Abstract
Patients with post-traumatic stress disorder (PTSD) can show declines in working memory. A dual-task design was used to determine if these impairments are linked to executive control limitations. Participants performed a Sternberg memory task with either one or four letters. In the dual-task condition, the maintenance period was filled with an arrow flanker task. PTSD patients were less accurate on the working memory task than controls, especially in the dual-task condition. In the single-task condition, both groups showed similar patterns of brain potentials from 300–500 ms when discriminating old and new probes. However, when taxed with an additional task, the event-related potentials (ERPs) of the PTSD group no longer differentiated old and new probes. In contrast, interference resolution processes in both the single- and dual-task conditions of the flanker were intact. The lack of differentiation in the ERPs reflects impaired working memory performance under more difficult dual-task conditions. Exacerbated difficulty in performing a working memory task with concurrent task demands suggests a specific limitation in executive control resources in PTSD.
Keywords: Working memory, PTSD, ERP, dual task, executive function, Sternberg memory task
One critical aspect of executive control is the coordination of multiple cognitive processes. Executive control is required to maintain items relevant to current goals in memory and to selectively focus on goal-relevant items (Garcia-Larrea & Cezanne-Bert, 1998). Top-down attention influences the selection of visual stimuli based on previous experience and current goals, while filtering out distractor stimuli (Bledowski, Prvulovic, Goebel, Zanella, & Linden, 2004; Corbetta & Shulman, 2002; Hopf & Mangun, 2000; Lavie, Hirst, de Fockert, & Viding, 2004). Working memory plays a critical role in guiding these top-down attentional processes by keeping current goals in mind (de Fockert, Rees, Frith, & Lavie, 2001; Downing, 2000; Soto, Heinke, Humphreys, & Blanco, 2005). The interaction between working memory and attention suggests that as working memory load increases, attentional capacity decreases, and in turn, causes working memory performance to decline (Gazzaley, 2011; Pratt, Willoughby, & Swick, 2011).
An ongoing question in cognitive neuroscience is the extent to which different executive control processes can be functionally and neuroanatomically dissociated. For example, factor analysis has demonstrated that response inhibition, set shifting, and working memory updating are separable processes (Miyake et al., 2000). Neuroimaging studies have investigated the “unity and diversity” of executive functions, finding both overlapping and distinct patterns of activation for different interference resolution tasks (e.g., Wager et al., 2005; Nee et al., 2007). One executive function that has been less investigated in these sorts of studies is dual task performance (Miyake & Friedman, 2012) or multitasking, a key function in daily life. Studies of patient populations can reveal potential dissociations in performance relative to controls, providing a powerful method for examining the structure of cognition (Henry, 2006; Pantelis & Maruff, 2002). In the current study, we were interested in how an anxiety disorder might influence cognitive control processes. We used a dual-task design with working memory and conflict monitoring components. By using emotionally neutral stimuli, we can examine whether more general limitations in executive control are seen in individuals with difficulty regulating emotion..
Post-traumatic stress disorder (PTSD) is a specific anxiety disorder that occurs following a traumatic event. PTSD is characterized by three symptom clusters: 1) intrusive memories, such as flashbacks and nightmares, 2) avoidance of activities, people or places as well as general feelings of emotional numbing and 3) hyperarousal symptoms such as increased startle to unexpected noises, bursts of anger and decreased ability to sleep (American Psychiatric Association: DSM-IV, 1994). These symptoms are often associated with decreased motivation, comorbid depression, and blunt affective disposition (Danckwerts & Leathem, 2003). In addition, individuals with PTSD also show impairments in coordinating, inhibiting, and monitoring cognition and behavior (Koso & Hansen, 2006; Leskin & White, 2007; Swick, Honzel, Larsen, Ashley, & Justus, 2012; Vasterling et al., 2012). These limitations in executive control can lead to impairments in multiple aspects of cognition. However, the effects of PTSD on executive control have not been as consistently documented as the well-known difficulties in regulating emotional memory (e.g., Rauch, Shin, & Phelps, 2006). Some studies have reported deficits in working memory (WM) and attention in PTSD (Elzinga & Bremner, 2002; Koso & Hansen, 2006; Vasterling, Brailey, Constans, & Sutker, 1998), while other studies have shown little to no impairment in performance (Brenner, et al., 2010; Neylan, et al., 2003). The current experiment set out to determine if cognitive impairment in WM is linked to executive control limitations by examining performance on a WM task alone and when a secondary attention task was performed during the maintenance period. Exacerbated difficulty while performing a WM task with concurrent task demands would suggest executive control dysfunction in PTSD rather than a general decline in memory (Baddeley, 1996).
The severity of PTSD symptomatology is often related to cognitive dysfunction, specifically to a decline in cognitive control and memory performance (Bremner, et al., 1993; Drag, Spencer, Walker, Pangilinan, & Bieliauskas, 2012; Elzinga & Bremner, 2002; Vasterling, et al., 1998; Vasterling, et al., 2012). Bremner et al. (1993) found a significant decline in both immediate and delayed recall in patients with PTSD compared to military controls using the Wechsler Memory scale. The impairment in WM performance was strongly correlated with symptom severity of re-experiencing the traumatic event (Elzinga & Bremner, 2002). In addition, other studies indicate that re-experiencing is significantly related to impairments in inhibitory control (Swick, et al., 2012; Vasterling, et al., 1998). Deficits in cognitive control, impulsivity and working memory may relate to dysfunction in networks that mediate emotional control (Etkin & Wager, 2007; Aupperle, Allard, Grimes et al., 2012).
The prefrontal cortex (PFC) is thought to be critical for efficient functioning of executive control (Wager & Smith, 2003). Some evidence suggests that PTSD is related to frontal dysfunction because performance on certain tasks is similar to performance of patients with frontal lobe injury, specifically on memory tasks (Vasterling, et al., 1998). Frontal patients may perform well on certain tasks when performed singly, but show difficulty in coordinating multiple processes as evidenced by declines in dual-task performance (Cowey & Green, 1996; Dreher, Koechlin, Tierney, & Grafman, 2008). Other studies have not observed this pattern, however (Andrés & Van der Linden, 2002; Vilkki et al., 1996; Roussel et al., 2012). Regardless of the neuroanatomical underpinnings, cognitive difficulties in patients with PTSD might not necessarily be apparent when testing only one cognitive domain, but might instead be more prominent in tasks that require coordination of multiple elements. However, no studies have examined dual-task performance in PTSD. Here, we focus on WM retrieval and how it is affected by the performance of a demanding visual attention task during the retention interval.
To determine the nature of the neurophysiological changes that might underlie any memory deficits in PTSD patients, we also examined event-related potentials (ERPs) to the probe stimulus in the WM task. Alteration of a relatively early electrophysiological component in the patients might be indicative of problems with item recognition, while later ERP changes could reflect difficulties with decision or post-retrieval monitoring processes (Folstein & van Petten, 2011; Wilding & Herron, 2006). A specific neural marker of memory retrieval processes is the ERP old/new effect. This electrophysiological response consists of a positive shift in the waveform to previously presented items that are correctly recognized, relative to new items that are correctly rejected (Rugg & Curran, 2007). Although typically examined using experimental designs such as study/test list learning (Rugg & Doyle, 1992; Smith, 1993) and continuous recognition (Friedman, 1990; Swick & Knight, 1997), the old/new effect has also been examined using WM and Sternberg tasks (Tays, Dywan, Capuana, & Segalowitz, 2011; Tays, Dywan, Mathewson, & Segalowitz, 2008). In those studies, an array of letters or words was presented, followed after a delay by a probe stimulus. A probe that was correctly identified as being contained within the array (“old”) elicited a greater positivity from approximately 350 to 600 ms than a probe that was not in the array (“new”).
Thus far, no studies have examined ERP old/new effects in individuals with PTSD, either under single- or dual-task conditions. In addition to examining verbal WM performance, the present study incorporated a distracting secondary task to tax cognitive control processes while maintaining a smaller or larger memory set. We adjusted the set size by manipulating the number of items to be remembered (either 1 or 4), and then used a modified version of the Eriksen Flanker task (Eriksen & Eriksen, 1974) to engage selective attention and conflict resolution processes. Participants performed both tasks (Sternberg and Flanker) alone and in conjunction. We predicted that high working memory load would affect attentional performance. Specifically, if items are being maintained in working memory, then fewer resources would be available for the flanker task, thereby resulting in decreased ability to resolve response conflict and diminished accuracy to incongruent flankers (Lavie & de Fockert, 2005). We also predicted that PTSD patients would show a disproportionate decline in WM performance in the dual-task condition. Electrophysiological measures were expected to reflect this decline in performance by showing a reduction in the amplitude of the old/new effect in the dual-task condition, suggesting that the secondary task would disrupt WM retrieval processes in PTSD.
Methods
Participants
Participants were 18 Iraq and Afghanistan combat Veterans diagnosed with PTSD (17 male, 1 female) and 16 Iraq and Afghanistan control Veterans matched in age and gender (15 male, 1 female). None of the enrolled participants reported significant substance abuse or a history of other psychological disorders, excluding depression (as in Seal, et al., 2008). One Veteran with PTSD was unable to complete the experiment and was subsequently dropped from analysis leaving the PTSD group at n=17. For the flanker analysis, five participants (three in the patient group and two in the control group) were excluded due to incorrect task performance. These five participants mistakenly responded to the arrow in the center of the flanker array and not to the centrally presented arrow and were therefore excluded. The final analysis for the working memory tasks yielded n=17 patients and n=16 for controls, while the flanker data yielded n=14 for each group. Fourteen of the seventeen participants with PTSD had attended a clinic for traumatic brain injury (TBI); however, all participants reported no history of TBI involving loss of consciousness greater than 1–2 minutes or any other pre-existing neurological disease. Within the patient group, four participants reported no loss of consciousness, while the remaining 13 reported feeling dazed or experiencing a brief loss of consciousness no longer than 1–2 minutes in duration. A semi-structured clinical interview was conducted, and mild TBI was diagnosed based on patient self-report of the following criteria from the VA/DoD Clinical Practice Guidelines – loss of consciousness (LOC) 30 min or less or altered mental status (e.g., feeling dazed, disoriented, or confused), with post-traumatic amnesia less than 24 hrs (The Management of Concussion/mTBI Working Group, 2009). PTSD diagnosis was based on semi-structured clinical interview using DSM-IV criteria. The diagnoses of mTBI and PTSD were corroborated with available VA medical records, to the fullest extent possible.
All participants were given the PTSD Checklist, Military Version (PCL-M) for DSM-IV (Weathers, et al., 1994) and the Beck Depression Inventory (BDI). The PCL-M is an accepted diagnostic tool for measuring PTSD (Blanchard, et al., 1996). The PCL-M is a 17-item self-report tool that was used to establish the presence of PTSD symptoms in combat-exposed veterans. It has three clusters or subsets: re-experiencing, numbing, and hyperarousal. The BDI is one of the most commonly used self-report screens for major depression which has been validated with well-established psychometric properties (Beck, et al., 1988). As expected, the two groups showed highly divergent scores on these questionnaires (Table 1), indicating greater levels of PTSD and depression symptoms in the patients The groups did not significantly differ in age (PTSD: mean age 33.5 ± 7.2 years; Controls: mean age 36.4 ± 8.6 years, t(31) = 1.070, p=0.29). However, there was a significant difference for education (PTSD: mean years of education: 13.7 ± 1.1; Controls: 14.9 ± 1.9, t(31) = 2.269, p=0.03). See Table 1 for details on demographic data.
Table 1.
Demographic information and self-rating scores for the PTSD Patients and the Controls.
| Patients (n=17) | Controls (n=16) | |
|---|---|---|
| Age (yrs) | 33.5 ± 7 (n.s.) | 36.4 ± 7 |
| Education (yrs) | 13.68 ± 1.10 (**) | 14.94 ± 1.95 |
| Handedness | 16 R, 1 L | 15 R, 1 L |
| Deployed (n) | 17 | 6 |
| Combat (n) | 17 | 2 |
| PCL-M | 57.1 ± 13.0(***) | 24.8 ± 7.3 |
| BDI | 18.5 ± 7.6 (***) | 4.8 ± 5.4 |
Note: The mean ± standard deviation are given for age, education, PCL-M, and BDI. n.s. = not significantly different from controls;
significantly different from controls at p<.005;
significantly different from controls at p<.0001;
R = right; L = left; PCL-M = PTSD checklist, military version; BDI = Beck Depression Inventory.
The Institutional Review Board of the VA Northern California Health Care System approved the experimental protocol, and all participants gave informed consent prior to beginning the experiment. They were paid for transportation plus $20/hour for their participation.
Stimuli and Tasks
Single-Task Condition (Sternberg Memory Task)
Participants were seated in a darkened, sound-attenuated room and were instructed to limit blinking and fixate at the center of a screen. Participants were asked to remember either one consonant (presented for 2000 ms) or four consonants (presented for 3500 ms). After an 8500 ms delay, another consonant was presented (probe). Participants responded with a button press to indicate whether the probe was part of the previous memory set (old) or whether the probe was not part of the memory set (new). For each trial, the set size (one or four) as well as the probe type (old or new) was determined randomly with equal probabilities. There were ten blocks of ten trials each (100 total).
Single-Task Condition (Arrow Flanker Task)
Participants were instructed to respond with a button press to indicate, as quickly and accurately as possible, whether the central arrowhead pointed to the left or the right. Flanking arrows, positioned either above, below, or both above and below the central arrow, could point in either the same (congruent) direction (40% of trials) or different (incongruent) direction (60% of trials). Each flanker stimulus was presented for 200 ms, with the next trial beginning 300 to 500 ms after a response was made. If there was no response, the next trial began 900 ms after stimulus onset. Each participant completed 10 blocks of 60 flanker trials.
Dual-Task Condition (Sternberg Memory Task +Arrow Flanker)
In the dual-task condition, participants were required to perform the arrow flanker task during the delay interval of the Sternberg memory task. Nine flanker trials began 300 to 500 ms following the presentation of each Sternberg memory set. The stimulus parameters were the same as for the single-task flanker described above. The Sternberg probe was then presented 500 ms following the final flanker trial, and participants responded with a button press to indicate whether this item was in the previous memory set. Each of the ten blocks contained ten Sternberg trials, each with nine flanker trials embedded during each delay interval, for a total of 100 Sternberg trials and 900 arrow flankers. The task order was counterbalanced across participants.
EEG Recording
Continuous EEG was recorded from 64 scalp electrodes and two electrodes on the left and right mastoids using the ActiveTwo Biosemi electrode system (BioSemi, Amsterdam, The Netherlands). Four electrodes placed lateral to and below the right and left eyes recorded blinks and eye movements. The EEG was sampled at 512 Hz. Off-line analysis was completed using Brain Vision Analyzer software (Brainproducts, Munich). Data were re-referenced to the averaged mastoids and bandpass filtered from 0.1 to 30 Hz. The EEG was segmented for each trial from 100 ms pre-stimulus to 900 ms post-stimulus onset. EEG was corrected for blinks; eye movements and extraneous artifacts exceeding 150 microvolts were rejected.
Statistical Analysis
Behavioral Performance
Behavioral analyses examined reaction time (RT) and accuracy using repeated measure ANOVAs. Only correct responses to Sternberg probes were used in the RT analysis. The RT data were analyzed using a 2 x 2 x 2 x 2 design with within-subjects factors Task (single or dual), Set Size (one or four), and Probe (old or new), and between-subjects factor Group (PTSD or control). The accuracy analysis examined percentage of correct responses using the same factor design as the RT analysis. The Flanker data were analyzed using a 2 x 3 factor design: Congruence (congruent or incongruent) x Load (single flanker, set size 1, or set size 4). The accuracy analysis examined percentage of correct responses using the same factor design as the RT analysis. In addition, any significant group effects were also followed up with an analysis of covariance to evaluate the effect of education.
Electrophysiological Analysis
The old/new effect was analyzed in 100 ms intervals. The Sternberg probe ERPs were analyzed by taking the mean amplitude of six midline electrodes over time windows of 300–400 ms, 400–500 ms, 500–600 ms, and 600–700 ms, with the factors Task (single or dual), Set Size (one or four), Probe (old or new), Electrode (Fz, FCz, Cz, CPz, Pz, or POz), and Group (controls or PTSD) for correct responses only. To ensure that each averaged ERP represented a sufficient number of artifact-free segments (mean > 40, minimum > 20), effects of Set Size were examined in analyses that collapsed across Probe, and effects of Probe were examined in analyses that collapsed across Set Size. Therefore, we performed two analyses at each time interval using a four way ANOVA (1: Task, Set Size, Electrode X Group; 2: Task, Probe, Electrode X Group).
Results
Behavioral Results: Sternberg
Individuals with PTSD were less accurate than controls on the Sternberg WM task, and their performance was disproportionately impaired in the dual-task condition (Figure 1). This was supported by a main effect of Group [F(1,31)=5.55, p=0.03] and a Task by Group interaction [F(1,31)=4.42, p=0.04]. The PTSD patients were not significantly different from controls in the single-task condition [F(1,31)=2.49, p=.12] but were significantly less accurate on the Sternberg task in the dual-task condition [F(1,31)=6.42, p=0.02], when the demanding flanker task occurred during the WM delay (Figure 1). Accuracy in the PTSD patient group dropped from 93.7% in the single task to 86.7% in the dual task [F(1,16)=13.49, p=.002]. The controls also showed a decline in accuracy, yet the decrease in performance was smaller (single task: 96.5%; dual task: 93.9%), [F(1,15)=11.79, p=.004]. In addition, all participants were less accurate in the dual task compared to the single task, and for new probes compared to old probes (Table 2), as indicated by significant main effects of Task [F(1,31)=20.81, p<0.0001] and Probe [F(1,31)=8.97, p=0.005]. The main effect of Set Size (p=0.11) and the Set Size by Group interaction (p=0.74) were not significant.
Figure 1.
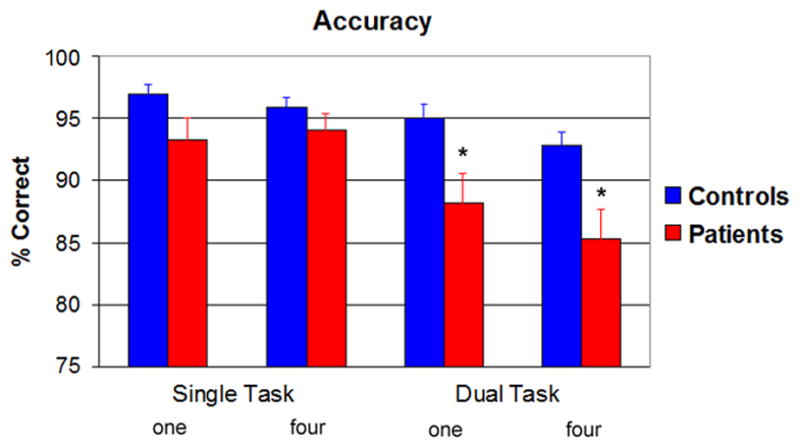
Mean percent correct responses to Sternberg probe items, as a function of Task (single, dual), Set Size (one, four), and Group (controls, patients). Individuals with PTSD were less accurate than controls were at classifying Sternberg probes as old vs. new, particularly for the dual task (p<.05 indicated by *).
Table 2.
Accuracy (percent correct ± SEM) and reaction time (mean ± SEM, in ms) for the controls (n=16) and the participants with PTSD (n=17) in the Sternberg Task.
| Accuracy - Sternberg | ||||
|---|---|---|---|---|
| Single Task | ||||
| Set Size 1 old | Set Size 1 new | Set Size 4 old | Set Size 4 new | |
| Controls | 98.0 ± 0.6 | 95.6 ± 1.3 | 97.4 ± 1.0 | 94.5 ± 1.1 |
| PTSD | 93.9 ± 1.8 | 92.7 ± 2.8 | 97.1 ± 1.3 | 91.1 ± 1.9 |
| Dual Task | ||||
| Set Size 1 old | Set Size 1 new | Set Size 4 old | Set Size 4 new | |
| Controls | 96.6 ± 1.3 | 93.4 ± 2.0 | 95.0 ± 1.4 | 90.6 ± 1.4 |
| PTSD | 90.2 ± 3.7 | 86.1 ± 3.3 | 89.3 ± 2.5 | 81.3 ± 3.8 |
| Reaction Time - Sternberg | ||||
|---|---|---|---|---|
| Single Task | ||||
| Set Size 1 old | Set Size 1 new | Set Size 4 old | Set Size 4 new | |
| Controls | 769 ± 46 | 882 ± 60 | 1049 ± 60 | 1135 ± 95 |
| PTSD | 978 ± 82 | 1102 ± 91 | 1216 ± 84 | 1210 ± 85 |
| Dual Task | ||||
| Set Size 1 old | Set Size 1 new | Set Size 4 old | Set Size 4 new | |
| Controls | 1027 ± 79 | 1119 ± 73 | 1279 ± 72 | 1360 ± 92 |
| PTSD | 1141 ± 91 | 1206 ± 110 | 1391 ± 87 | 1394 ± 115 |
To account for the discrepancy in education between the groups, a covariant analysis was used to examine differences in accuracy in the dual task condition. After adjusting for education, there was a marginal effect between groups [F(1,29) = 3.616, p=0.067]. Adjusted mean accuracy scores suggest PTSD patients performed worse (87.5%) than controls (93.4%). In addition, the correlation between years of education and accuracy in the dual task condition was not significant (r = 0.27, p=.14).
In contrast, the two groups did not differ in their RTs to the memory probe [F(1,31)=1.44, p=.24], nor did Group interact with Task (p=.19), Set Size (p=.16), or Probe (p=.45). Only significant main effects of Task [F(1,31)=42.69, p<0.0001], Set Size [F(1,31)=120.80, p<0.0001], and Probe [F(1,31) = 5.90, p=0.02] were observed (Table 2). Responses were faster in the single task than in the dual task, faster for set size one than for set size four, and faster for old probes than for new probes.
Behavioral Results: Flanker
Table 3 illustrates that the two groups showed highly similar performance. For RTs, the significant main effect of Congruence reflected the classic flanker interference effect: all participants were faster to respond to congruent flankers than to incongruent flankers [F(1,26) = 241.89, p<.0001]. There was neither a main effect of Load (p=.81) nor an interaction between Congruence and Load [F(2,52) = 2.23, p=.12], the latter suggesting that the addition of the working memory task did not significantly alter the flanker interference effect. Importantly, there were no differences between the PTSD patients and controls: Group (p=.99), Load x Group (p=.94), and Congruence x Group (p=.80) for RT.
Table 3.
Accuracy (percent correct ± SEM) and reaction time (mean ± SEM, in ms) for the controls (n=14) and the participants with PTSD (n=14) in the Arrow Flanker Task.
| Accuracy - Flanker | ||||
|---|---|---|---|---|
| Single Task | ||||
| Congruent | Incongruent | |||
| Controls | 95.5 ± 0.7 | 83.6 ± 2.1 | ||
| PTSD | 96.1 ± 0.8 | 83.0 ± 3.6 | ||
| Dual Task | Set Size 1 | Set Size 4 | ||
| Congruent | Incongruent | Congruent | Incongruent | |
| Controls | 97.6 ± 0.5 | 89.3 ± 1.6 | 97.6 ± 0.5 | 89.4 ± 1.6 |
| PTSD | 98.4 ± 0.4 | 86.4 ± 3.1 | 98.8 ± 0.5 | 88.5 ± 1.8 |
| Reaction Time - Flanker | ||||
|---|---|---|---|---|
| Single Task | ||||
| Congruent | Incongruent | |||
| Controls | 453 ± 14 | 508 ± 14 | ||
| PTSD | 455 ± 18 | 504 ± 18 | ||
| Dual Task | Set Size 1 | Set Size 4 | ||
| Congruent | Incongruent | Congruent | Incongruent | |
| Controls | 456 ± 19 | 511 ± 21 | 457 ± 18 | 510 ± 20 |
| PTSD | 450 ± 16 | 512 ± 19 | 454 ± 16 | 516 ± 19 |
The flanker interference effect was also seen for accuracy, with a main effect of Congruence [F(1,26) = 88.59, p<.0001]. In addition, there was a main effect of Load [F(2,52) = 7.39, p=.002]. However, this did not reflect a decline in performance for the dual task, but for a decline in performance in the single task. Although slightly unexpected, this could be explained by the greater number of flanker trials presented in the single task (each block consisting of 60 flanker trials) compared to the dual task (nine consecutive flanker trials between probe and set presentation). Load did not interact with Congruence [F(2,52) = 2.23, p=.23]. Once again, the PTSD patients did not differ from controls: Group (p=.84), Load x Group (p=.36), and Congruence x Group (p=.80).
ERP Results
Beginning with the 300–400 ms window, large effects of Task began to emerge. ERPs were more positive in the dual-task condition compared to the single-task condition across all participants [F(1, 31)=37.6, p<.001]. This Task effect interacted with Electrode [F(5, 155)=32.4, p<.001], being largest at Cz and FCz. Further, ERPs to old probes were more positive in amplitude than those to new probes [F(1, 31)=9.6, p=.004]. This Probe effect interacted with Task and Electrode [F(5, 155)=2.8, p=.05], such that Probe effects were larger at Cz and FCz in the single task, but were more uniform in the dual task. Finally, the analysis including the factor Set Size confirmed that ERPs to set size one were more positive than those to set size four [F(1, 31)=6.2, p=.02]. This Set Size effect interacted with Electrode [F(5, 155)=3.5, p=.03], being largest at Fz.
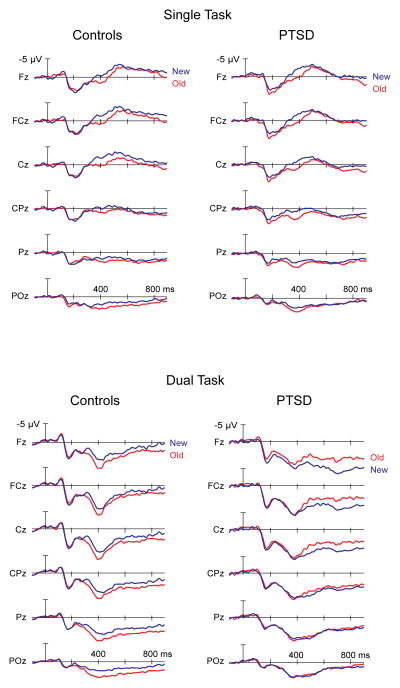
The major finding was that the PTSD patients did not show any differences between ERPs to old and new probes in the dual-task condition. This was supported by a three-way interaction between Task, Probe, and Group [F(1, 31)=12.3, p=.001]. This interaction was explored in follow-up analyses conducted separately on the single- and dual-task conditions. For the single task alone, a strong effect of Probe was observed [F(1, 31)=12.5, p=.001], with more positive measurements for old probes. This effect did not interact with Group for the single task [p=.36] (Figures 2 and 3). For the dual task alone, a main effect of Probe [F(1, 31)=4.0, p=.05] interacted with Group [F(1, 31)=5.3, p=.03]. This interaction was in turn followed up in separate analyses for each Group, which showed that, in the dual-task condition, controls demonstrated a significant effect of Probe [F(1, 15)=7.6, p=.02], consistent with single-task performance in which old probes produced a more positive shift in the waveform (Figure 2). However, individuals with PTSD did not show any distinction between old and new probes in the dual-task condition [p=.81] (Figure 3).
Figure 2.
Event-related potentials time-locked to the onset of the Sternberg probe item, as a function of Task (single, dual), Electrode (six midline electrodes), Probe (old, new), and Group (controls, PTSD). The ERP old/new effect – the positive shift for previously presented (old) probes that are correctly recognized, relative to new probes that are correctly rejected – was observed beginning at 300 ms for both groups in the single-task condition, but only for the controls in the dual-task condition.
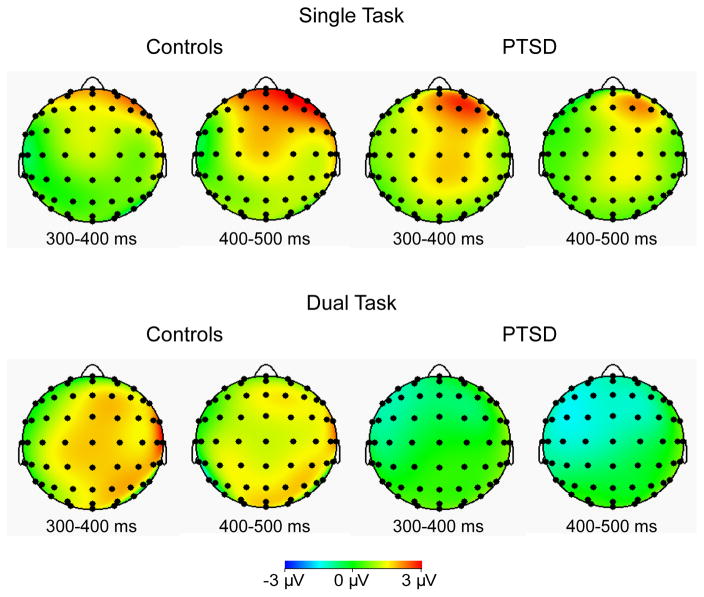
Figure 3.
Topographic plots illustrating the old-new difference wave as a function of Task (single, dual) and Group (controls, PTSD). More positive measurements for previously presented (old) probes, relative to new probes, are indicated by warmer colors.
Largely similar effects and interactions were observed for the 400–500, 500–600, and 600–700 ms window, as shown in Table 4. The main effect of Task, and its interaction with Electrode, remained significant across all the later time windows. The critical interaction between Task, Probe, and Group remained significant through 600 ms, after which it reduced to a trend (Table 4). Follow-up analyses demonstrated a consistent pattern from 300 to 500 ms, such that the interaction was driven by the ERPs of the PTSD group, who demonstrated a statistically flat effect of Probe during the dual-task condition.
Table 4.
| Table 4a. Event-related potential time window analysis - Task, Probe, Electrode X Group Note: Main effects of Electrode are not reported. The remaining interactions not listed above were not significant (all p values > .10). | ||||
|---|---|---|---|---|
| 300–400 ms | 400–500 ms | 500–600 ms | 600–700 ms | |
| Task | F=37.6, p<.001 | F=64.6, p<.001 | F=30.4, p<.001 | F=15.8, p<.001 |
| Task x Electrode | F=32.4, p<.001 | F=38.0, p<.001 | F=14.6, p<.001 | F=11.6, p=.001 |
| Probe | F=9.6, p=.004 | F=5.2, p=.03 | n.s. | n.s. |
| Task x Probe | n.s. | F=7.3, p=.01 | n.s. | n.s. |
| Task x Probe x Electrode | F=2.8, p=.05 | F=3.2, p=.03 | F=2.5, p=.08 | n.s. |
| Group | n.s. | n.s. | n.s. | n.s. |
| Probe x Group | n.s. | F=3.2, p=.08 | F=4.0, p=.05 | F=5.2, p=.03 |
| Task x Probe x Group | F=12.3, p=.001 | F=6.0, p=.02 | F=5.0, p=.03 | F=3.2, p=.08 |
| Probe, single task | F=12.5, p=.001 | F=13.4, p=.001 | n.s. | n.s. |
| Probe x Group, single task | n.s. | n.s. | n.s. | n.s. |
| Probe, dual task | F=4.0, p=.05 | n.s. | n.s. | n.s. |
| Probe x Group, dual task | F=5.3, p=.03 | F=6.3, p=.02 | F=7.9, p=.009 | F=8.6, p=.006 |
| Probe, dual task, controls | F=7.6, p=.02 | F=5.1, p=.04 | F=4.9, p=.04 | F=5.8, p=.03 |
| Probe, dual task, PTSD | n.s. | n.s. | F=3.2, p=.09 | F=3.4, p=.08 |
| Table 4b. Event-related potential time window analysis - Task, Set Size, Electrode X Group Note: Main effects and interactions involving Task, Electrode, and Group are comparable to the preceding analysis and are not repeated here. All remaining interactions were not significant (all p values > .10). | ||||
|---|---|---|---|---|
| 300–400 ms | 400–500 ms | 500–600 ms | 600–700 ms | |
| Set Size | F=6.2, p=.02 | F=7.1, p=.01 | F=3.1, p=.09 | F=8.1, p=.008 |
| Set Size x Electrode | F=3.5, p=.03 | F=4.1, p=.01 | n.s. | F=2.7, p=.07 |
This was also followed up with a covariant analysis to account for group differences in education. After adjusting for education, there remained a significant difference between groups in the magnitude of the old/new effect from 300 to 400 in the dual task condition [F(1,29) = 9.959, p=0.004]. Additionally, there was no significant correlation between years of education and the ERP old/new effect in the dual task condition (r = −0.112, p=0.506).
Discussion
PTSD patients performed similarly to controls and showed comparable electrophysiological differences between old and new probes in the single-task WM condition. However, in the dual-task condition, the patients showed declines in both recognition accuracy and the ERP old/new effect. This finding suggests that limitations in central executive resources contributed to the PTSD patients’ impaired performance in the dual-task condition. The ERP results indicate that the electrophysiological activity underlying working-memory retrieval was intact in the patients, but the addition of a secondary task interfered with the neural processes that support probe recognition. In contrast, the patients were not impaired on either single-task or dual-task versions of the flanker interference task. Interestingly, this intact performance on the flanker task suggests that some forms of inhibitory control were spared in the veterans with PTSD. Furthermore, these results provide evidence for a dissociation between interference resolution processes and dual-task performance during WM.
Baddeley (1996) found that patients with Alzheimer’s disease performed similarly to controls on working memory alone, but showed a significant decline in accuracy with a concurrent attention task. This finding signified that the disruption of performance was related to executive control dysfunction and not necessarily impairment in verbal working memory capacity (Baddeley, 1996; Baddeley, Logie, Bressi, Della Sala, & Spinnler, 1986). Our current findings show similar intact performance on working memory when tested in isolation, yet significant decreases in accuracy when performed in a dual-task condition. This pattern of impaired multitasking performance, compared to relatively preserved performance on the single task and flanker interference task, therefore suggests that individuals with PTSD show disruption to some subset of executive control processes.
Given the intact performance in the flanker interference task in patients with PTSD, the results do not support the concept of a unitary central executive, which is not endorsed by Baddeley (Baddeley, 2000). Indeed, another conception of executive control is that the different functions are fractionated and anatomically dissociable as evidenced by neuroimaging and neuropsychological studies (Stuss, 2011). A recent meta-analysis of the neuroimaging literature identified four different executive component processes within working memory (Nee, et al., 2012). As previously mentioned, latent variable analysis has differentiated working memory updating from task switching and response inhibition, which are considered separate executive functions (Miyake, et al., 2000). The limitations found in the PTSD patient population also support separate, but potentially overlapping, executive control functions. Findings from the current study found multitasking deficits, but only for the working memory condition, and not the attention condition. This suggests that the emotional impairments commonly associated with PTSD deplete resources associated with working memory when participants are required to complete a concurrent task.
One explanation for the current results is that the patients were better able to maintain the items in working memory when there was no distraction, but had difficulties doing so when asked to divide their limited executive resources between the working memory and flanker tasks. The single-task condition relies on only the storage component of WM, while the dual-task condition also invokes distractor resistance, an executive component of WM (Nee et al., 2012). Previous findings have suggested that patients with PTSD rely more strongly on repeating the last few items on a word list as indicated by an increase in recency scores on memory tests compared to controls (Johnsen & Asbjornsen, 2009). If patients with PTSD were more reliant on a rote encoding strategy in the current task, and less efficient at maintaining the stimuli in a longer-term store that would be less susceptible to interference, then the secondary task could have reduced their ability to explicitly rehearse the encoded information. This view is supported by theories suggesting that PTSD symptoms can cause deficits in learning and memory due to an inability to disengage from trauma-related memories, even on neutral, non-trauma related tasks (Vasterling, et al., 1998). In other words, the traumatic memories occupy a central portion of working memory, and an added cognitive task has to compete with the processing of emotionally charged material.
This interpretation suggests that the mechanisms required for maintaining items in working memory during distraction can be compromised by ongoing symptoms of emotional stress or anxiety. The results extend the processing efficiency theory developed by Eysenck & Calvo (1992) to a clinical population. This theory states that trait anxiety reduces the processing and storage capacity of WM, especially when the central executive is required. In turn, greater effort is expended as a compensatory strategy to maintain performance (a reduction in processing efficiency). High-anxious individuals can typically maintain performance effectiveness but are less efficient than controls. In contrast, the current results suggest that the clinically significant anxiety of PTSD causes a reduction in performance effectiveness than cannot be overcome by increased effort, since WM updating was impaired when executive demands were high. Future studies in PTSD patients can examine the operation of top-down attentional control mechanisms (Eysenck et al., 2007; Falies et al., 2008) in this light.
The resolution of response conflict is another major executive control function (Nee et al., 2007). We expected that the PTSD population would have difficulties with an interference resolution task, especially in light of their previously demonstrated deficit in motor response inhibition (Swick et al., 2012). Therefore, we predicted that the patients would show greater RT interference and decreased accuracy relative to controls in the flanker task. However, the patients performed as well as controls in both single- and dual-task versions of the flanker. New research has emerged that supports overlapping, but distinct neural networks associated with different aspects of inhibitory control (Swick, Ashley & Turken, 2011). Sebastiain et al. (2012) suggested that response inhibition processes can be divided into interference inhibition, withholding action response, and canceling current actions (Sebastian, Pohl, Kloppel, et al., 2012). It may be that patients with PTSD show difficulties withholding action responses in a Go/NoGo task (Swick et al., 2012), but not with interference inhibition as demonstrated by intact performance in the flanker task.
Our current results tend to support the view that separate cognitive systems are involved in implementing executive control. Deficits in only one aspect of executive control may contribute to inconsistent neuropsychological testing reported previously (Polak et al., 2012). For example, patients with PTSD performed worse than trauma-exposed controls on the trail-making test and Wisconsin card sorting task, but not on the Stroop task or digit-span backward (Polak et al., 2012). However, the current findings could also reflect differential prioritization of common executive resources in the dual task condition, where performance in the flanker task was more important to the patients than accurate performance in the working memory task. Comparing and contrasting performance in various attention control and inhibition tasks is a new and understudied area of PTSD and executive control research. One challenge to this approach is disentangling task-specific effects from common underlying cognitive processes. Electrophysiological and hemodynamic imaging methods can be helpful in this regard.
We used ERPs to investigate the neural dynamics of WM. Accurate recognition of previously encoded items is generally reflected as a positive shift in the ERP waveform starting around 300 ms (Rugg & Curran, 2007). In the present study, both groups showed comparable ERP effects from 300 ms to 500 ms when distinguishing between old and new probes in the single-task condition, similar to previous reports on the ERP old/new effect (Danker, et al., 2008; Tays, et al., 2011). However, the PTSD group no longer produced ERP differences between old and new probes when taxed with an additional flanker task during the maintenance period. The early onset of the ERP deficit in the patients suggests that their decreased accuracy was a direct result of retrieval difficulties in the dual-task condition, as opposed to problems with later decision processes. This is generally consistent with Weber et al.’s (2005) study examining WM in PTSD patients using a variable target task. ERPs associated with WM updating showed a diminished positive wave in PTSD patients starting around 300 ms over frontal and parietal regions. Weber et al. (2005) suggested that diminished ERP responses from 300 to 900 ms reflected abnormal frontal and parietal activation in patients with PTSD. Specifically, the authors argued that reductions in both frontal and parietal activity suggest that patients with PTSD have difficulties integrating information into WM (Weber, et al., 2005).
In the current study, differences between the PTSD and control groups were found in the frontal-parietal network, but only for the ERPs associated with distinguishing old versus new items under the dual-task condition. The topographic maps indicated a more frontally distributed effect in the single-task condition in both groups. In controls, this effect shifts to central and posterior regions when distinguishing old and new probes in the dual-task condition. PTSD patients show similar frontal scalp distributions in the single-task condition, but fail to show a shift in scalp topography in the dual-task condition. This result may suggest that the frontal and parietal networks necessary to maintain information in WM during distraction, and to successfully retrieve information from WM, are functioning at a limited capacity in PTSD. Specifically, the lack of frontal and parietal activation during the ERP old/new waveform indicates an impairment in recognition when taxed with an additional task. Our findings extend previous reports by Weber and colleagues and suggest that dual-task performance exacerbates WM difficulties often found in PTSD patients.
Previous reports have specifically compared WM deficits in patients with PTSD to patients with frontal lobe damage (Vasterling, et al., 1998; Weber, et al., 2005). Knight and colleagues (1998, 1999) have observed diminished ERPs in patients with dorsolateral PFC damage when updating events in WM (Chao & Knight, 1998; Nielsen-Bohlman & Knight, 1999). Our current findings also suggest diminished cortical activation in both the frontal and parietal lobes in WM updating. However, these results were specific for the dual-task condition.
The deficits in the dual-task condition cannot be attributed solely to task difficulty, because there was no interaction with WM set size nor were deficits found for the flanker task in the dual-task condition. All participants showed increased response times to probes when maintaining a larger set size compared to a smaller set size. Although we expected set size to affect patient performance, our results instead suggest that significant WM impairment was observed only when coordinating more than one task, and was not caused by a general decline in WM capacity, at least for set sizes of one versus four items. Many previous studies have associated WM impairment with PTSD, but have usually used immediate free recall tests, such as California Verbal Learning Test (CVLT), which are more difficult and typically require more than four items to be maintained in WM (for a review see Johnsen & Asbjornsen, 2008). Future studies using dual-task designs may consider increasing the set size to determine whether an interaction between set size and task exists.
One limitation of the present study is the lack of a distractor condition in which participants passively view arrow flanker stimuli during the maintenance period. The current findings are unclear as to whether the disruption in WM performance was due to performance of the secondary flanker task or to the presence of visual distractors. Nonetheless, resistance to external distraction is also considered an executive component of WM (Gazzaley, 2011; Nee, et al., 2012). In accord with this view, patients with prefrontal lesions were impaired in a match-to-sample working memory task only when distractors intervened between the study item and the probe (Chao & Knight, 1998). Future studies including passive presentations of visual distractor stimuli will be critical in evaluating the extent of cognitive impairments using a dual-task design.
Another limitation of the current study is the inability to determine if the deficits in WM dual-task condition are specific to PTSD or the combination of PTSD and depression. The comorbidity rate of depression in patients with PTSD is extremely high (Seal, et al., 2008), and that was also the case in our current population, according to self-report. The focus of this study was to recruit patients with PTSD. Future studies would greatly benefit from taking the comorbidity into account and even including a major depression group with no PTSD. Including a psychiatric control group would better increase our understanding of the deficits associated with PTSD and the deficits that could be attributed to depression. In addition, it would be beneficial to also match the groups based on combat exposure. In the current study, we did not obtain information related to severity of combat exposure. Future studies would benefit from incorporating this into the methods and analysis.
Conclusion
Patients with PTSD were able to perform a WM task in isolation, showing no significant difference from controls. However, when the maintenance period was filled with a distracting task, the PTSD patients declined significantly in WM accuracy and the associated ERPs no longer differentiated old and new probes. However, the patients showed no performance declines in the flanker interference task, either in isolation or in the dual-task condition. This supports research suggesting distinct and separable processes of executive control (Miyake et al., 2000). Deficits in WM dual task performance may suggest that ongoing traumatic memories in PTSD patients are interfering with a portion of working memory. Limitations in WM processes were found in PTSD patients when an added cognitive task was included and the required resources then had to compete with the processing of emotionally charged material. Impairments in executive control have great clinical importance because even subtle deficits can influence coping style and cognitive reappraisal strategies (Vasterling & Verfaellie, 2009). Previous results indicate that dual-task performance is reflective of real-world functioning (McDowell, et al., 1997). Limitations in executive processing may contribute to the inability of individuals with PTSD to disengage from traumatic memories (re-experiencing) and to modulate emotional responses (hyperarousal). These in turn may lead to withdrawal from situations in which executive control is likely to fail (avoidance and numbing) (Aupperle, Melrose, Stein, & Paulus, 2012). The dual-task design presented here is a useful experimental representation of the real-world multitasking deficits and suggests that emotional impairments from an anxiety disorder like PTSD can produce distinct limitations in executive control.
Acknowledgments
We would like to thank Dr. Jary Larsen and Victoria Ashley for their assistance in recruiting our Veteran population. In addition, we would like to thank Devin Adair and Julien Cayton for their assistance with scheduling and running participants, and Adrian Willoughby for task design. This work was supported by the U.S. Army Medical Research and Materiel Command under W81XWH-08-2-0086 and a VA Merit Review grant from Clinical Science Research and Development.
Footnotes
The authors declare that they have no conflicts of interest. The information in this manuscript and the manuscript itself has never been published either electronically or in print. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- Andrés P, Van der Linden M. Are central executive functions working in patients with focal frontal lesions? Neuropsychologia. 2002;40(7):835–45. doi: 10.1016/s0028-3932(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, Cissell SH, Twamley EW, Thorp SR, Norman SB, Paulus MP, Stein MB. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012 Apr;69(4):360–71. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93(24):13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Q J Exp Psychol A. 1986;38(4):603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56(6):893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behaviour research and therapy. 1996;34(8):669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DE. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. The Journal of neuroscience. 2004;24(42):9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, et al. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry. 1993;150(7):1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Terrio H, Homaifar BY, Gutierrez PM, Staves PJ, Harwood JE, et al. Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology. 2010;24(2):160–167. doi: 10.1037/a0017966. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10(2):167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002 Mar;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowey CM, Green S. The hippocampus: a “working memory” structure? The effect of hippocampal sclerosis on working memory. Memory. 1996 Jan;4(1):19–30. doi: 10.1080/741940668. [DOI] [PubMed] [Google Scholar]
- Danker JF, Hwang GM, Gauthier L, Geller A, Kahana MJ, Sekuler R. Characterizing the ERP Old-New effect in a short-term memory task. Psychophysiology. 2008;45(5):784–793. doi: 10.1111/j.1469-8986.2008.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckwerts A, Leathem J. Questioning the link between PTSD and cognitive dysfunction. Neuropsychology Review. 2003;13(4):221–235. doi: 10.1023/b:nerv.0000009485.76839.b7. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychological Science. 2000;11(6):467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- Drag LL, Spencer RJ, Walker SJ, Pangilinan PH, Bieliauskas LA. The contributions of self-reported injury characteristics and psychiatric symptoms to cognitive functioning in OEF/OIF veterans with mild traumatic brain injury. J Int Neuropsychol Soc. 2012;18(3):576–584. doi: 10.1017/S1355617712000203. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3(9):e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70(1):1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & psychophysics. 1974;16(1):143–149. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164:10, 1476. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition & Emotion. 1992;6(6):409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007 May;7(2):336–53. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008 Sep;8(3):239–53. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Folstein JR, van Petten C. After the P3: late executive processes in stimulus categorization. Psychophysiology. 2011;48(6):825–841. doi: 10.1111/j.1469-8986.2010.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biol Psychol. 1990;30(1):61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Cézanne-Bert G. P3, positive slow wave and working memory load: a study on the functional correlates of slow wave activity. Electroencephalogr Clin Neurophysiol. 1998;108(3):260–73. doi: 10.1016/s0168-5597(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49(6):1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD. A meta-analytic review of Wisconsin Card Sorting Test and verbal fluency performance in obsessive-compulsive disorder. Cogn Neuropsychiatry. 2006 Mar;11(2):156–76. doi: 10.1080/13546800444000227. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR. Shifting visual attention in space: an electrophysiological analysis using high spatial resolution mapping. Clinical Neurophysiology. 2000;111(7):1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Johnsen GE, Asbjørnsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111(1):74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Johnsen GE, Asbjørnsen AE. Verbal learning and memory impairments in posttraumatic stress disorder: the role of encoding strategies. Psychiatry research. 2009;165(1):68–77. doi: 10.1016/j.psychres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Koso M, Hansen S. Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. Eur Psychiatry. 2006;21(3):167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, De Fockert J. The role of working memory in attentional capture. Psychonomic Bulletin & Review. 2005;12(4):669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133(3):339. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Leskin LP, White PM. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology. 2007;21(3):275–284. doi: 10.1037/0894-4105.21.3.275. [DOI] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D’Esposito M. Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia. 1997;35(10):1341–1353. doi: 10.1016/s0028-3932(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, et al. A Meta-analysis of Executive Components of Working Memory. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Jasiukaitis PA, Lenoci M, Scott JC, Metzler TJ, Weiss DS, et al. Temporal instability of auditory and visual event-related potentials in posttraumatic stress disorder. Biol Psychiatry. 2003;53(3):216–225. doi: 10.1016/s0006-3223(02)01450-6. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal cortical involvement in visual working memory. Brain Res Cogn Brain Res. 1999;8(3):299–310. doi: 10.1016/s0926-6410(99)00035-x. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Maruff P. The cognitive neuropsychiatric approach to investigating the neurobiology of schizophrenia and other disorders. J Psychosom Res. 2002 Aug;53(2):655–64. doi: 10.1016/s0022-3999(02)00434-8. [DOI] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: A systematic review. Journal of affective disorders. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Pratt N, Willoughby A, Swick D. Effects of working memory load on visual selective attention: behavioral and electrophysiological evidence. Frontiers in Human Neuroscience. 2011:5. doi: 10.3389/fnhum.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Roussel M, Dujardin K, Hénon H, Godefroy O. Is the frontal dysexecutive syndrome due to a working memory deficit? Evidence from patients with stroke. Brain. 2012;135(Pt 7):2192–201. doi: 10.1093/brain/aws132. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Doyle MC. Event-related potentials and recognition memory for low- and high-frequency words. Journal of Cognitive Neuroscience. 1992;4:69–79. doi: 10.1162/jocn.1992.4.1.69. [DOI] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Maguen S, Gima K, Chu A, Marmar CR. Getting beyond “Don’t ask; don’t tell”: an evaluation of US Veterans Administration postdeployment mental health screening of veterans returning from Iraq and Afghanistan. Am J Public Health. 2008;98(4):714–720. doi: 10.2105/AJPH.2007.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Pohl MF, Klöppel S, Feige B, Lange T, Stahl C, Tüscher O. Disentangling common and specific neural subprocesses of response inhibition. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66(10):1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgments. Journal of Cognitive Neuroscience. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Soto D, Heinke D, Humphreys GW, Blanco MJ. Early, involuntary top-down guidance of attention from working memory. Journal of Experimental Psychology: Human Perception and Performance. 2005;31(2):248. doi: 10.1037/0096-1523.31.2.248. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17(5):759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126(Pt 11):2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Swick D, Honzel N, Larsen J, Ashley V, Justus T. Impaired Response Inhibition in Veterans with Post-Traumatic Stress Disorder and Mild Traumatic Brain Injury. J Int Neuropsychol Soc. 2012:1–10. doi: 10.1017/S1355617712000458. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight RT. Event-related potentials differentiate the effects of aging on word and nonword repetition in explicit and implicit memory tasks. J Exp Psychol Learn Mem Cogn. 1997;23(1):123–142. doi: 10.1037//0278-7393.23.1.123. [DOI] [PubMed] [Google Scholar]
- Tays WJ, Dywan J, Capuana LJ, Segalowitz SJ. Age-related differences during simple working memory decisions: ERP indices of early recognition and compensation failure. Brain Res. 2011;1393:62–72. doi: 10.1016/j.brainres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Tays WJ, Dywan J, Mathewson KJ, Segalowitz SJ. Age differences in target detection and interference resolution in working memory: an event-related potential study. J Cogn Neurosci. 2008;20(12):2250–2262. doi: 10.1162/jocn.2008.20158. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12(1):125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Proctor SP, Kane R, Heeren T, Franz M. Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br J Psychiatry. 2012;201(3):186–192. doi: 10.1192/bjp.bp.111.096461. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M. Introduction-posttraumatic stress disorder: a neurocognitive perspective. J Int Neuropsychol Soc. 2009;15(6):826–829. doi: 10.1017/S1355617709990683. [DOI] [PubMed] [Google Scholar]
- Vilkki J, Virtanen S, Surma-Aho O, Servo A. Dual task performance after focal cerebral lesions and closed head injuries. Neuropsychologia. 1996 Nov;34(11):1051–6. doi: 10.1016/0028-3932(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CYC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Huska JA, Keane TM. PTSD Checklist—Military Version (PCL-M) for DSM-IV. Boston: National Center for PTSD—Behavioral Science Division; 1994. [Google Scholar]
- Weber DL, Clark CR, McFarlane AC, Moores KA, Morris P, Egan GF. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Res. 2005;140(1):27–44. doi: 10.1016/j.pscychresns.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Herron JE. Electrophysiological measures of episodic memory control and memory retrieval. Clin EEG Neurosci. 2006;37(4):315–321. doi: 10.1177/155005940603700409. [DOI] [PubMed] [Google Scholar]