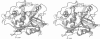Abstract
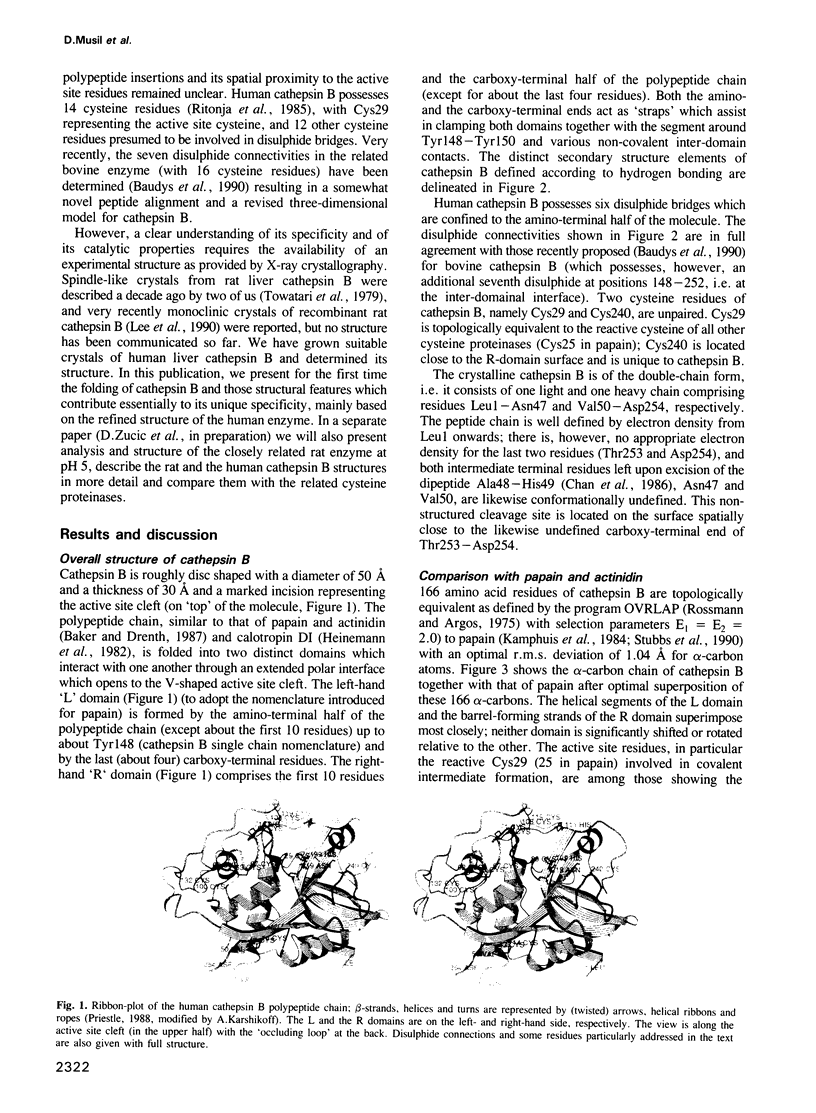
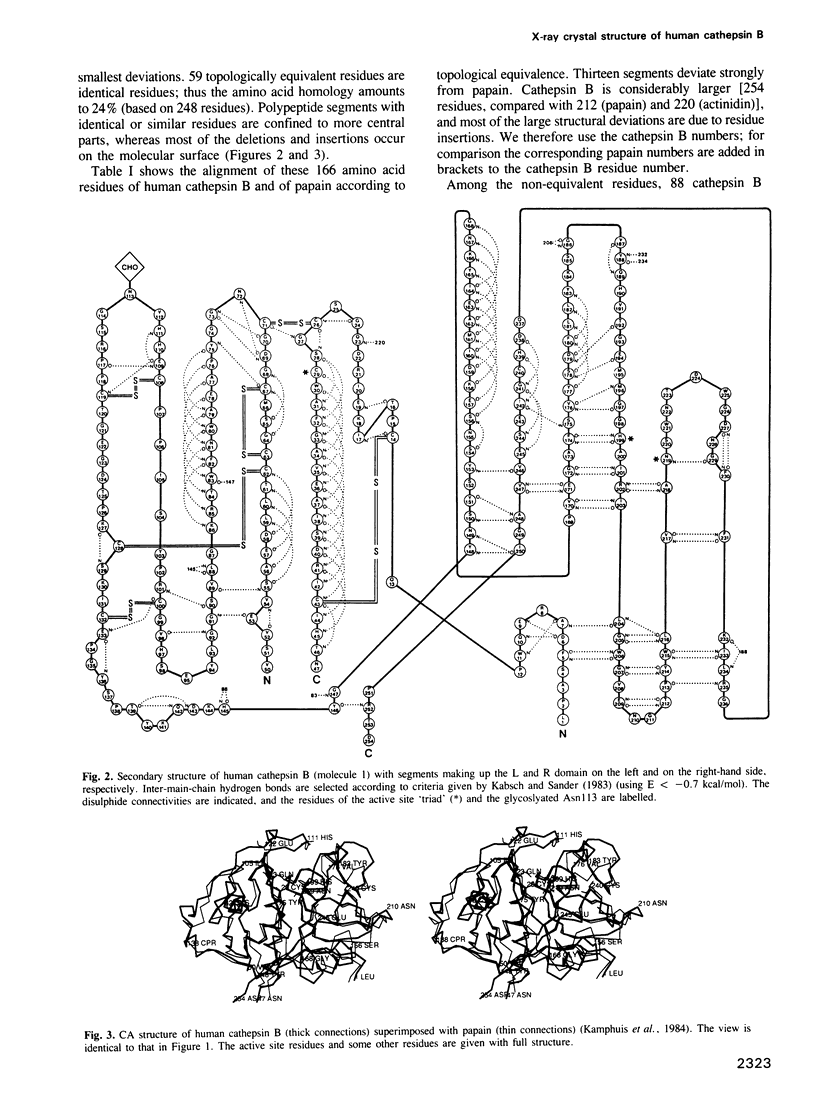
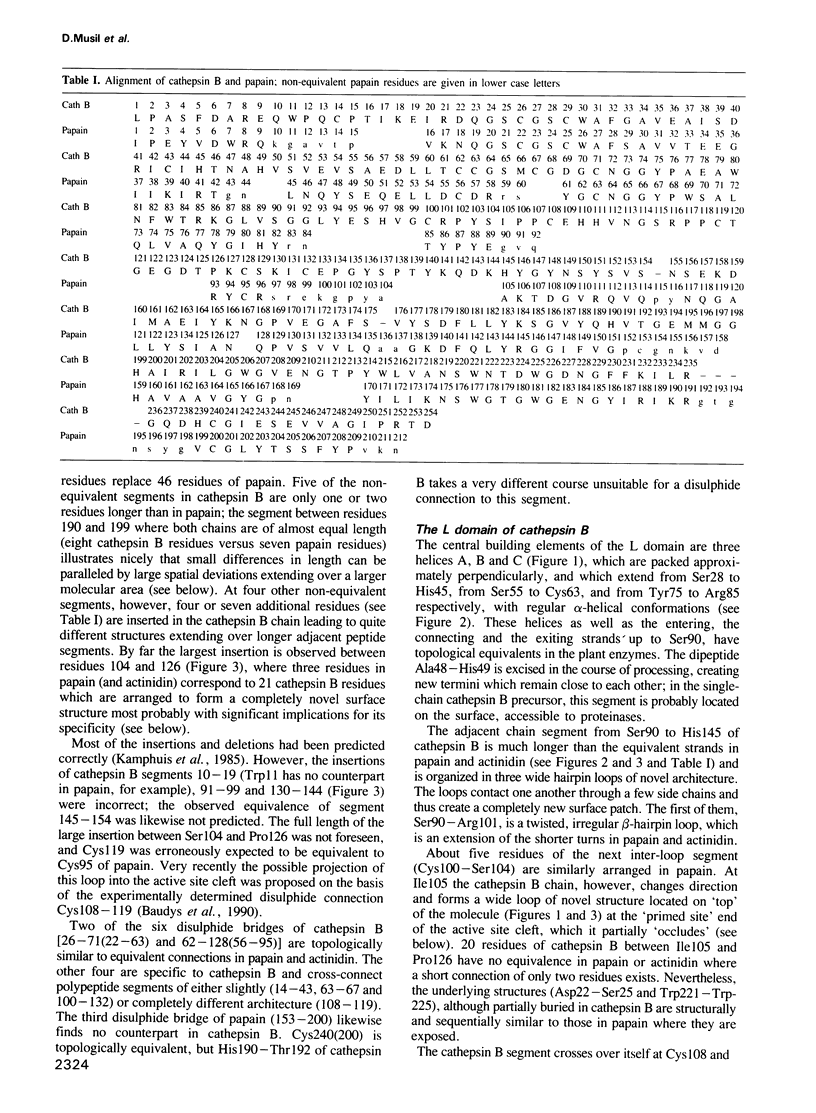
From the lysosomal cysteine proteinase cathepsin B, isolated from human liver in its two-chain form, monoclinic crystals were obtained which contain two molecules per asymmetric unit. The molecular structure was solved by a combination of Patterson search and heavy atom replacement methods (simultaneously with rat cathepsin B) and refined to a crystallographic R value of 0.164 using X-ray data to 2.15 A resolution. The overall folding pattern of cathepsin B and the arrangement of the active site residues are similar to the related cysteine proteinases papain, actinidin and calotropin DI. 166 alpha-carbon atoms out of 248 defined cathepsin B residues are topologically equivalent (with an r.m.s. deviation of 1.04 A) with alpha-carbon atoms of papain. However, several large insertion loops are accommodated on the molecular surface and modify its properties. The disulphide connectivities recently determined for bovine cathepsin B by chemical means were shown to be correct. Some of the primed subsites are occluded by a novel insertion loop, which seems to favour binding of peptide substrates with two residues carboxy-terminal to the scissile peptide bond; two histidine residues (His110 and His111) in this "occluding loop' provide positively charged anchors for the C-terminal carboxylate group of such polypeptide substrates. These structural features explain the well-known dipeptidyl carboxypeptidase activity of cathepsin B. The other subsites adjacent to the reactive site Cys29 are relatively similar to papain; Glu245 in the S2 subsite favours basic P2-side chains. The above mentioned histidine residues, but also the buried Glu171 might represent the group with a pKa of approximately 5.5 near the active site, which governs endo- and exopeptidase activity. The "occluding loop' does not allow cystatin-like protein inhibitors to bind to cathepsin B as they do to papain, consistent with the reduced affinity of these protein inhibitors for cathepsin B compared with the related plant enzymes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Barrett A. J. The specificity of cathepsin B. Hydrolysis of glucagon at the C-terminus by a peptidyldipeptidase mechanism. Biochem J. 1978 Jun 1;171(3):759–765. doi: 10.1042/bj1710759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Baudys M., Meloun B., Gan-Erdene T., Pohl J., Kostka V. Disulfide bridges of bovine spleen cathepsin B. Biol Chem Hoppe Seyler. 1990 Jun;371(6):485–491. doi: 10.1515/bchm3.1990.371.1.485. [DOI] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. S., Barrett A. J. Degradation of fructose-1,6-bisphosphate aldolase by cathepsin B. Biochem J. 1980 Jul 1;189(1):17–25. doi: 10.1042/bj1890017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- Ferrara M., Wojcik F., Rhaissi H., Mordier S., Roux M. P., Béchet D. Gene structure of mouse cathepsin B. FEBS Lett. 1990 Oct 29;273(1-2):195–199. doi: 10.1016/0014-5793(90)81083-z. [DOI] [PubMed] [Google Scholar]
- Fong D., Calhoun D. H., Hsieh W. T., Lee B., Wells R. D. Isolation of a cDNA clone for the human lysosomal proteinase cathepsin B. Proc Natl Acad Sci U S A. 1986 May;83(9):2909–2913. doi: 10.1073/pnas.83.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashida S., Towatari T., Kominami E., Katunuma N. Inhibitions by E-64 derivatives of rat liver cathepsin B and cathepsin L in vitro and in vivo. J Biochem. 1980 Dec;88(6):1805–1811. doi: 10.1093/oxfordjournals.jbchem.a133155. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Pal G. P., Hilgenfeld R., Saenger W. Crystal and molecular structure of the sulfhydryl protease calotropin DI at 3.2 A resolution. J Mol Biol. 1982 Nov 15;161(4):591–606. doi: 10.1016/0022-2836(82)90410-7. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Kalk K. H., Swarte M. B., Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233–256. doi: 10.1016/0022-2836(84)90467-4. [DOI] [PubMed] [Google Scholar]
- Kominami E., Tsukahara T., Hara K., Katunuma N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988 Apr 11;231(1):225–228. doi: 10.1016/0014-5793(88)80736-1. [DOI] [PubMed] [Google Scholar]
- Lee X., Ahmed F. R., Hirama T., Huber C. P., Rose D. R., To R., Hasnain S., Tam A., Mort J. S. Crystallization of recombinant rat cathepsin B. J Biol Chem. 1990 Apr 15;265(11):5950–5951. [PubMed] [Google Scholar]
- Marks N., Berg M. J., Benuck M. Preferential action of rat brain cathepsin B as a peptidyl dipeptidase converting pro-opioid oligopeptides. Arch Biochem Biophys. 1986 Sep;249(2):489–499. doi: 10.1016/0003-9861(86)90026-3. [DOI] [PubMed] [Google Scholar]
- Meloun B., Baudys M., Pohl J., Pavlík M., Kostka V. Amino acid sequence of bovine spleen cathepsin B. J Biol Chem. 1988 Jul 5;263(19):9087–9093. [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Extracellular presence of the lysosomal proteinase cathepsin B in rheumatoid synovium and its activity at neutral pH. Arthritis Rheum. 1984 May;27(5):509–515. doi: 10.1002/art.1780270505. [DOI] [PubMed] [Google Scholar]
- Ménard R., Khouri H. E., Plouffe C., Dupras R., Ripoll D., Vernet T., Tessier D. C., Lalberté F., Thomas D. Y., Storer A. C. A protein engineering study of the role of aspartate 158 in the catalytic mechanism of papain. Biochemistry. 1990 Jul 17;29(28):6706–6713. doi: 10.1021/bi00480a021. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Barrett A. J. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984 Oct 1;223(1):245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Kato K. Intracellular transport and processing of lysosomal cathepsin B. Biochem Biophys Res Commun. 1987 Oct 14;148(1):254–259. doi: 10.1016/0006-291x(87)91103-x. [DOI] [PubMed] [Google Scholar]
- Pohl J., Davinic S., Bláha I., Strop P., Kostka V. Chromophoric and fluorophoric peptide substrates cleaved through the dipeptidyl carboxypeptidase activity of cathepsin B. Anal Biochem. 1987 Aug 15;165(1):96–101. doi: 10.1016/0003-2697(87)90205-3. [DOI] [PubMed] [Google Scholar]
- Polgár L., Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J Biol Chem. 1987 Oct 25;262(30):14448–14453. [PubMed] [Google Scholar]
- Popović T., Brzin J., Kos J., Lenarcic B., Machleidt W., Ritonja A., Hanada K., Turk V. A new purification procedure of human kidney cathepsin H, its properties and kinetic data. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):175–183. [PubMed] [Google Scholar]
- Ritonja A., Popovic T., Turk V., Wiedenmann K., Machleidt W. Amino acid sequence of human liver cathepsin B. FEBS Lett. 1985 Feb 11;181(1):169–172. doi: 10.1016/0014-5793(85)81136-4. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. A comparison of the heme binding pocket in globins and cytochrome b5. J Biol Chem. 1975 Sep 25;250(18):7525–7532. [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- Shaw E., Kettner C. The specificity of cathepsin B. Acta Biol Med Ger. 1981;40(10-11):1503–1511. [PubMed] [Google Scholar]
- Shaw E., Wikstrom P., Ruscica J. An exploration of the primary specificity site of cathepsin B. Arch Biochem Biophys. 1983 Apr 15;222(2):424–429. doi: 10.1016/0003-9861(83)90540-4. [DOI] [PubMed] [Google Scholar]
- Sloane B. F. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990 Apr;1(2):137–152. [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Dehdarani A. H., Schmidt P. G., Tang J. Cathepsins B and H from porcine spleen. Purification, polypeptide chain arrangements, and carbohydrate content. J Biol Chem. 1984 Aug 10;259(15):9874–9882. [PubMed] [Google Scholar]
- Takahashi T., Dehdarani A. H., Yonezawa S., Tang J. Porcine spleen cathepsin B is an exopeptidase. J Biol Chem. 1986 Jul 15;261(20):9375–9381. [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele U., Assfalg-Machleidt I., Machleidt W., Auerswald E. A. N-terminal variants of recombinant stefin B: effect on affinity for papain and cathepsin B. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):125–136. [PubMed] [Google Scholar]
- Towatari T., Kawabata Y., Katunuma N. Crystallization and properties of cathepsin B from rat liver. Eur J Biochem. 1979 Dec;102(1):279–289. doi: 10.1111/j.1432-1033.1979.tb06290.x. [DOI] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochem J. 1985 Apr 15;227(2):521–528. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]