenetic map of the central bearded dragon, Pogona vitticeps (Squamata: Agamidae). Chromosome Res 21:361–374. 10.1007/s10577-013-9362-z [DOI] [PubMed] [Google Scholar]
- 50. Johnson Pokorná M, Rovatsos M, Kratochvíl L (2014) Sex chromosomes and karyotype of the (nearly) mythical creature, the Gila monster, Heloderma suspectum (Squamata: Helodermatidae). PLoS ONE 9:e104716 10.1371/journal.pone.0104716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koubová M, Johnson Pokorná M, Rovatsos M, Farkačová K, Altmanová M, Kratochvíl L (2014) Sex determination in Madagascar geckos of the genus Paroedura (Squamata: Gekkonidae): are differentiated sex chromosomes indeed so evolutionary stable? Chromosome Res 22:441–452. 10.1007/s10577-014-9430-z [DOI] [PubMed] [Google Scholar]
- 52. Pokorná M, Rens W, Rovatsos M, Kratochvíl L (2014) A ZZ/ZW sex chromosome system in the thick-tailed gecko (Underwoodisaurus milii; Squamata: Gekkota: Carphodactylidae), a member of the ancient gecko lineage. Cytogenet Genome Res 142:190–196. 10.1159/000358847 [DOI] [PubMed] [Google Scholar]
- 53. Rojo V, Giovannotti M, Naveira H, Nisi Cerioni P, González-Tizón AM, Caputo Barucchi V, et al. (2014) Karyological characterization of the endemic Iberian rock lizard, Iberolacerta monticola (Squamata, Lacertidae): insights into sex chromosome evolution. Cytogenet Genome Res 142:28–39. 10.1159/000356049 [DOI] [PubMed] [Google Scholar]
- 54. Matthey R (1931) Chromosomes des reptiles, sauriens, ophidiens, chéloniens. L’évolution de la formule chromosomiale chez les sauriens. Rev Suisse Zool 38:117–186. [Google Scholar]
- 55. Gorman GC (1973) The chromosomes of the Reptilia, a cytotaxonomic interpretation In: Chiarelli AB, Capanna E, editors. Cytotaxonomy and Vertebrate Evolution. New York: Academic Press; pp. 349–424. [Google Scholar]
- 56. King M (1991) Chromosome change and speciation in lizards In: Atchley WR, Woodruff DS, editors. Evolution and Speciation. Cambridge: Cambridge University Press; pp. 262–285. [Google Scholar]
- 57. Giovannotti M, Caputo V, O'Brien PC, Lovell FL, Trifonov V, Cerioni PN, et al. (2009) Skinks (Reptilia: Scincidae) have highly conserved karyotypes as revealed by chromosome painting. Cytogenet Genome Res 127:224–231. 10.1159/000295002 [DOI] [PubMed] [Google Scholar]
- 58. Pokorná M, Giovannotti M, Kratochvíl L, Kasai F, Trifonov VA, O'Brien PC, et al. (2011) Strong conservation of the bird Z chromosome in reptilian genomes is revealed by comparative painting despite 275 million years divergence. Chromosoma 120:455–468. 10.1007/s00412-011-0322-0 [DOI] [PubMed] [Google Scholar]
- 59. Pokorná M, Giovannotti M, Kratochvíl L, Caputo V, Olmo E, Ferguson-Smith MA, et al. (2012) Conservation of chromosomes syntenic with avian autosomes in squamate reptiles revealed by comparative chromosome painting. Chromosoma 121:409–418. 10.1007/s00412-012-0371-z [DOI] [PubMed] [Google Scholar]
- 60. Trifonov VA, Giovannotti M, O'Brien PCM, Wallduck M, Lovell F, Rens W, et al. (2011) Chromosomal evolution in Gekkonidae. I. Chromosome painting between Gekko and Hemidactylus species reveals phylogenetic relationships within the group. Chromosome Res 19:843–855. 10.1007/s10577-011-9241-4 [DOI] [PubMed] [Google Scholar]
- 61. Srikulnath K, Nishida C, Matsubara K, Uno Y, Thongpan A, Suputtitada S, et al. (2009) Karyotypic evolution in squamate reptiles: comparative gene mapping revealed highly conserved linkage homology between the butterfly lizard (Leiolepis reevesii rubritaeniata, Agamidae, Lacertilia) and the Japanese four-striped rat snake (Elaphe quadrivirgata, Colubridae, Serpentes). Chromosome Res 17:975–986. 10.1007/s10577-009-9101-7 [DOI] [PubMed] [Google Scholar]
- 62. Srikulnath K, Uno Y, Nishida C, Matsuda Y (2013) Karyotype evolution in monitor lizards: cross-species chromosome mapping of cDNA reveals highly conserved synteny and gene order in the Toxicofera clade. Chromosome Res 21:805–819. 10.1007/s10577-013-9398-0 [DOI] [PubMed] [Google Scholar]
- 63. Gamble T, Geneva AJ, Glor RE, Zarkower D (2014) Anolis sex chromosomes are derived from a single ancestral pair. Evolution 68:1027–1041. 10.1111/evo.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rovatsos M, Altmanová M, Pokorná M, Kratochvíl L (2014) Conserved sex chromosomes across adaptively radiated Anolis lizards. Evolution 68:2079–2085. 10.1111/evo.12357 [DOI] [PubMed] [Google Scholar]
- 65. Rovatsos M, Pokorná M, Altmanová M, Kratochvíl L (2014) Cretaceous park of sex determination: sex chromosomes are conserved across iguanas. Biol Lett 10:20131093 10.1098/rsbl.2013.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rovatsos M, Altmanová M, Johnson Pokorná M, Kratochvíl L (2014) Novel X-linked genes revealed by qPCR in the green anole, Anolis carolinensis . G3-Genes Genom Genet 4:2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pokorná M, Rábová M, Ráb P, Ferguson-Smith MA, Rens W, Kratochvíl L (2010) Differentiation of sex chromosomes and karyotypic evolution in the eye-lid geckos (Squamata: Gekkota: Eublepharidae), a group with different modes of sex determination. Chromosome Res 18:809–820. 10.1007/s10577-010-9154-7 [DOI] [PubMed] [Google Scholar]
- 68. Ijdo JW, Wells RA, Baldini A, Reeders ST (1991) Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res 19:4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org
- 70. Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 13:93 10.1186/1471-2148-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rovatsos MT, Marchal A, Romero-Fernández I, Cano-Linares M, Fernández FJ, Giagia-Athanasopoulou EB, et al. (2014) Molecular and physical characterization of the complex pericentromeric heterochromatin of the vole species Microtus thomasi . Cytogenet Genome Res 144:131–141. 10.1159/000368648 [DOI] [PubMed] [Google Scholar]
- 72. Völker M, Backström N, Skinner BM, Langley EJ, Bunzey SK, Ellegren H, et al. (2010) Copy number variation, chromosome rearrangement, and their association with recombination during avian evolution. Genome Res 20:503–511. 10.1101/gr.103663.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Skinner BM, Griffin DK (2012) Intrachromosomal rearrangements in avian genome evolution: evidence for regions prone to breakpoints. Heredity 108:37–41. 10.1038/hdy.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lithgow PE, O'Connor R, Smith D, Fonseka G, Al Mutery A, Rathje C, et al. (2014) Novel tools for characterising inter and intra chromosomal rearrangements in avian microchromosomes. Chromosome Res 22:85–97. 10.1007/s10577-014-9412-1 [DOI] [PubMed] [Google Scholar]
- 75. Alföldi J, Di Palma F, Grabherr M, Williams C, Kong L, Mauceli E, et al. (2011) The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477:587–591. 10.1038/nature10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Johnson Pokorná M, Trifonov VA, Rens W, Ferguson-Smith MA, Kratochvíl L (2015) Low rate of interchromosomal rearrangements during old radiation of gekkotan lizards (Squamata: Gekkota). Chromosome Res, in press. 10.1007/s10577-015-9468-6 [DOI] [PubMed] [Google Scholar]
- 77. Ezaz T, Deakin JE (2014) Repetitive sequence and sex chromosome evolution in vertebrates. Adv Evol Biol 2014:1–9. [Google Scholar]
- 78. Gomes NMV, Shay JW, Wright WE (2010) Telomere Biology in Metazoa. FEBS Lett 584:3741–3751. 10.1016/j.febslet.2010.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Christiansen JL, Johnson JC, Henderson ER, Budke BJ, Lynch M (2001) The relationship between telomeres, telomerase, reptilian lifespan, and reptilian tissue regeneration. Proceedings of the Iowa Space Grant Consortium 2001:1–10. [Google Scholar]
- 80. Ujvari B, Madsen T (2009) Short telomeres in hatchling snakes: erythrocyte telomere dynamics and longevity in tropical pythons. PLoS ONE 4:e7493 10.1371/journal.pone.0007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laguna MM, Amaro RC, Mott T, Yonenaga-Yassuda Y, Rodrigues MT (2010) Karyological study of Amphisbaena ridleyi (Squamata, Amphisbaenidae), an endemic species of the Archipelago of Fernando de Noronha, Pernambuco, Brazil. Genet Mol Biol 33:57–61. 10.1590/S1415-47572010005000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.