Abstract
The three-dimensional crystallization of bacteriorhodopsin was systematically investigated and the needle-shaped crystal form analysed. In these crystals the M-intermediate forms 10 times faster and decays 15 times more slowly than in purple membranes. Polarized absorption spectra of the crystals were measured in the dark and light adapted states. A slight decrease in the angle between the transition moment and the membrane plane was detected during dark adaptation. The crystallization of a mutated bacteriorhodopsin, in which the aspartic acid at residue 96 was replaced by asparagine, provided crystals with a long lived M-intermediate. This allowed polarized absorption measurements of the M-chromophore. The change in the polarization ratio upon formation of the M-intermediate indicates an increase in the angle between the main transition dipole and the membrane plane by 2.2 degrees +/- 0.5, corresponding to a 0.5 A displacement of one end of the chromophore out of the membrane plane of the bacteriorhodopsin molecule.
Full text
PDF

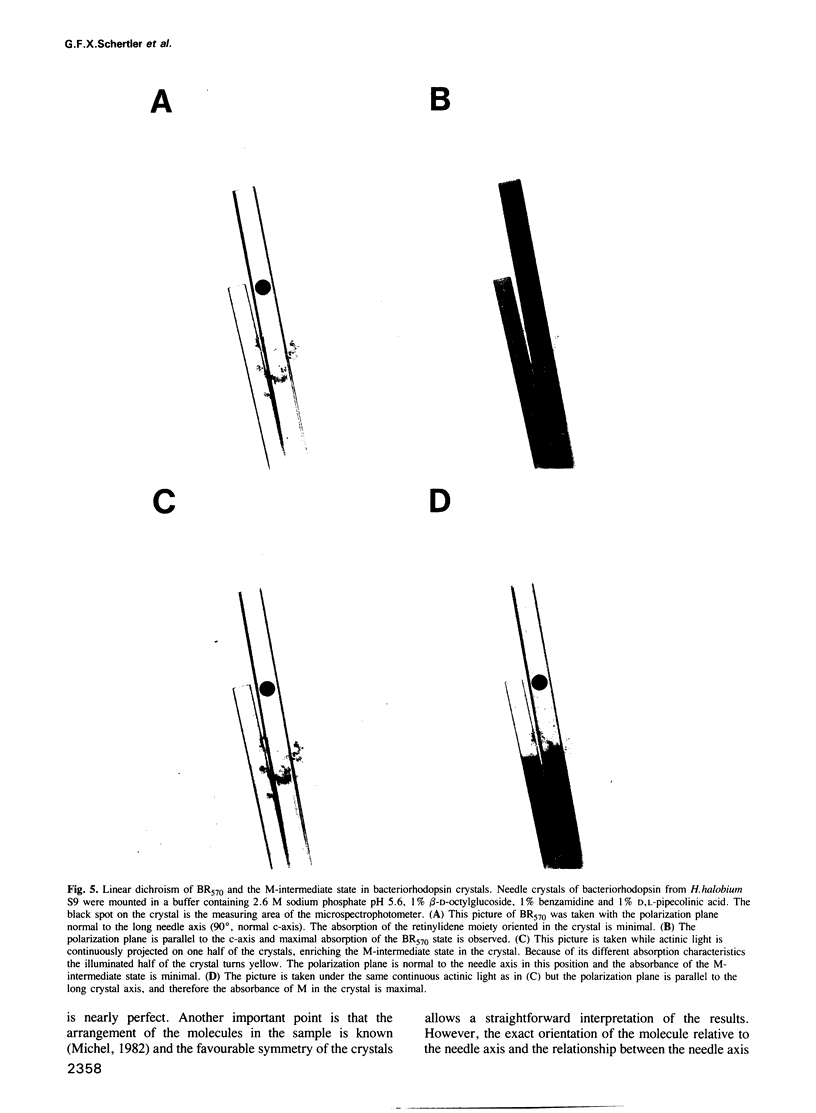
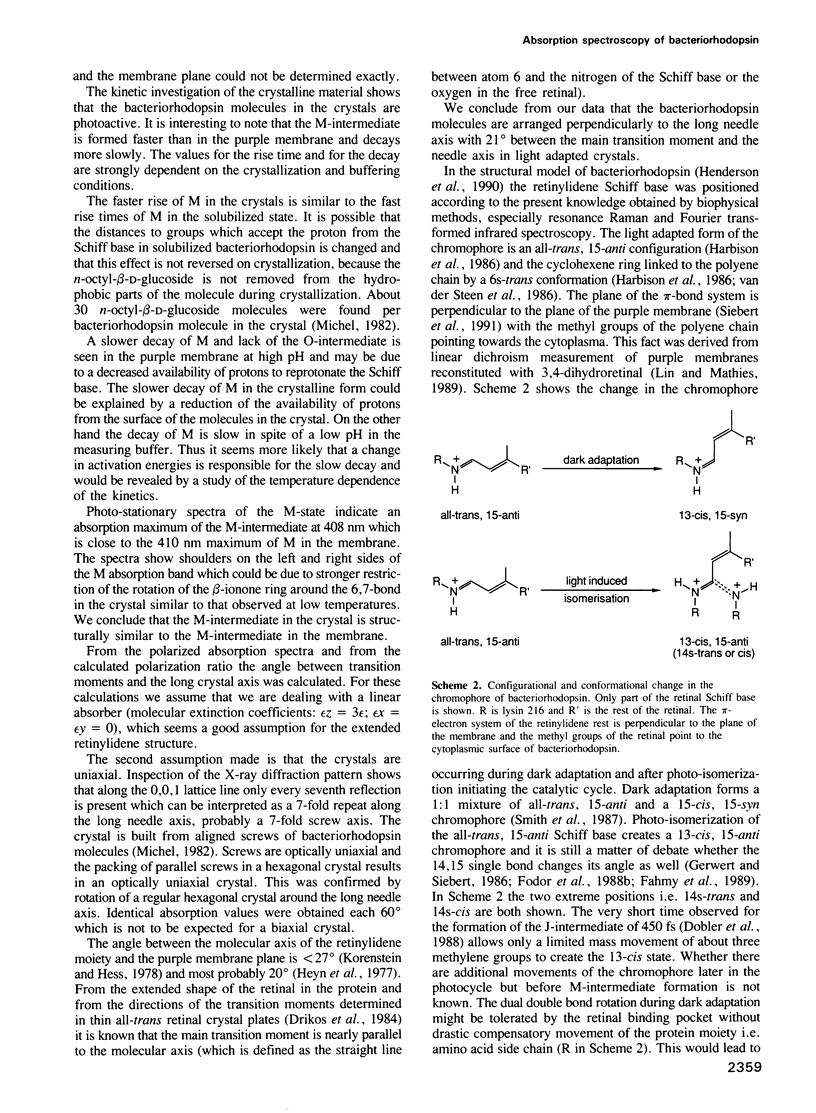


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Henderson R., Beckman E., Zemlin F. Images of purple membrane at 2.8 A resolution obtained by cryo-electron microscopy. J Mol Biol. 1988 Aug 5;202(3):585–591. doi: 10.1016/0022-2836(88)90288-4. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest T. N., Roepe P., Braiman M. S., Gillespie J., Rothschild K. J. Orientation of the bacteriorhodopsin chromophore probed by polarized Fourier transform infrared difference spectroscopy. Biochemistry. 1986 Dec 2;25(24):7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Pollard W. T., Gebhard R., van den Berg E. M., Lugtenburg J., Mathies R. A. Bacteriorhodopsin's L550 intermediate contains a C14-C15 s-trans-retinal chromophore. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2156–2160. doi: 10.1073/pnas.85.7.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Westerhausen J., Wallat I., Seiff F. High-sensitivity neutron diffraction of membranes: Location of the Schiff base end of the chromophore of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2146–2150. doi: 10.1073/pnas.85.7.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Eaton W. A. Linear dichroism of biological chromophores. Annu Rev Biophys Bioeng. 1976;5:511–560. doi: 10.1146/annurev.bb.05.060176.002455. [DOI] [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Immobilization of bacteriorhodopsin and orientation of its transition moment in purple membrane. FEBS Lett. 1978 May 1;89(1):15–20. doi: 10.1016/0014-5793(78)80512-2. [DOI] [PubMed] [Google Scholar]
- Michel H. Characterization and crystal packing of three-dimensional bacteriorhodopsin crystals. EMBO J. 1982;1(10):1267–1271. doi: 10.1002/j.1460-2075.1982.tb00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D. Three-dimensional crystals of membrane proteins: bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1283–1285. doi: 10.1073/pnas.77.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Kölling E., Oesterhelt D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):651–662. doi: 10.1016/S0006-3495(86)83692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M., Gurel O., Zaccaï G. Tertiary structure of bacteriorhodopsin. Positions and orientations of helices A and B in the structural map determined by neutron diffraction. J Mol Biol. 1989 Dec 20;210(4):829–847. doi: 10.1016/0022-2836(89)90111-3. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Braiman M. S., Mogi T., Stern L. J., Khorana H. G. Conserved amino acids in F-helix of bacteriorhodopsin form part of a retinal binding pocket. FEBS Lett. 1989 Jul 3;250(2):448–452. doi: 10.1016/0014-5793(89)80774-4. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Hornung I., van der Steen R., Pardoen J. A., Braiman M. S., Lugtenburg J., Mathies R. A. Are C14-C15 single bond isomerizations of the retinal chromophore involved in the proton-pumping mechanism of bacteriorhodopsin? Proc Natl Acad Sci U S A. 1986 Feb;83(4):967–971. doi: 10.1073/pnas.83.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppa J., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. I. The 5-bromo-2'-deoxyuridine selection as a method to isolate point mutants in halobacteria. J Biol Chem. 1989 Aug 5;264(22):13043–13048. [PubMed] [Google Scholar]
- Soppa J., Otomo J., Straub J., Tittor J., Meessen S., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. II. Characterization of mutants. J Biol Chem. 1989 Aug 5;264(22):13049–13056. [PubMed] [Google Scholar]
- Wagner G., Hartmann R., Oesterhelt D. Potassium uniport and ATP synthesis in Halobacterium halobium. Eur J Biochem. 1978 Aug 15;89(1):169–179. doi: 10.1111/j.1432-1033.1978.tb20909.x. [DOI] [PubMed] [Google Scholar]