Abstract
Background
Access site hematomas and pseudoaneurysms are the most frequent complications of peripheral vascular intervention (PVI); however, their incidence and risk factors remain unclear.
Methods and Results
We retrospectively analyzed data from the multicenter Vascular Quality Initiative® on 22,226 patients who underwent 27,048 PVI from August 2007 to May 2013. Primary endpoints included incidence and predictors of access site complications (ASC), length of postprocedural hospitalization, discharge status, and 30-day and 1-year mortality. ASC complicated 936 procedures (3.5%). Of these, 74.4% were minor complications, 9.7% were moderate requiring transfusion, 5.4% were moderate requiring thrombin injection, and 10.5% were severe requiring surgery. Predictors of ASC were age >75 years, female gender, white race, no prior PVI, nonfemoral arterial access site, >6-Fr sheath size, thrombolytics, arterial dissection, fluoroscopy time >30 minutes, nonuse of vascular closure device, bedridden preoperative ambulatory status, and urgent indication. Mean hospitalization was longer after procedures complicated by ASC (1.2 ± 1.6 days vs. 1.9 ± 1.9 days; range 0-7 days; p=0.002). Severity of ASC correlated with higher rates of discharge to rehabilitation/nursing facilities compared to home discharge. Patients with severe ASC had higher 30-day mortality (6.1% vs. 1.4%; p<0.001), and those with moderate ASC requiring transfusion had elevated 1-year mortality (12.1%, vs. 5.7%; p<0.001).
Conclusions
Several factors independently predict access site complication following peripheral vascular intervention. Appropriate use of antithrombotic therapies and vascular closure device in patients at increased risk of ASC may improve post-PVI outcomes.
Keywords: peripheral vascular intervention, pseudoaneurysm, hematoma, mortality
Approximately 8.5 million Americans over the age of 40 have peripheral artery disease, a disease that increases morbidity and mortality.1 Recent advances in peripheral vascular intervention (PVI) have improved safety and vessel patency, increasing the popularity of percutaneous endovascular treatment modalities for peripheral artery disease over traditional open surgical approaches associated with higher morbidity.2 Since 1995 there has been a tenfold growth in rate of PVI and a simultaneous decrease in surgical vascular interventions.3 Access site complications (ASC), including hematoma associated with and without pseudoaneurysm, is the most frequent PVI complication, occuring in 1.0% to 11% of procedures.4-8 Proposed risk factors of this complication include female gender, advanced age, prior anemia, prior heart failure, low creatinine clearance, rest pain, heparin use and nonuse of a closure device.9 Due to incomplete analysis, inconsistent bleeding definitions and small study populations of patients undergoing PVI, ASC predictors and outcomes are not fully elucidated in the literature. Accordingly, this study evaluated the incidence, predictors and outcomes of periprocedural access site complications in an unselected real-world patient population who underwent PVI.
Methods
Study Population
This retrospective study analyzed data on 22,226 patients who underwent 27,048 PVI procedures from August 2007 to May 2013 in more than 130 centers participating in the Society for Vascular Surgery's Vascular Quality Initiative® (VQI). A description of the VQI has been previously published.10 Complications are site determined, and based on examination of the medical record documentation. Basic automated validation occurs when data field are empty or when a data is outside preset parameters. Further validation occurs by comparing data entered into the VQI database with billing information. There is no external validation done on the data entered into VQI at this time. The Aurora Health Care IRB prospectively approved this study of unidentified data.
Definitions
ASC is defined by the VQI as the presence of a hematoma at the procedural puncture site associated with or without pseudoaneurysm, prior to discharge and classified as one of four types: minor with no therapy employed, moderate necessitating blood transfusion, moderate necessitating thrombin injection, or major for which an operation was performed. Procedural urgency was considered emergent if the patient was treated within hours of presentation, urgent if treatment was expected in the same hospital stay, and elective if it was scheduled on an outpatient basis. Distal embolization was defined as any vascular embolization occurring after PVI and prior to discharge related to either the endovascular procedure or the access site closure. Similarly, access site occlusion refers to access site stenosis or occlusion after PVI and prior to discharge.
Follow-Up
Immediate and in-hospital events were collected by personnel or providers involved in each patient's care at each center participating in the VQI, or via retrospective chart review by designated data entry personnel. Patient mortality rates at 30 days and 1 year were determined by hospital chart review, inclusion in the Social Security Death Index or ascertained in long-term follow-up. The database was searched for all PVI and includes multiple procedures performed on single individuals.
Statistical Methods
Continuous variables are presented as mean ± standard deviation. Chi-square and Fisher's exact tests were used for analysis of categorical variables when appropriate, and the Student's t-test was used for the analysis of continuous variables. Unequal- variance for t test was used to compare length of hospitalization. A multivariable logistic regression analysis was performed to determine the predictors of ASC. For patients with multiple procedures, only data from the first PVI was included in the modeling. Variables significant at the univariate probability level of < 0.05 were entered into the model. These variables included gender, age, race, body mass index, diabetes, dialysis dependence, preoperative ambulatory status, urgency, arterial access site, vascular closure device, access site sheath size used, fluoroscopy time, the use of heparin or bivalirudin, pharmacologic thrombolysis and prior history of PVI. The model was adjusted to account for patients who were not candidates for closure devices (non-femoral vascular access and 4 F sheaths). The presence of ASC, arterial dissection and arterial perforation in addition to the variables utilized in the aforementioned model were entered in a separate model to identify predictors of 30-day and 1-year mortality. Flouroscopy time was included in the survival analysis as a marker of disease severity while vascular closure device and access sheath size used were excluded as they similarly reflect the extent of vascular disease. For all analysis, alpha level ≤ 0.05 was considered significant. All statistical analysis was done using SAS Version 9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline Demographics
Patient demographics are described in Table 1. ASC occurred in 936 (3.5%) PVI procedures, of which 696 (74.4%) were minor, 91 (9.7%) were moderate necessitating transfusion, 51 (5.5%) were moderate necessitating thrombin injection, and 98 (10.5%) were major necessitating operation. Patients with ASC were older and more likely to be white, nondiabetic and have no prior PVI. Female patients had a higher incidence of ASC compared to males (4.28 vs. 2.87; p<0.001). Those on peritoneal dialysis or hemodialysis had lower rates of ASC than other patients (2.51% vs. 3.52; p=0.02).
Table 1.
Patient Demographic and Clinical Characteristics at Time of Procedure
| No Access Site Complication (n=26,112) | Access Site Complication (n=936) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 67.81 ± 11.35 | 69.68 ± 11.37 | <0.0001 |
| ≤ 65 years, % | 38.7 | 33.0 | |
| 65-75 years, % | 31.9 | 31.4 | |
| ≥ 75 years, % | 29.4 | 35.6 | |
| Gender | <0.0001 | ||
| Male, % | 58.5 | 48.2 | |
| Female, % | 41.5 | 51.8 | |
| Race | <0.0001 | ||
| White, % | 83.8 | 88.7 | |
| Black, % | 13.0 | 8.3 | |
| Other, % | 3.3 | 3.0 | |
| Body mass index | 0.0014 | ||
| < 18.5, % | 4.1 | 5.8 | |
| 18.5-24.9, % | 29.6 | 30.7 | |
| 25-29.9, % | 34.1 | 36.4 | |
| > 30.0, % | 32.2 | 27.0 | |
| Smoking status | 0.17 | ||
| No smoking, % | 20.2 | 21.9 | |
| Prior, % | 40.7 | 41.9 | |
| Current, % | 39.2 | 36.3 | |
| History of hypertension, % | 87.3 | 87.2 | 0.94 |
| Diabetic, % | 48.6 | 42.7 | <0.001 |
| Chronic obstructive pulmonary disease, % | 23.8 | 25.9 | 0.15 |
| Coronary artery disease | 0.64 | ||
| None/Asymptomatic, % | 68.0 | 68.7 | |
| Stable angina, % | 10.2 | 10.7 | |
| Prior myocardial infarction or unstable angina, % | 21.8 | 20.6 | |
| Peritoneal dialysis or hemodialysis, % | 7.0 | 5.0 | 0.043 |
| Congestive heart failure | 0.65 | ||
| None/Asymptomatic, % | 93 | 93.1 | |
| Mild, % | 5.1 | 4.6 | |
| Moderate, % | 0.9 | 1.3 | |
| Severe, % | 1.0 | 1.0 | |
| Preoperative ambulatory status | 0.001 | ||
| Ambulatory, % | 79.5 | 79.1 | |
| Ambulatory with assistance, % | 14.8 | 14.8 | |
| Wheelchair bound, % | 5.1 | 4.3 | |
| Bedridden, % | 0.7 | 1.8 | |
| Prior subinguinal bypass, % | 17.5 | 18.3 | 0.53 |
| Prior peripheral vascular intervention, % | 40.2 | 36.8 | 0.038 |
| Prior major or minor lower extremity amputation, % | 4.7 | 3.3 | 0.16 |
| Prior percutaneous coronary intervention, % | 23.0 | 21.6 | 0.43 |
| Prior coronary artery bypass graft, % | 22.0 | 20.9 | 0.58 |
| Preoperative anticoagulants, % | 0.86 | ||
| None, % | 16.8 | 16.6 | |
| Aspirin, % | 72.6 | 72.4 | |
| P2Y12 antagonists, % | 7.4 | 8.1 | |
| Chronic anticoagulation, % | 3.1 | 2.9 |
SD indicates standard deviation.
Procedural Characteristics
Procedures complicated by ASC more often had an indication of claudication, and urgent procedures had higher rates of ASC (6.9%) compared to emergent (3.7%) and elective (3.3%) procedures (Table 2). ASC occurred less frequently in unilateral femoral artery access compared to bilateral femoral artery access sites (3.1% vs. 4.7%; p<0.001), and ASC occurred in 7.9% of procedures in which an arm was used for arterial access. ASC was more frequent in procedures wherein the largest sheath size used was 7-8 Fr compared with those in which 4-6 Fr was used (4.5% vs. 3.2%; p<0.001). Rates of ASC were higher in procedures with fluoroscopy time > 30 minutes compared with those of less duration (4.7% vs. 2.9%; p<0.001). ASC was less frequent in PVI with concomitant common femoral artery endarterectomy than those without (3.6% vs. 2.3%; p=0.003) and in procedures which included pharmacologic thrombolysis (7.5% vs 3.8%; p<0.001). ASC was more common in procedures complicated by arterial perforation (9.4% vs. 3.4%; p<0.001) or arterial dissection (5.1% vs. 3.2%; p<0.001). The use of access guidance, type of pathology and number of arteries treated and technical outcome did not influence ASC.
Table 2.
Procedural Characteristics
| No Access Site Complication (n=26,112) | Access Site Complication (n=936) | P Value | |
|---|---|---|---|
| Indication | 0.042 | ||
| Asymptomatic, % | 3.4 | 2.0 | |
| Claudication, % | 46.7 | 51.4 | |
| Rest pain, % | 12.4 | 13.6 | |
| Tissue loss, % | 28.1 | 23.1 | |
| Acute ischemia, % | 9.4 | 10.0 | |
| Urgency | <0.001 | ||
| Elective, % | 83.5 | 80.3 | |
| Urgent, % | 14.6 | 15.8 | |
| Emergent, % | 1.9 | 4.0 | |
| Type of pathology | 0.081 | ||
| None, % | 3.0 | 2.1 | |
| Occlusive, % | 95.2 | 95.8 | |
| Aneurysmal, % | 1.6 | 2.2 | |
| Both, % | 0.2 | 0.0 | |
| Access guidance | 0.09 | ||
| None, % | 27.1 | 25.6 | |
| Ultrasound, % | 33.0 | 31.1 | |
| Fluoroscopy, % | 32.9 | 36.9 | |
| Cut-down, % | 7.0 | 6.3 | |
| Access site | <0.001 | ||
| Unilateral femoral artery, % | 82.9 | 74.8 | |
| Bilateral femoral artery, % | 14.5 | 20.0 | |
| Arm, % | 1.5 | 3.7 | |
| Graft, % | 0.5 | 0.6 | |
| Popliteal artery, % | 0.3 | 0.1 | |
| Other, % | 0.3 | 0.8 | |
| Number of arteries treated, mean (range) | 1.67 (1 to 6) | 1.73 (1 to 6) | 0.052 |
| Concomitant CFA endarterectomy, % | 7.5 | 4.9 | 0.003 |
| Best technical outcome | 0.97 | ||
| Successful, % | 95.8 | 95.8 | |
| Stenosis ≥ 30% or 10-mm gradient, % | 1.8 | 1.7 | |
| Technical failure, % | 2.4 | 2.5 | |
| Pharmacological Thrombolysis | 3.8 | 7.5 | <0.001 |
| Largest sheath size | 0.003 | ||
| 4 Fr, % | 3.6 | 1.9 | |
| 5 Fr, % | 16.0 | 13.2 | |
| 6 Fr, % | 58.9 | 56.8 | |
| 7 Fr, % | 19.3 | 25.6 | |
| 8 Fr, % | 2.1 | 2.5 | |
| Fluoroscopy time | <0.001 | ||
| < 10 minutes, % | 29.3 | 24.5 | |
| 10-30 minutes, % | 55.2 | 54.4 | |
| > 30 minutes, % | 15.5 | 21.1 | |
| Arterial perforation | <0.001 | ||
| None, % | 99.0 | 97.2 | |
| Iliac artery, % | 0.2 | 1.2 | |
| Femoral or popliteal artery, % | 0.5 | 1.3 | |
| Tibial artery, % | 0.2 | 0.3 | |
| Arterial dissection | <0.001 | ||
| None, % | 94.9 | 92.1 | |
| Iliac artery, % | 1.0 | 2.3 | |
| Femoral or popliteal artery, % | 3.6 | 5.0 | |
| Tibial artery, % | 0.5 | 0.6 | |
| Postprocedural LOS, mean days ± SD (range) | 1.2 ± 1.6 (0-7) | 1.9 ± 1.9 (0-7) | 0.002 |
CFA indicates common femoral artery; LOS, length of stay; SD, standard deviation.
Antithrombotic Medications and Vascular Closure Devices
ASC occurred less frequently in procedures in which bivalirudin antithrombotic therapy was used compared with heparin therapy (3.1% vs. 4.2%; p=0.041). Rates of distal embolization were similar between bivalirudin and heparin use (Table 3), whereas access site occlusions were less frequent in procedures in which bivalirudin was used (0.5% vs. 1.2%; p=0.020). ASC were less frequent in heparinized patients who received vascular closure devices compared with those who did not (2.5% vs. 4.2%; p<0.001) and in patients who received bivalirudin and vascular closure device compared with those who did not (1.5% vs. 2.5%; p=0.008). Closure devices were associated with low rates of all ASC types while only the minor ASC rate was lower in patients who received bivalirudin (Table 4).
Table 3.
Procedural Complications by Antithrombotic and Bleeding Avoidance Strategy
| Heparin | Bivalirudin | Heparin + VCD | Bivalirudin + VCD | |
|---|---|---|---|---|
| Access Site Complication, % | 4.41 | 3.23* | 2.54* | 1.47** |
| Distal embolization, % | 1.87 | 1.17 | 1.85 | 2.83*** |
| Access site occlusion, % | 1.22 | 0.51* | 0.99 | 0.73 |
VCD indicates vascular closure device.
Lower rate than in heparin group (p<0.05).
Lower rate of access site complication than all other groups (p<0.05).
Higher rate than in heparin group (p<0.05).
Table 4.
Complication Rates by Closure Device use and Antithrombotic Agent
| Closure Device | Antithrombotic Agent | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | OR (95% CI) | p | Heparin | Bivalirudin | OR (95% CI) | P | |
| Minor, % | 3.15 | 1.90 | 1.69(1.44-1.98) | <0.001 | 2.68 | 1.78 | 1.53(1.07-2.17) | 0.019 |
| Mod, Transfusion, % | 0.45 | 0.22 | 2.10(1.34-3.30) | 0.001 | 0.33 | 0.38 | 0.89(0.41-1.92) | 0.76 |
| Mod, Thrombin, % | 0.25 | 0.12 | 2.13(1.17-3.89) | 0.014 | 0.21 | 0.11 | 1.96(0.48-8.06) | 0.35 |
| Severe, Surgery, % | 0.47 | 0.25 | 1.92(1.25-2.94) | 0.003 | 0.38 | 0.22 | 1.77(0.65-4.84) | 0.26 |
Mod indicates moderate ASH. OR, odds ratio
Predictors of Access Site Complication
Multivariable analysis of baseline and procedural factors showed that predictors of ASC were age > 75 years, female gender, white race, no prior PVI, nonfemoral artery access site, sheath size greater than 6 Fr, pharmacologic thrombolysis fluoroscopy time > 30 minutes, and nonuse of vascular closure device (Figure 1). The most powerful indicators of ASC were bedridden preoperative ambulatory status (odds ratio 2.40, 95% confidence interval (CI) 1.39 - 4.14), and thrombolytic use (odds ratio 2.00, 95% CI 1.48 - 2.70).
Figure 1.
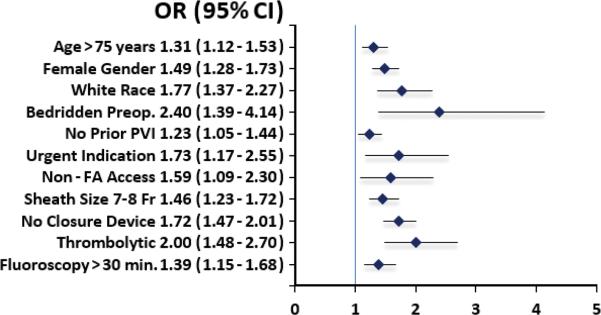
Predictors of access site complication. CI indicates confidence interval; FA, femoral artery; OR, odds ratio; PVI, peripheral vascular intervention.
Patient Outcomes
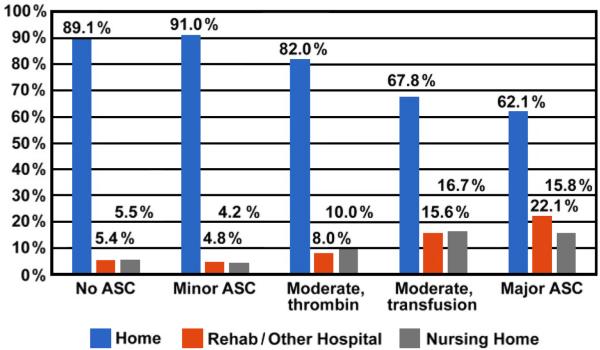
Average hospitalization was longer after procedures complicated by ASC than those that were not (1.9 ± 1.9 days vs. 1.2 ± 1.6 days; range 0-7 days; p=0.002). ASC severity correlated with higher rates of discharge to rehabilitation and nursing homes in patients who were living at home prior to admission (Figure 2).
Figure 2.
Discharge status by type of access site complication (ASC).
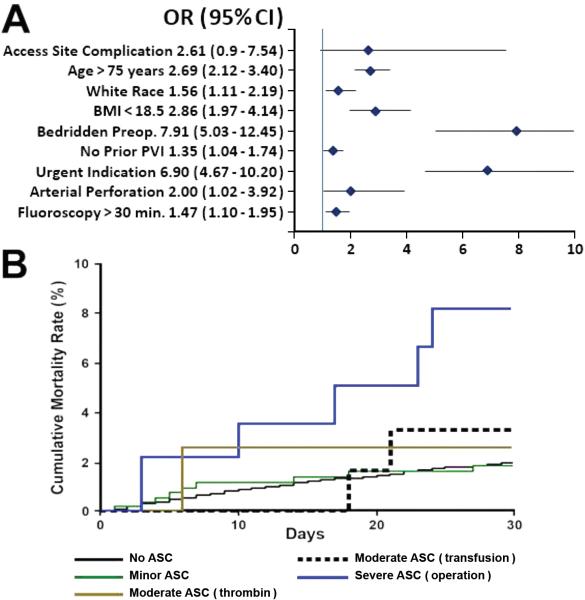
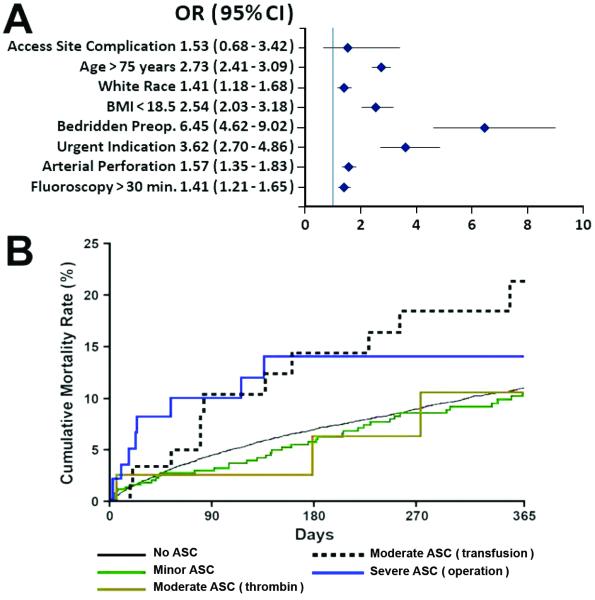
Patients with severe ASC had a fourfold increase in 30-day mortality rate compared with the rest of the population (6.1% vs. 1.4%; OR 4.33; CI 2.07-10.92; p<0.001). At 1 year, the highest mortality rate was observed in patients with ASC requiring transfusion (12.1% vs. 5.7%; p=0.001). Multivariable analysis of baseline and procedural characteristics revealed that ASC (both overall and when analyzed by severity) was not an independent predictor of 30-day or 1-year mortality. Baseline, clinical and procedural characteristics that predicted 30-day and 1-year mortality are summarized in Figures 3 and 4.
Figure 3.
A, Predictors of 30-day mortality. B, Kaplan-Meier curves for 30-day mortality by type of access site complication (ASC). BMI indicates body mass index; CI, confidence interval; OR, odds ratio.
Figure 4.
A, Predictors of 1-year mortality. B, Kaplan-Meier curves for 1-year mortality by type of access site complication (ASC). BMI indicates body mass index; CI, confidence interval; DM, diabetes mellitus; FA, femoral artery; OR, odds ratio.
Discussion
In our study of 27,048 PVI procedures (in 22,226 patients) derived from the VQI database, we observed a relatively low rate of ASC (3.5%) and found that only 0.9% of PVI are complicated by ASC requiring treatment (thrombin injection, blood transfusion or operation). In comparison, access site complication occurred in 7.0% and 8.9% of patients in prior PVI studies.5,6 The small sample sizes and difference in access site bleeding definitions used in the previous studies may account for the difference in complication rates.
Several baseline, clinical and procedural characteristics independently predicted ASC. Some of these easily identifiable factors may be useful in evaluating the patient risk of developing ASC to guide the use of more beneficial antithrombotic therapy, vascular closure device and the use of smaller access site sheaths. These predictors have not been studied or identified previously in post-PVI populations. A valid comparison between the predictors of ASC complicating PVI to those complicating percutaneous coronary intervention (PCI) is difficult due to differences in registry definitions,11-13 however, the distinct patient characteristics intrinsic to peripheral artery disease and coronary artery disease, the procedural differences between PVI and PCI, including the use of pharmacologic thrombolysis, and the similarities in antithrombotic therapies, endovascular approaches and patient comorbidities warrant an assessment.13 Older age, female gender, peripheral artery disease, use of heparin instead of bivalirudin, no prior PCI antithrombotic therapy and prolonged procedure duration are predictors of post-PCI bleeding that are comparable to predictors identified in our study.14-22 Also similar to PCI, higher rates of access site complication occurred in patients with a body mass index of < 18.5; however, low BMI is not an independent predictor of ASC in PCI on multivariable analysis.23
Other predictors of post-PCI bleeding not shared in our analysis of PVI procedures include impaired creatinine clearance, hypertension and heart failure.15,16 Furthermore, several PCI studies identified diabetes as an independent predictor of bleeding, whereas our study found ASC was less frequent in diabetics. Other predictors unique to the acute coronary syndrome population undergoing PCI include presence of ST-elevation myocardial infarction, cardiogenic shock and intra-aortic balloon pump, and could contribute to the higher rate of ASC (and possibly other bleeding complications) in PCI procedures.12,19,24
In the VQI dataset, 4.2% of PVI procedures in which heparin was used were complicated by ASC compared with 3.1%, 2.4% and 1.5% of procedures in which bivalirudin, heparin plus a vascular closure device, and bivalirudin plus a vascular closure device were utilized, respectively. The rate of thrombotic complications was similar between heparin and bivalirudin. Additionally, use of heparin over bivalirudin (odds ratio 1.40, 95% CI 1.03-1.91) and nonuse of vascular closure device (odds ratio 1.74, 95% CI 1.49-2.02) were predictors of ASC. These observations concur with results from prior PVI studies,5,9 the ACUITY trial16 and an observational study based on the National Cardiovascular Data Registry®.25 This shows that despite distinct populations, pathologies, procedures and bleeding definitions, bivalirudin is associated with reduced risk of bleeding complications compared to heparin while having the same efficacy in reducing thrombotic events. Finally, the reduction of specific types of ASC by vascular closure devices and bivalirudin mirrors the PCI literature.26,27 The trends of bivalirudin use were beyond the scope of this study and therefore associations between bivalirudin and ASC may be due to selection bias. The ENDOvascular interventions with angioMAX (ENDOMAX) trial may clarify the intrinsic risk of hemorrhagic complication difference between heparin and bivalirudin.28
We found that average postprocedure hospitalization was longer after PVI complicated by ASC and that the presence and severity of hematoma were related to higher rates of discharge to rehabilitation and nursing homes. This has not been previously reported. A nearly twofold increase in 1-year mortality in patients whose ASC required blood transfusion compared to all other patients was found. Vavalle et al. also reported an increase in 1-year mortality rate in post-PCI hematoma requiring transfusion (including non-access site hematomas), which they determined to be an independent predictor of 30-day and 1-year mortality.14 Our data reveal that ASC necessitating blood transfusion was not an independent predictor of 30-day or 1-year mortality. This suggests that although ASC requiring blood transfusion is causally related to poor outcomes in some patients, it may only be an indicator of patients at higher risk for poor outcomes.29 This hypothesis is further supported by the predictors found to predict both ASC and mortality. We observed that patients with severe hematomas had a fourfold increase in 30-day mortality rate compared to the rest of the study population, which mirrors the PCI literature that has repeatedly found higher mortality rates in patients with major bleeding complications at 30 days, 6 months, and 1 year.15-19 Explanations of the association between bleeding complications and mortality in PCI patients that may apply to severe ASC post-PVI include: the discontinuation of antithrombotic therapy, which may result in adverse cardiac outcomes; bleeding in the presence of other comorbidities precipitating decompensated heart failure; and blood transfusion complications leading to increased mortality.15 Finally, complications related to the surgical management of severe ASC and prolonged hospitalization may explain the increased 30-day mortality in this group.
Study Limitations
Strengths of the current study include its large sample size, the amount of data collected for each procedure and its unselected real-world population. However, certain limitations must be considered when interpreting the results. First, the current study only addressed hematomas at the arterial access site associated with and without pseudoaneurysm and no other ASC, including pseudoaneurysm without hematomas, retroperitoneal bleeding or other bleeding from other site. Institutional variability may result in underreporting of post-PVI ASC and as the analyzed data was de-identified, clustering of patients within hospitals was not done. In addition, other procedure-related bleeding complications are not adjudicated by the VQI. Furthermore, the retrospective nature of the study conveys inherent limitations, and the multivariable analysis performed may not have accounted for all relevant variables. Another limitation is that no analysis was performed to identify factors that may have influenced the administration of bivalirudin and placement of vascular closure devices, or variables that led to the treatment of ASC with thrombin injections, blood transfusions or surgeries. In addition, hematomas were more frequent in patients with arterial access in the arm, however the VQI does not specify which specific upper extremity artery was accessed. Since only newer extra-long equipment provides the means to treat above-the-knee lesions by a transradial approach, we postulate that many of those procedures involved brachial access.30 The PCI literature has demonstrated that transbrachial access is associated with a higher rate of puncture site complication, and that transradial access is safer than the femoral approach.31,32 Future studies are necessary to determine if there is a similar difference in PVI. Lastly, it was not possible to explore the relationship between bleeding and dosage, timing and duration of antithrombotic agents. This may be an important factor, as a recent analysis of post-PCI bleeding determined that maximum in-laboratory activated clotting time was the second most important predictor of bleeding in that population.33 Future studies are necessary to elucidate these questions.
Conclusions
The results of this study suggest that access site hematoma requiring transfusion or operation had a significant, independent, negative impact on mortality in patients undergoing peripheral vascular intervention. ASC complications also were associated with prolonged hospitalization and more adverse discharge status. Several patient and procedural variables independently predict ASC, including treatment with heparin rather than bivalirudin and the nonuse of a vascular closure device. Risk assessment for the potential development of ASC with knowledge of these findings could facilitate the appropriate selection of antithrombotic therapy and the use of closure devices to improve post-PVI outcomes.
Supplementary Material
Acknowledgments
The authors express their gratitude to the health care professionals involved in the Vascular Quality Initiative, including Jo Ann Simmons, RN, BSN, and Jennifer Farrell, RN, BSN. The authors also thank the editorial assistance of Joe Grundle and Katie Klein and the figure preparation of Brian Miller and Brian Schurrer, all of Aurora Cardiovascular Services.
Sources of Funding
Dr. Jahangir's work is partly supported by National Heart, Lung and Blood Institute R01 grants HL089542 and HL101240.
Footnotes
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White CJ, Gray WA. Endovascular therapies for peripheral arterial disease an evidence-based review. Circulation. 2007;116:2203–2215. doi: 10.1161/CIRCULATIONAHA.106.621391. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PL, Gelijns A, Moskowitz A, Arons R, Gupta L, Weinberg A, Faries PL, Nowygrod R, Kent KC. Understanding trends in inpatient surgical volume: vascular interventions, 1980-2000. J Vasc Surg. 2004;39:1200–1208. doi: 10.1016/j.jvs.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Shammas NW, Shammas GA, Jerin M, Dippel EJ, Shammas AN. In-hospital safety and effectiveness of bivalirudin in percutaneous peripheral interventions: data from a real-world registry. J Endovasc Ther. 2010;17:31–36. doi: 10.1583/09-2810.1. [DOI] [PubMed] [Google Scholar]
- 5.Sheikh IR, Ahmed SH, Mori N, Gupta A, Mewissen M, Allaqaband S, Bajwa T. Comparison of safety and efficacy of bivalirudin versus unfractionated heparin in percutaneous peripheral intervention: a single-center experience. JACC Cardiovasc Interv. 2009;2:871–876. doi: 10.1016/j.jcin.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Walker S, Beasley C, Reeves M. A retrospective study on the use of heparin for peripheral vascular intervention. Perspect Vasc Surg Endovasc Ther. 2012;24:63–69. doi: 10.1177/1531003512459889. [DOI] [PubMed] [Google Scholar]
- 7.Krajcer Z, Howell MH. Update on endovascular treatment of peripheral vascular disease: new tools, techniques, and indications. Tex Heart Inst J. 2000;27:369–385. [PMC free article] [PubMed] [Google Scholar]
- 8.Kasapis C, Gurm HS, Chetcuti SJ, Munir K, Luciano A, Smith D, Aronow HD, Kassab EH, Knox MF, Moscucci M, Share D, Grossman PM. Defining the optimal degree of heparin anticoagulation for peripheral vascular interventions: insight from a large, regional, multicenter registry. Circ Cardiovasc Interv. 2010;3:593–601. doi: 10.1161/CIRCINTERVENTIONS.110.957381. [DOI] [PubMed] [Google Scholar]
- 9.Shammas NW. Complications in peripheral vascular interventions: emerging role of direct thrombin inhibitors. J Vasc Interv Radiol. 2005;16:165–171. doi: 10.1097/01.RVI.0000147548.66405.84. [DOI] [PubMed] [Google Scholar]
- 10.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, Bell WR, Knatterud G, Robertson TL, Terrin ML. Thrombolysis in Myocardial Infarction (TIMI) Trial--phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- 12.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP, National Cardiovascular Data Registry Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–229. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 13.Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, Topol EJ, Manoukian SV. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100:1364–1369. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Vavalle JP, Rao SV. Impact of bleeding complications on outcomes after percutaneous coronary interventions. Interv Cardiol. 2009;1:51–62. [Google Scholar]
- 15.Yatskar L, Selzer F, Feit F, Cohen HA, Jacobs AK, Williams DO, Slater J. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2007;1:961–966. doi: 10.1002/ccd.21087. [DOI] [PubMed] [Google Scholar]
- 16.Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, 3rd, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;27:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs S, Kornowski R, Teplitsky I, Brosh D, Lev E, Vaknin-Assa H, Ben-Dor I, Iakobishvili Z, Rechavia E, Battler A, Assali A. Major bleeding complicating contemporary primary percutaneous coronary interventions-incidence, predictors, and prognostic implications. Cardiovasc Revasc Med. 2009;10:88–93. doi: 10.1016/j.carrev.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 20.Sulzbach-Hoke LM1, Ratcliffe SJ, Kimmel SE, Kolansky DM, Polomano R. Predictors of complications following sheath removal with percutaneous coronary intervention. J Cardiovasc Nurs. 2010;25:E1–8. doi: 10.1097/JCN.0b013e3181c83f4b. [DOI] [PubMed] [Google Scholar]
- 21.Ayhan E1, Isik T, Uyarel H, Ergelen R, Cicek G, Ghannadian B, Halil Tanboga I, Ergelen M, Eren M. Femoral pseudoaneurysm in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: incidence, clinical course and risk factors. Int Angiol. 2012;31:579–585. [PubMed] [Google Scholar]
- 22.Badr S1, Kitabata H, Torguson R, Chen F, Suddath WO, Satler LF, Pichard AD, Waksman R, Bernardo NL. Incidence and Correlates in the Development of Iatrogenic Femoral Pseudoaneurysm after Percutaneous Coronary Interventions. J Interv Cardiol. 2014;27:212–216. doi: 10.1111/joic.12091. [DOI] [PubMed] [Google Scholar]
- 23.Delhaye C, Wakabayashi K, Maluenda G, Belle L, Ben-Dor I, Gonzalez MA, Gaglia MA, Jr, Torguson R, Xue Z, Suddath WO, Satler LF, Kent KM, Lindsay J, Pichard AD, Waksman R. Body mass index and bleeding complications after percutaneous coronary intervention: does bivalirudin make a difference? Am Heart J. 2010;159:1139–1146. doi: 10.1016/j.ahj.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Manoukian SV, Voeltz MD, Eikelboom J. Bleeding complications in acute coronary syndromes and percutaneous coronary intervention: predictors, prognostic significance, and paradigms for reducing risk. Clin Cardiol. 2007;30:II24–34. doi: 10.1002/clc.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS, National Cardiovascular Data Registry Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 26.Gurm HS, Hosman C, Share D, Moscucci M, Hansen BB, Blue Cross Blue Shield of Michigan Cardiovascular Consortium Comparative safety of vascular closure devices and manual closure among patients having percutaneous coronary intervention. Ann Intern Med. 2013;159:660–666. doi: 10.7326/0003-4819-159-10-201311190-00004. [DOI] [PubMed] [Google Scholar]
- 27.Sanborn TA, Ebrahimi R, Manoukian SV, Ebrahimi R, Manoukian SV, McLaurin BT, Cox DA, Feit F, Hamon M, Mehran R, Stone GW. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:57–62. doi: 10.1161/CIRCINTERVENTIONS.109.896704. [DOI] [PubMed] [Google Scholar]
- 28.Wayangankar SA, Abu-Fadel MS, Aronow HD, Kennedy KF, Gupta R, Yeh RW, Gray WA, Rosenfield K, Hennebry TA. Hemorrhagic and ischemic outcomes after bivalirudin versus unfractionated heparin during carotid artery stenting: a propensity score analysis from the NCDR. Circ Cardiovasc Interv. 2013;6:131–138. doi: 10.1161/CIRCINTERVENTIONS.112.974857. [DOI] [PubMed] [Google Scholar]
- 29.Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, Goodman SG, Budaj A, FitzGerald G, Fox KA, GRACE Investigators Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation. 2007;116:2793–2801. doi: 10.1161/CIRCULATIONAHA.107.694273. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzoni R, Roffi M. Transradial access for peripheral and cerebrovascular interventions. J Invasive Cardiol. 2013;25:529–536. [PubMed] [Google Scholar]
- 31.Baklanov DV, Kim S, Marso SP, Subherwal S, Rao SV. Comparison of bivalirudin and radial access across a spectrum of preprocedural risk of bleeding in percutaneous coronary intervention: analysis from the national cardiovascular data registry. Circ Cardiovasc Interv. 2013;6:347–353. doi: 10.1161/CIRCINTERVENTIONS.113.000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997;29:1269–1275. doi: 10.1016/s0735-1097(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 33.Hillegass WB, Brott BC, Chapman GD, Phillips HR, Stack RS, Tcheng JE, Califf RM. Relationship between activated clotting time during percutaneous intervention and subsequent bleeding complications. Am Heart J. 2002;144:501–507. doi: 10.1067/mhj.2002.123143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.