Summary
Sensory neurons continually adapt their processing properties in response to changes in the sensory environment or the brain’s internal state. Neuromodulators are thought to mediate such adaptation through a variety of receptors and their action has been implicated in processes such as attention, learning and memory, aggression, reproductive behaviour and state-dependent mechanisms. Here, we review recent work on neuromodulation of electrosensory processing by acetylcholine and serotonin in the weakly electric fish Apteronotus leptorhynchus. Specifically, our review focuses on how experimental application of these neuromodulators alters excitability and responses to sensory input of pyramidal cells within the hindbrain electrosensory lateral line lobe. We then discuss current hypotheses on the functional roles of these two neuromodulatory pathways in regulating electrosensory processing at the organismal level and the need for identifying the natural behavioural conditions that activate these pathways.
Keywords: acetylcholine, serotonin, weakly electric fish
Introduction
In order to optimally process stimuli with widely varying spatiotemporal characteristics, the response properties of sensory neurons must be continually adjusted (Abbott, 2005; Wark et al., 2007). Neuromodulators, such as serotonin (5-HT) and acetylcholine (ACh), are important mediators of these adjustments. Indeed, neuromodulators have been shown to significantly alter processing across several sensory systems and are thought to impose dynamic filters whose properties are tied to environmental events and/or the brain’s internal state rather than the detailed information about the ongoing stimulus (Devore and Linster, 2012; Hurley et al., 2004). As such, the effects of neuromodulators are slower, longer lasting and more spatially diffuse than those of classical neurotransmitters. Neuromodulators often act through multiple receptors that can have opposite effects on membrane excitability and the response to sensory input, which greatly complicates understanding their functional roles (Hurley et al., 2004). Significant progress has been made in unravelling the relationships between the effects of neuromodulators at the cellular and at the systems level, i.e. the processing of sensory stimuli and its consequences for behaviour (for reviews, see Birmingham and Tauck, 2003; Edeline, 2012; Hurley et al., 2004; Hurley and Sullivan, 2012; Witkovsky, 2004). For example, at the single-cell level, neuromodulators have been implicated in facilitation of evoked responses, increases in signal-to-noise ratio, and improved functional properties of sensory neurons in the visual, auditory and somatosensory systems (for reviews, see Edeline, 2012; Hurley et al., 2004; Hurley and Sullivan, 2012). In invertebrates, neuromodulators have been shown to enhance sensitivity of, and odour discrimination by, ensembles of olfactory neurons (Dacks et al., 2009) and to modulate spike timing precision in mechanosensory neurons (Billimoria et al., 2006). Dopamine is known to play a key role in the adaptation of retinal processing to different light levels as well as circadian changes in retinal physiology (e.g. Herrmann et al., 2011; Witkovsky, 2004; Zhang et al., 2011). However, more work is required to better understand the links between cellular and circuit effects of neuromodulators, the consequences for the processing of sensory information, and their role in the natural ecological context of an organism’s life.
Here we review recent work on serotonergic and cholinergic neuromodulation in the electrosensory system of the gymnotiform wave-type weakly electric fish Apteronotus leptorhynchus. Weakly electric fish provide an excellent experimental model to study effects of neuromodulators at cellular, network and behavioural levels in a sensory system, first and foremost because of the direct linkage between neuronal and circuit properties and their role in specific behaviours. In addition, the anatomy and physiology of the electrosensory system have been well characterized. Further, the electrosensory system is closely related to the mechanosensory lateral line and thus displays many similarities with other eighth nerve systems such as the auditory and vestibular systems (Coombs and Montgomery, 2005; Modrell et al., 2011).
Our review is organized as follows. First, we present the relevant anatomy and physiology of the electrosensory system. Second, we review current knowledge on cholinergic input to the electrosensory lateral line lobe (ELL) of the hindbrain and describe the distribution of ACh receptors in this area. We then focus on electrosensory pyramidal neurons and show how cholinergic downregulation of A-type potassium currents can lead to a greater response to low-frequency input. Third, we review the current knowledge on serotonergic input within the electrosensory system. We then describe the known distribution of serotonergic fibres, focusing on the ELL, and show how serotonergic downregulation of M and small-conductance calcium-activated potassium (SK) channels can significantly alter pyramidal cell responses to sensory input. We conclude by highlighting some potential functions of cholinergic and serotonergic input onto pyramidal cells for regulating behavioural responses to sensory input.
Review of relevant anatomy and physiology of the electrosensory system
Weakly electric fish generate an oscillating electric field around their body by discharging a specialized electric organ [electric organ discharge (EOD)]. They sense perturbations of their self-generated electric field through tuberous electroreceptors distributed all over the skin. These perturbations can arise from the presence of nearby objects such as prey and other fish or from the EODs of conspecifics (Chacron et al., 2011; Heiligenberg, 1991; Nelson and MacIver, 1999; Bullock et al., 2005). One set of primary electrosensory afferents relays information on amplitude modulations of the EOD to the ELL in the hindbrain of the fish, and it is this amplitude-coding pathway that is the focus of the present review. We henceforth refer to amplitude modulations of the EOD as stimuli. Electrosensory stimuli can vary in temporal frequency content from 0 to ~400 Hz. In A. leptorhynchus, prey stimuli as well as those generated by the presence of same-sex conspecifics typically contain low temporal frequencies [<30Hz (Nelson and Maciver, 1999)] whereas stimuli generated by the presence of opposite-sex conspecifics as well as electrocommunication stimuli called chirps displayed during agonistic and courtship behaviours (Zakon et al., 2002) typically contain high frequencies [>50Hz (Zupanc and Maler, 1993)].
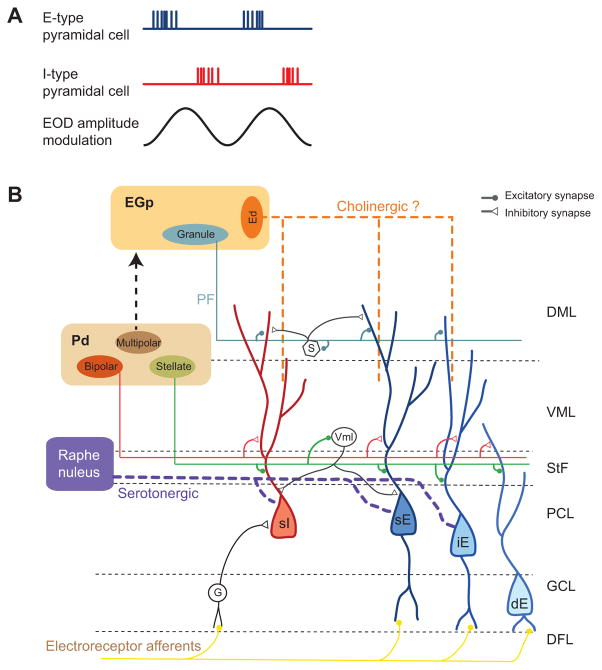
Upon entering the hindbrain, each primary afferent trifurcates and synapses onto pyramidal neurons (Fig. 1) in three different segments of the ELL: the centromedial segment (CMS), the centrolateral segment (CLS) and the lateral segment (LS) (Carr et al., 1982; Heiligenberg and Dye, 1982). Within each ELL segment there are two morphologically and physiologically distinguishable types of pyramidal neurons. I-type pyramidal neurons are characterized by the absence of a basilar dendrite and respond to amplitude downstrokes, while E-type pyramidal cells have basilar dendrites and respond to amplitude upstrokes (Maler, 1979; Saunders and Bastian, 1984) (Fig. 1A). Both types of neurons have apical dendrites that extend to the ventral and dorsal molecular layers of the ELL, where they receive feedback input (see below).
Fig. 1.
Diagram of the relevant circuitry of the electrosensory lateral line lobe (ELL) in gymnotiform weakly electric fish. Electroreceptor afferents respond to amplitude modulations of the electric organ discharge (EOD) and synapse onto basilar dendrites of the pyramidal cells and granule cells (G). According to the dorso-ventral position of their soma in the layers of the ELL, pyramidal cells are classified as: superficial (s), intermediate (i) and deep (d). Pyramidal cells are also classified as I-type (I) and E-type (E) cells depending on their response to EOD amplitude modulations. I-type cells lack basilar dendrites and receive indirect input from electroreceptor afferents through synapses from granule cells. E-type cells present basilar dendrites where electroreceptors make direct synapses. Here we indicate only the position of the superficial I cell (sI), but intermediate and deep I-type pyramidal cells are also present in the ELL (Maler, 2009a). Pyramidal cells receive feedback from bipolar cells and stellate cells in the nucleus praeeminentialis dorsalis (Pd). Multipolar cells project to granule cells in the eminentia granularis posterior (EGp) of the caudal lobe of the cerebellum. Multipolar cells make further feedback onto pyramidal cells via parallel fibres both directly and indirectly through molecular layer stellate cells (S), which are inhibitory interneurons. Eurydendroid (Ed) cells within the EGp are thought to be the source of cholinergic input via vertical fibres to the dendrites of pyramidal cells (Phan and Maler, 1983). Serotonergic input enters the ELL through a fibre bundle at the ventromedial edge of the ELL. Both neuromodulators affect both E- and I-type pyramidal cells. DFL, deep fibre layer; DML, dorsal molecular layer; GCL, granule cell layer; PCL, pyramidal cell layer; StF, stratum fibrosum; VML, ventral molecular layer; Vml: VML interneuron (adapted from Berman and Maler, 1999).
E- and I-type pyramidal cells are further subdivided into superficial, intermediate and deep cells according to the dorso-ventral position of their soma (Bastian and Courtright, 1991; Bastian et al., 2004). Superficial pyramidal cells have the largest apical dendritic trees, show low spontaneous firing rates and receive the largest amount of feedback (Bastian et al., 2004). This feedback has various functional roles such as gain control (Bastian, 1986), rejection of self-generated stimuli (Bastian, 1999), as well as controlling frequency tuning (Chacron et al., 2005b), burst firing and correlated activity (Chacron and Bastian, 2008; Litwin-Kumar et al., 2012). In contrast, deep pyramidal neurons have the smallest apical dendritic trees, high spontaneous firing rates (Bastian and Nguyenkim, 2001; Bastian et al., 2002) and receive little to no feedback (Bastian et al., 2004; Chacron et al., 2005a). Intermediate pyramidal cells are intermediate with respect to these properties (for reviews, see Maler, 2009a; Maler, 2009b). The source of electrosensory feedback to the apical dendrites of superficial and intermediate pyramidal cells is the nucleus praeeminentialis dorsalis (Pd), which is innervated by the axons of the deep pyramidal cells only (Bastian et al., 2004). The so-called direct feedback pathway consists of Pd stellate cells that make reciprocal and topographic connections and bipolar cells that project in a spatially diffuse manner onto pyramidal cell apical dendrites. The indirect pathway relies on Pd multipolar cells that project to the eminentia granularis posterior (EGp) of the caudal lobe of the cerebellum. Parallel fibres originating from EGp granule cells terminate in the ELL molecular layers on pyramidal cell apical dendrites as well as inhibitory interneurons (Fig. 1B) [for a review of the circuitry, see Berman and Maler (Berman and Maler, 1999)] The ELL thus constitutes a cerebellum-like structure as discussed, for example, by Bell et al. (Bell et al., 2008). We also note that there are no direct lateral connections between pyramidal cells within and between maps (Maler, 1979).
All types of pyramidal neurons from all three segments project to the torus semicircularis (TS) (Fig. 1B) (Maler, 1979; Bell and Maler, 2005). The three segments receive identical afferent input but differ in their processing characteristics. For instance, the receptive fields of pyramidal neurons in the LS have the largest size and those in the CMS the smallest (Maler, 2009a; Shumway, 1989), E-type pyramidal cells of LS show high-pass filtering of amplitude modulations, those of CMS display low-pass tuning and those of CLS switch their tuning properties depending on behavioural context (Chacron et al., 2003; Chacron, 2006; Krahe et al., 2008). During spatially extended stimuli they act as high-pass or bandpass filters, and for spatially localized stimuli as low-pass filters. The I-cells of all three maps are much more homogenous and act as low-pass filters (Krahe et al., 2008; Shumway, 1989). LS cells are also the most responsive to chirp stimuli (Marsat and Maler, 2010; Marsat et al., 2009; Marsat et al., 2012; Vonderschen and Chacron, 2011). From a functional point of view, CMS has been shown to be both necessary and sufficient for the jamming avoidance response, which is a shift of the animal’s own EOD frequency away from similar frequencies of a conspecific. LS, in contrast, is required for chirping behaviour (Metzner and Juranek, 1997). The physiological differences between the pyramidal cells of the three segments have been shown to be related to differences in cell-intrinsic and network properties (Ellis et al., 2007b; Fernandez et al., 2005; Krahe et al., 2008; Mehaffey et al., 2006; Mehaffey et al., 2008a; Rashid et al., 2001a; Rashid et al., 2001b).
Pyramidal cells also display burst firing through an intrinsic bursting mechanism that relies on a somatodendritic interaction (Ellis et al., 2007b; Fernandez et al., 2005; Lemon and Turner, 2000; Mehaffey et al., 2006; Rashid et al., 2001b; Turner et al., 2002) (for review, see Krahe and Gabbiani, 2004). Superficial pyramidal cells display the lowest spontaneous firing rates and the highest tendency to burst, whereas deep pyramidal cells display the highest spontaneous firing rates and the lowest tendency to burst in vivo (Bastian and Nguyenkim, 2001; Maler, 2009a). Bursting behaviour is in part controlled by the direct and indirect feedback pathways mentioned above (Chacron and Bastian, 2008; Mehaffey et al., 2005; Marsat et al., 2009; Marsat and Maler, 2012). The presence or absence of burst firing can have significant consequences on information processing by pyramidal cells, including the gating of sensory information (Toporikova and Chacron, 2009). Further, bursts can code for specific stimulus features such as low-frequency events (Avila-Akerberg and Chacron, 2011; Avila-Akerberg et al., 2010; Gabbiani et al., 1996; Krahe et al., 2002; Metzner et al., 1998; Oswald et al., 2004) as well as chirps (Marsat et al., 2009; Marsat et al., 2012).
Distribution of muscarinic ACh receptors and downregulation of A-type potassium channels
In the central nervous system, ACh acts on two categories of receptors, nicotinic and muscarinic, the latter being the most abundant and functionally predominant ACh receptor type in the central nervous system (Sarter et al., 2005). Muscarinic receptors act via activation of G proteins and are grouped into two families based on the type of second messenger that is used. Muscarinic receptor types 1, 3 and 5 stimulate phospholipase C, while muscarinic receptor types 2 and 4 inhibit adenylyl cyclase (Caulfield, 1993).
Distribution of cholinergic input to ELL pyramidal cells
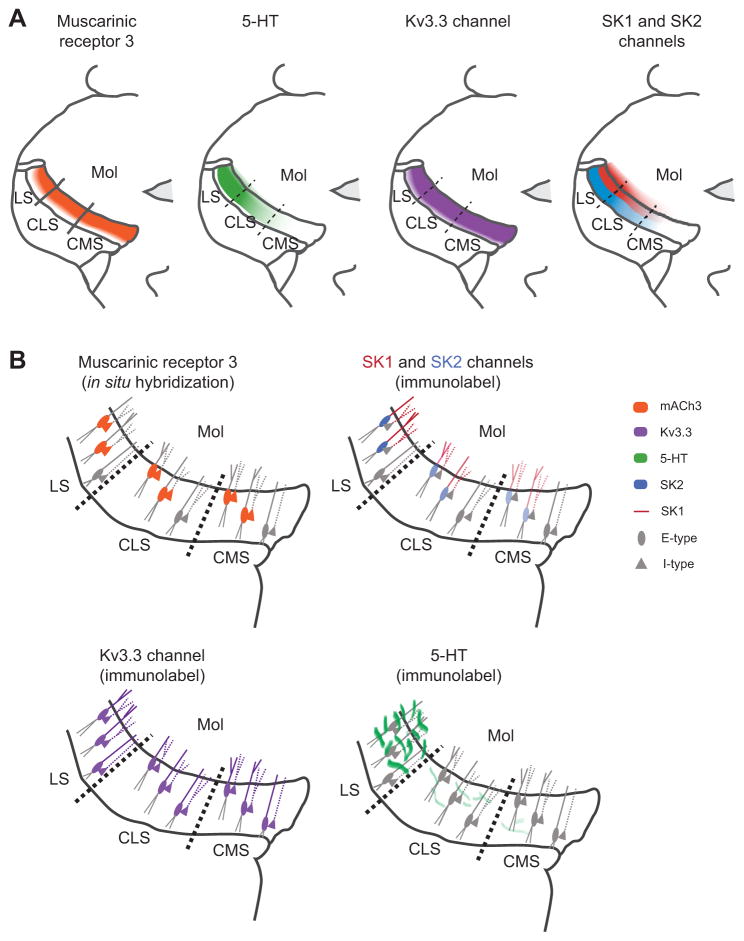
Cholinergic input to the ELL was originally demonstrated histologically by the presence of ACh-esterase as well as muscarinic receptors and is thought to originate from eurydendroid cells within the EGp (Fig. 1B) (Maler et al., 1981; Phan and Maler, 1983). These cerebellar eurydendroid cells are thought to perform functions similar to those of deep cerebellar nuclei neurons in tetrapods (Finger, 1978; Ikenaga et al., 2006). A recent in situ hybridization study (Toscano-Márquez et al., 2013) showed that M3 is the only muscarinic receptor present in the ELL and that its mRNA is homogenously distributed across pyramidal cells in all three tuberous ELL segments (Fig. 2A). Moreover, M3 receptors are present in superficial and intermediate pyramidal cells, but not deep pyramidal cells (Toscano-Márquez et al., 2013) (Fig. 2B). Only one type of ELL interneuron, likely corresponding to polymorphic cells, was labeled by the M3 probe (Toscano-Márquez et al., 2013). These cells may also be affected by cholinergic input to their apical dendrites.
Fig. 2.
(A) Graphical representation of the distribution of muscarinic receptor 3 (mAChR3) (Toscano-Márquez et al., 2013), serotonergic fibres (5-HT) (Deemyad et al., 2011), Kv3.3 channels (Rashid et al., 2001a) and small-conductance calcium-activated potassium channels (SK) channels (SK1 and SK2) (Ellis et al., 2008) in the three tuberous maps of the ELL. mACh3 and Kv3.3 channels present a homogeneous distribution within the three segments while 5-HT and SK channels show a higher expression in the lateral segment (LS), almost none in the centromedial segment (CMS) and intermediate expression in the centrolateral segment (CLS). (B) Graphical representation of the distribution of mAChR3, 5-HT fibres, SK and Kv3.3 channels in the six types of pyramidal cells across the three segments of the ELL. mAChR3 and Kv3.3 are expressed in superficial and intermediate cells of both E- and I-type pyramidal cells. Additionally, Kv3.3 is present in deep pyramidal cells of the three segments. Serotonin fibres reach both E- and I-type pyramidal cells, but their density differs between segments. SK1 channels are localized in the apical dendrites of both E- and I-type cells with differential distribution in the three segments. SK2 channels are present only in the somata of E-type pyramidal cells, with a higher expression in the LS. Mol, molecular layer.
Activation of muscarinic receptors alters pyramidal cell excitability and responses to sensory input
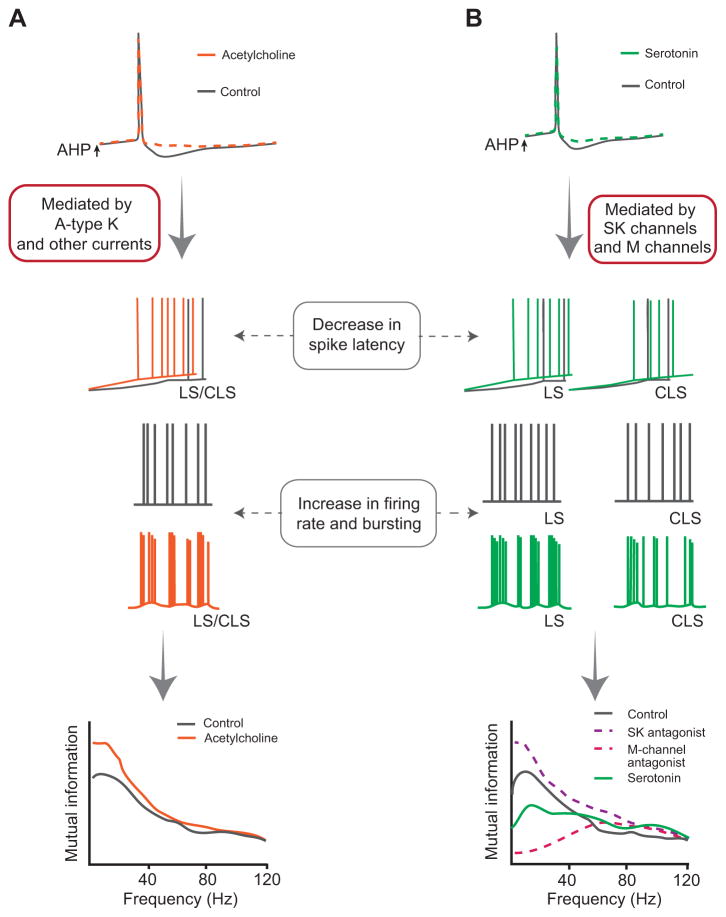
A previous study showed that application of the cholinergic agonist carbachol in the ELL in vivo increased excitability and burst firing of CLS and LS pyramidal cells (Ellis et al., 2007a) (Fig. 3A). This effect was due to muscarinic receptor activation because prior application of the selective muscarinic antagonist atropine occluded the effect of carbachol. Based on the recent in situ hydridization results (Toscano-Márquez et al., 2013), we conclude that the observed effects were most likely due to activation of M3 receptors, although more selective antagonists were not used. Activation of muscarinic input to CLS and LS pyramidal cells also altered their responses to sensory input. Indeed, Ellis et al. (Ellis et al., 2007a) found that such activation led to an increased response to low-frequency (<40Hz) stimuli (Fig. 3A). It should be noted that atropine application did not alter pyramidal cell excitability or responses to sensory input, thus suggesting that muscarinic input onto pyramidal cells is not constitutively active (Ellis et al., 2007a). Although physiological data for cholinergic effects on CMS pyramidal neurons are not available, we hypothesize that carbachol application in CMS would result in effects similar to those seen in CLS and LS cells: increased excitability and burst firing and enhanced low-frequency information transmission. This hypothesis is based on the fact that in situ hybridization studies have shown that M3 receptors are expressed equally across the three ELL maps (Toscano-Márquez et al., 2013).
Fig. 3.
Schematic representation of the effects of acetylcholine and 5-HT on the pyramidal cells of the ELL. (A) Application of an acetylcholine agonist decreases the spike afterhyperpolarization (AHP) of pyramidal cells. By acting on an A-type potassium current, it also decreases first-spike latency and increases the firing rate as well as burst firing. As a consequence, the response to low-frequency (<40Hz) sensory stimuli is enhanced as quantified by mutual information (Ellis et al., 2007a). (B) Application of 5-HT onto pyramidal cells also reduces the spike AHP, but this effect is mediated by the downregulation of SK channels and the downregulation of M-type channels. The downregulation of these two channels also significantly decreases spike latency and significantly increases the firing rate as well as burst firing, but the overall effect on information transmission is a whitening of the mutual information curves (Deemyad et al., 2011). Blocking either M or SK channels leads to opposite effects on information transmission (Deemyad et al., 2012). M-channel blockage decreases low-frequency responses while slightly increasing high-frequency responses. SK-channel blockade increases low-frequency responses (Deemyad et al., 2011).
Mechanisms underlying the cholinergic modulation of ELL pyramidal cells
Application of the cholinergic agonist carbachol to an in vitro preparation of the ELL led to increased excitability accompanied by an increase in firing rate as well as burst firing, all of which were occluded by prior application of atropine (Ellis et al., 2007a). Ellis et al. (Ellis et al., 2007a) also found that carbachol application depolarized the membrane potential, decreased membrane conductance, decreased the first spike latency and decreased the spike afterhyperpolarization (AHP) (Fig. 3A).
Further experiments found that the low-threshold potassium channel antagonist 4-aminopyridine (4-AP) reproduced most of the effects of carbachol, thereby suggesting that activation of muscarinic receptors downregulates a low-threshold potassium current (Ellis et al., 2007a). Together with the effect on first spike latency, these results suggest that muscarinic receptor activation leads to the downregulation of A-type potassium currents that are present in ELL pyramidal cells (Mathieson and Maler, 1988). Atype currents are known to activate transiently in the subthreshold range of membrane potential and inactivate during depolarization (Akins et al., 1990). As such, these channels delay the appearance of the first action potential in response to depolarization and also contribute to action potential repolarization (Kang et al., 2000) and burst firing (Nakajima et al., 1986). They thus exert a powerful control over excitability including action potential back propagation, synaptic integration and plasticity (Baranauskas, 2007; Hoffman et al., 1997; Johnston et al., 2003).
These results are consistent with those found in other systems as M3 receptors couple preferentially to G proteins of the Gq/11 family, which generally results in postsynaptic excitation through the inhibition of potassium and calcium currents (Brown, 2010; Shapiro et al., 2001). Typically, muscarinic receptor activation has been found to alter the firing properties of individual neurons through the modulation of a number of individual ionic conductances (Chen and Johnston, 2004; Delgado-Lezama et al., 1997; Stocker et al., 1999). As Ellis et al. (Ellis et al., 2007a) did not perform occlusion experiments, we cannot exclude that other conductances besides the A-type current are affected by carbachol. Indeed, the fact that carbachol application led to a decreased AHP, which cannot be explained by the downregulation of an A-type current, suggests that muscarinic receptor activation affects other membrane conductances that are associated with the AHP (Ellis et al., 2007a; Mehaffey et al., 2008b) as is discussed below.
The AHP can be mediated by Ca2+-activated K+ channels (Faber and Sah, 2007; Power and Sah, 2008) as well as M-type potassium channels (Faber and Sah, 2007). Although it has been shown that the latter currents are inhibited by activation of muscarinic receptors in other systems (Adams et al., 1982; Marrion, 1997), this does not appear to be the case in ELL pyramidal cells. This is because the effects of carbachol application differ from those of the selective M-current antagonists linopyridine and XE-991. Indeed, while carbachol application increases pyramidal cell responses to low-frequency stimuli as described above (Ellis et al., 2007a), XE-991 and linopyridine application both decreased pyramidal cell responses to these stimuli (Deemyad et al., 2012). Instead, the effects of carbachol application on the AHP are similar to those obtained with the selective SK channel antagonist apamin (Ellis et al., 2007b; Mehaffey et al., 2008b), suggesting that activation of muscarinic receptors onto ELL pyramidal cells also downregulates SK channels. However, this has not yet been confirmed experimentally.
Alternatively, it is possible that activation of muscarinic receptors of ELL pyramidal cells will affect membrane conductances generated by Kv3.3 channels, which have been shown to be present in ELL pyramidal cells (Rashid et al., 2001a) (Fig. 2) and can give rise to AHPs (Rudy and McBain, 2001; McMahon et al., 2004). The distribution of these voltage-gated ion channels mirrors that of muscarinic receptors in the ELL. Their blockade in a slice preparation of the ELL results in a lower threshold for burst generation, which is consistent with the results of carbachol application (Ellis et al., 2007a). The characteristic effect of the Kv3.3 channel of enhancing burst generation makes it a plausible additional candidate for controlling burst generation by ACh and, with it, sensory information transmission.
In summary, the activation of muscarinic input onto ELL pyramidal cells increases excitability and responses to low-frequency sensory input via downregulation of A-type potassium channels and possibly other channels. As the effects of carbachol application in vitro and in vivo were very similar, we expect that the effects of ACh in ELL pyramidal cells are largely mediated by cell-intrinsic mechanisms. Nevertheless, some presynaptic actions of ACh appear likely, because one type of ELL interneuron, presumably polymorphic cells, showed strong staining for M3 mRNA. Given that muscarinic receptors have a relatively homogeneous distribution across segments, it is expected that activation of the cholinergic system will lead to these effects across all segments. However, because the cholinergic system does not appear to be constitutively active (Ellis et al., 2007a), cholinergic modulation is likely stimulus-specific and/or may depend on behavioural state. Future studies should investigate the role of ACh in more natural conditions, considering blocking the endogenous liberation of acetylcholine instead of artificially applying the agonist. Also, it should be interesting to characterize the types of stimuli and/or behavioural contexts that activate the cholinergic pathway onto ELL pyramidal cells.
Distribution of serotonergic fibres, downregulation of M and SK currents, and consequences for behaviour
5-HT is a powerful modulator of social behaviour throughout the animal kingdom (Berger et al., 2009). 5-HT has been shown to activate at least 15 different receptors. These receptors are grouped into seven families based on signaling mechanisms, and all but two [5-HT (3A) and 5-HT (3B)] are G-protein coupled receptors (Hoyer et al., 2002).
Distribution of serotonergic input to the ELL pyramidal cells
Serotonergic fibres project from the nucleus raphe dorsalis into the ELL. They enter the ELL through a fibre bundle located at the ventromedial edge of the ELL (Johnston et al., 1990; Wong, 1997). A recent and more sensitive immunohistochemical study revealed that the different ELL segments receive differential 5-HT innervation (Deemyad et al., 2011). Indeed, while 5-HT innervation is highest in LS, it is almost non-existent in the CMS and intermediate in the CLS (Fig. 2A). It also seems that 5-HT innervates different ELL layers across segments as the immunoreactive fibres were mostly confined to the granule cell layer in the CMS whereas in the LS dense immunoreactivity was observed in the pyramidal cell layer and extended to the ventral molecular layer (Deemyad et al., 2011). Thus, unlike the homogeneous pattern of muscarinic receptors, 5-HT innervation is quite different across the ELL segments. Further, 5-HT innervation is greatest for superficial pyramidal cells and weakest for deep pyramidal cells (Fig. 2B).
Activation of serotonergic receptors alters pyramidal cell excitability and responses to sensory input
A recent study performed in vitro has started investigating the effects of 5-HT on ELL pyramidal cells across segments. It was found that 5-HT increases E- and I-type pyramidal cell excitability and burst firing across segments, although it has the greatest effect in the LS and the least effect in the CMS, consistent with the patterns of 5-HT innervation described above (Deemyad et al., 2011). 5-HT also altered pyramidal cell responses to mimics of sensory input in vitro. Indeed, 5-HT led to whitening of the tuning curve in response to broadband current injection, i.e. the response strength became more evenly distributed across stimulus frequencies. Specifically, the response to the low-frequency stimulus components was slightly decreased and the response to the high-frequency components was increased (Deemyad et al., 2011) (Fig. 3B).
Mechanisms underlying the action of serotonergic input to ELL pyramidal cells
Studies performed in vitro revealed that increased excitability following 5-HT application was due to a decreased AHP (Deemyad et al., 2011). Further, as 5-HT application increased the membrane resistance, it was hypothesized that 5-HT downregulated K+ channels that mediate the AHP (Fig. 3B) (Deemyad et al., 2011). SK channels were considered an attractive candidate as their distribution is also graded across the ELL segments with greatest expression in LS (Fig. 2A) (Ellis et al., 2007b; Ellis et al., 2008). Occlusion experiments with the SK channel antagonist UCL-1684 (UCL) and 5-HT on LS pyramidal neurons indeed showed that 5-HT downregulates SK channels. However, as this effect only occurred in E-cells, it was hypothesized that another channel was responsible for the decreased AHP in I-type pyramidal cells. Occlusion experiments with the M-channel antagonists XE-991 and linopyridine revealed that 5-HT downregulates M currents in both E- and I-type pyramidal cells (Deemyad et al., 2011).
In contrast to ACh receptors, the distribution of 5-HT receptors in ELL is unknown. As such, it is currently not clear whether M and SK channels are downregulated through the effects of the same or different 5-HT receptors. The latter would provide enhanced control over frequency tuning, as a recent study has shown that M and SK channels have opposite effects on frequency tuning in ELL pyramidal cells (Deemyad et al., 2012). Future studies should investigate the receptors involved as well as the signaling cascades that lead to SK- and M-current downregulation in ELL pyramidal cells by 5-HT.
It should be noted that the experiments described above were performed in vitro. The effects of 5-HT on pyramidal cell activity in vivo are currently unknown and should be the focus of future studies. It is likely that they will differ slightly from those observed in vitro for several reasons. First, in situ hybridization studies have shown that ELL pyramidal cells possess two subtypes of SK channels: while SK2 channels are confined to the somatic regions of E-type pyramidal cells only, SK1 channels are instead found within the dendritic trees of both E- and I-type pyramidal cells (Fig. 2B). Both channel subtypes display the greatest/weakest levels of expression in LS/CMS, respectively (Ellis et al., 2008). Interestingly, SK channel antagonists applied in vitro only affect E-type pyramidal cells (Ellis et al., 2007b; Deemyad et al., 2011), whereas these same antagonists affect both E- and I-type pyramidal cells in vivo (Toporikova and Chacron, 2009). It is thus possible that 5-HT will downregulate SK1 channels in both E- and I-type pyramidal cells in vivo.
Second, like the muscarinic receptors discussed above and consistent with other systems (Berger et al., 2009), it is likely that 5-HT receptors will not be found solely on pyramidal cells but also on local interneurons as well as presynaptically in the ELL. 5-HT is thus likely to be able to exert a number of different effects on ELL pyramidal cells. For example, if the 5-HT receptors located on inhibitory interneurons were of a different type than the ones located on pyramidal cells, then activating the former might actually increase inhibition onto ELL pyramidal cells, thereby making them less excitable. Further studies investigating these potentially interesting effects in vivo are needed.
In summary, the activation of serotonergic input to ELL pyramidal cells increases excitability and alters responses to stimuli via downregulation of SK and M-type K+ channels. These effects are greatest in the LS and weakest in the CMS. Like cholinergic modulation, serotonergic modulation is likely to be highly dependent on behavioural context. Future studies should address the effect that dorsal raphe nucleus stimulation or local application of serotonin has on ELL pyramidal cell responses in an in vivo preparation.
Conclusions
The ELL is the only nucleus that receives and integrates direct input from peripheral electroreceptor afferents and thus constitutes the first brain area in which neuromodulators can alter the processing of sensory input based on changing behavioural contexts. Recent studies have made substantial progress towards understanding the effects of neuromodulators on sensory processing in ELL and the mechanisms that mediate them. However, many unanswered questions remain. In particular, it is possible that serotonergic and cholinergic neurons that innervate the ELL also express co-transmitters. If so, these co-transmitters could provide additional control of sensory processing by ELL neurons. Future studies will need to combine molecular, electrophysiological, as well as behavioural approaches in order to understand the behavioural contexts and relevant stimulus features that activate serotonergic and cholinergic inputs, as well as potential co-transmitters, to ELL pyramidal neurons and their consequences on sensory coding.
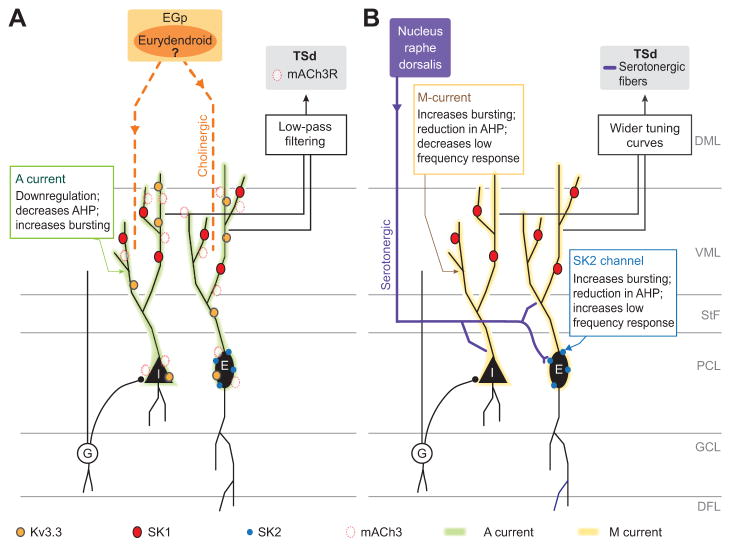
Earlier behavioural studies have shown that systemic application of 5-HT decreases aggressive behaviours in weakly electric fish and affects the waveform of the EOD in certain species (Maler and Ellis, 1987; Allee et al., 2008; Smith and Combs, 2008; Stoddard et al., 2006), but we do not yet know under which conditions intrinsic 5- HT release is modulated. Based on findings in other systems (Summers and Winberg, 2006), it is likely that agonistic encounters will activate the serotonergic system. In the case of ACh, application of atropine by itself does not alter the firing rate or bursting of pyramidal neurons, which suggests that the system is not constitutively active. Therefore, future studies should attempt to identify behavioural contexts and electrosensory stimulus conditions that activate the cholinergic as well as the serotonergic pathways in weakly electric fish. An additional important area of future research is to investigate how downstream areas decode altered sensory processing by ELL pyramidal neurons. In many cases, these areas receive cholinergic (Toscano-Márquez et al., 2013) as well as serotonergic (Johnston et al., 1990) inputs. This is, for example, the case for the midbrain TSd. Both cholinergic and serotonergic inputs are likely to have qualitatively different effects on different cell types in the TSd based on studies performed in other midbrain structures (Hurley et al., 2004; Hurley and Sullivan, 2012). Knowledge of the behavioural contexts and stimulus features that reliably activate serotonergic and cholinergic inputs to the ELL as well as other electrosensory structures will be essential for developing a functional theory of neuromodulation of sensory processing. The increase in burst firing and enhanced low-frequency information transmission caused by carbachol application suggests that the cholinergic modulation may play a role in specific behavioural contexts that involve low stimulus frequencies, such as foraging and/or communication with same-sex conspecifics. Based on the role 5-HT appears to play in aggressive interactions of A. leptorhynchus and the above-described findings on the effect of 5-HT on the response properties of ELL pyramidal cells, a first hypothesis for the behavioural role of 5-HT in electrosensory processing may be that subordinate males are brought into a ‘shut-up-and-listen’ mode, in which they do not produce communication signals (chirps), but are highly sensitive to stimuli caused by dominant conspecifics. The known and hypothesized effects of these two neuromodulators are summarized in Fig. 4.
Fig. 4.
Summary of the neuromodulation circuitry of the ELL. (A) Cholinergic system. Cholinergic input arising most likely from eurydendroid cells reaches the ELL molecular layer and affects both E- and I-type pyramidal cells. Acetylcholine acts through muscarinic receptor 3 (mAChR3) located in both types of pyramidal cells (Toscano-Márquez et al., 2013). This receptor downregulates an A-type potassium current leading to increased excitability. It also decreases the cell’s AHP, which increases bursting. Additional effects of acetylcholine via SK and Kv3.3 channels are possible. The modulation of pyramidal cell excitability is translated into enhanced processing of low-frequency stimuli. Additional cholinergic effects are expected in the target area of ELL pyramidal cells, the torus semicircularis dorsalis (TSd). (B) Serotonergic system. Serotonin input reaches the ELL through fibres originating in the nucleus raphe dorsalis. The fibres make synapses on the dendrites and somata of E- and I-type pyramidal cells. In both E- and I-cells they downregulate an M-type current. This downregulation increases bursting, reduces the AHP and reduces the response to low-frequency stimuli. Additionally to the M current, 5-HT downregulates SK2 channels in E-type cells. The downregulation of SK2 enhances the processing of low-frequency stimuli (Deemyad et al., 2011). Additionally, it is possible that serotonin modulates SK1 channels in the dendrites of I-type cells. The effects of serotonin on the excitability of pyramidal cells lead to a whitening of the tuning curve of the pyramidal cells. DFL, deep fibre layer; DML, dorsal molecular layer; EGp, eminentia granularis posterior; G, granular cell; GCL, granule cell layer; PCL, pyramidal cell layer; StF, stratum fibrosum; VML, ventral molecular layer.
Acknowledgments
The authors would like to thank L. Maler for useful discussions.
Funding
This research was supported by grants from the Fonds de Recherche sur la Nature et les Technologies du Québec (R.K., M.J.C.) and the Canada Research Chairs Program (M.J.C.). Fellowships were provided to B.T.M. by the Consejo Nacional de Ciencia y Tecnología and the Fonds de Recherche sur la Nature et les Technologies du Québec.
List of abbreviations
- 4-AP
4-aminopyridine
- 5-HT
serotonin
- ACh
acetylcholine
- AHP
afterhyperpolarization
- CLS
centrolateral segment
- CMS
centromedial segment
- EGp
eminentia granularis posterior
- ELL
electrosensory lateral line lobe
- EOD
electric organ discharge
- LS
lateral segment
- Pd
nucleus praeeminentialis dorsalis
- SK
small-conductance calcium-activated potassium (channel)
- TSd
torus semicircularis dorsalis
Footnotes
Author contributions
B.T.M, R.K. and M.J.C. conceived and wrote the manuscript.
Competing interests
No competing interests declared.
References
- Abbott LF. Where are the switches on this thing? In: Van Hemmen JL, Sejnowski TJ, editors. 23 Problems in Systems Neuroscience. New York, NY: Oxford University Press; 2005. pp. 423–431. [Google Scholar]
- Adams PR, Brown DA, Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Akerberg OA, Chacron MJ. In vivo conditions influence the coding of stimulus features by bursts of action potentials. J Comput Neurosci. 2011;31:369–383. doi: 10.1007/s10827-011-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–242. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- Allee SJ, Markham MR, Salazar VL, Stoddard PK. Opposing actions of 5HT1A and 5HT2-like serotonin receptors on modulations of the electric signal waveform in the electric fish Brachyhypopomus pinnicaudatus. Horm Behav. 2008;53:481–488. doi: 10.1016/j.yhbeh.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Akerberg O, Krahe R, Chacron MJ. Neural heterogeneities and stimulus properties affect burst coding in vivo. Neuroscience. 2010;168:300–313. doi: 10.1016/j.neuroscience.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G. Ionic channel function in action potential generation: current perspective. Mol Neurobiol. 2007;35:129–150. doi: 10.1007/s12035-007-8001-0. [DOI] [PubMed] [Google Scholar]
- Bastian J. Gain control in the electrosensory system mediated by descending inputs to the electrosensory lateral line lobe. J Neurosci. 1986;6:553–562. doi: 10.1523/JNEUROSCI.06-02-00553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J. Plasticity of feedback inputs in the apteronotid electrosensory system. J Exp Biol. 1999;202:1327–1337. doi: 10.1242/jeb.202.10.1327. [DOI] [PubMed] [Google Scholar]
- Bastian J, Courtright J. Morphological correlates of pyramidal cell adaptation rate in the electrosensory lateral line lobe of weakly electric fish. J Comp Physiol A. 1991;168:393–407. doi: 10.1007/BF00199600. [DOI] [PubMed] [Google Scholar]
- Bastian J, Nguyenkim J. Dendritic modulation of burst-like firing in sensory neurons. J Neurophysiol. 2001;85:10–22. doi: 10.1152/jn.2001.85.1.10. [DOI] [PubMed] [Google Scholar]
- Bastian J, Chacron MJ, Maler L. Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity. J Neurosci. 2002;22:4577–4590. doi: 10.1523/JNEUROSCI.22-11-04577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J, Chacron MJ, Maler L. Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron. 2004;41:767–779. doi: 10.1016/s0896-6273(04)00071-6. [DOI] [PubMed] [Google Scholar]
- Bell CC, Maler L. Central neuroanatomy of electrosensory systems in fish. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York, NY: Springer; 2005. pp. 68–111. [Google Scholar]
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman NJ, Maler L. Neural architecture of the electrosensory lateral line lobe: adaptations for coincidence detection, a sensory searchlight and frequency-dependent adaptive filtering. J Exp Biol. 1999;202:1243–1253. doi: 10.1242/jeb.202.10.1243. [DOI] [PubMed] [Google Scholar]
- Billimoria CP, DiCaprio RA, Birmingham JT, Abbott LF, Marder E. Neuromodulation of spike-timing precision in sensory neurons. J Neurosci. 2006;26:5910–5919. doi: 10.1523/JNEUROSCI.4659-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham JT, Tauck DL. Neuromodulation in invertebrate sensory systems: from biophysics to behavior. J Exp Biol. 2003;206:3541–3546. doi: 10.1242/jeb.00601. [DOI] [PubMed] [Google Scholar]
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci. 2010;41:340–346. doi: 10.1007/s12031-010-9377-2. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Hopkins CD, Popper AN, Fay RR. Electroreception. New York, NY: Springer; 2005. [Google Scholar]
- Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory nerves in gymnotiform fish. J Comp Neurol. 1982;211:139–153. doi: 10.1002/cne.902110204. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors – characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Chacron MJ. Nonlinear information processing in a model sensory system. J Neurophysiol. 2006;95:2933–2946. doi: 10.1152/jn.01296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacron MJ, Bastian J. Population coding by electrosensory neurons. J Neurophysiol. 2008;99:1825–1835. doi: 10.1152/jn.01266.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacron MJ, Doiron B, Maler L, Longtin A, Bastian J. Non-classical receptive field mediates switch in a sensory neuron’s frequency tuning. Nature. 2003;423:77–81. doi: 10.1038/nature01590. [DOI] [PubMed] [Google Scholar]
- Chacron MJ, Maler L, Bastian J. Electroreceptor neuron dynamics shape information transmission. Nat Neurosci. 2005a;8:673–678. doi: 10.1038/nn1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacron MJ, Maler L, Bastian J. Feedback and feedforward control of frequency tuning to naturalistic stimuli. J Neurosci. 2005b;25:5521–5532. doi: 10.1523/JNEUROSCI.0445-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Curr Opin Neurobiol. 2011;21:752–760. doi: 10.1016/j.conb.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Johnston D. Properties of single voltage-dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J Physiol. 2004;559:187–203. doi: 10.1113/jphysiol.2004.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Montgomery JC. Comparing octavolateralis sensory systems: what can we learn? In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York, NY: Springer; 2005. pp. 318–359. [Google Scholar]
- Dacks AM, Green DS, Root CM, Nighorn AJ, Wang JW. Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J Neurogenet. 2009;23:366–377. doi: 10.3109/01677060903085722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Maler L, Chacron MJ. Inhibition of SK and M channel-mediated currents by 5-HT enables parallel processing by bursts and isolated spikes. J Neurophysiol. 2011;105:1276–1294. doi: 10.1152/jn.00792.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Kroeger J, Chacron MJ. Sub- and suprathreshold adaptation currents have opposite effects on frequency tuning. J Physiol. 2012;590:4839–4858. doi: 10.1113/jphysiol.2012.234401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore S, Linster C. Noradrenergic and cholinergic modulation of olfactory bulb sensory processing. Front Behav Neurosci. 2012;6:52. doi: 10.3389/fnbeh.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM. Beyond traditional approaches to understand the functional role of neuromodulators in sensory cortices. Front Behav Neurosci. 2012;6:45. doi: 10.3389/fnbeh.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LD, Krahe R, Bourque CW, Dunn RJ, Chacron MJ. Muscarinic receptors control frequency tuning through the downregulation of an Atype potassium current. J Neurophysiol. 2007a;98:1526–1537. doi: 10.1152/jn.00564.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LD, Mehaffey WH, Harvey-Girard E, Turner RW, Maler L, Dunn RJ. SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons. J Neurosci. 2007b;27:9491–9502. doi: 10.1523/JNEUROSCI.1106-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LD, Maler L, Dunn RJ. Differential distribution of SK channel subtypes in the brain of the weakly electric fish Apteronotus leptorhynchus. J Comp Neurol. 2008;507:1964–1978. doi: 10.1002/cne.21597. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol. 2007;34:1077–1083. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- Fernandez FR, Mehaffey WH, Turner RW. Dendritic Na+ current inactivation can increase cell excitability by delaying a somatic depolarizing afterpotential. J Neurophysiol. 2005;94:3836–3848. doi: 10.1152/jn.00653.2005. [DOI] [PubMed] [Google Scholar]
- Finger TE. Efferent neurons of the teleost cerebellum. Brain Res. 1978;153:608–614. doi: 10.1016/0006-8993(78)90346-3. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Metzner W, Wessel R, Koch C. From stimulus encoding to feature extraction in weakly electric fish. Nature. 1996;384:564–567. doi: 10.1038/384564a0. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W. Neural Nets in Electric Fish. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- Heiligenberg W, Dye J. Labelling of electroreceptive afferents in a gymnotoid fish by intracellular injection of HRP: The mystery of multiple maps. J Comp Physiol A. 1982;148:287–296. [Google Scholar]
- Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, Caron MG, Eggers ED, Frishman LJ, McCall MA, et al. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–110. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR. From behavioral context to receptors: serotonergic modulatory pathways in the IC. Front Neural Circuits. 2012;6:58. doi: 10.3389/fncir.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Ikenaga T, Yoshida M, Uematsu K. Cerebellar efferent neurons in teleost fish. Cerebellum. 2006;5:268–274. doi: 10.1080/14734220600930588. [DOI] [PubMed] [Google Scholar]
- Johnston SA, Maler L, Tinner B. The distribution of serotonin in the brain of Apteronotus leptorhynchus: an immunohistochemical study. J Chem Neuroanat. 1990;3:429–465. [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc B. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J Neurophysiol. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- Krahe R, Kreiman G, Gabbiani F, Koch C, Metzner W. Stimulus encoding and feature extraction by multiple sensory neurons. J Neurosci. 2002;22:2374–2382. doi: 10.1523/JNEUROSCI.22-06-02374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe R, Bastian J, Chacron MJ. Temporal processing across multiple topographic maps in the electrosensory system. J Neurophysiol. 2008;100:852–867. doi: 10.1152/jn.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Turner RW. Conditional spike backpropagation generates burst discharge in a sensory neuron. J Neurophysiol. 2000;84:1519–1530. doi: 10.1152/jn.2000.84.3.1519. [DOI] [PubMed] [Google Scholar]
- Litwin-Kumar A, Chacron MJ, Doiron B. The spatial structure of stimuli shapes the timescale of correlations in population spiking activity. PLoS Comput Biol. 2012;8:e1002667. doi: 10.1371/journal.pcbi.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maler L. The posterior lateral line lobe of certain gymnotoid fish: quantitative light microscopy. J Comp Neurol. 1979;183:323–363. doi: 10.1002/cne.901830208. [DOI] [PubMed] [Google Scholar]
- Maler L. Receptive field organization across multiple electrosensory maps. I. Columnar organization and estimation of receptive field size. J Comp Neurol. 2009a;516:376–393. doi: 10.1002/cne.22124. [DOI] [PubMed] [Google Scholar]
- Maler L. Receptive field organization across multiple electrosensory maps. II. Computational analysis of the effects of receptive field size on prey localization. J Comp Neurol. 2009b;516:394–422. doi: 10.1002/cne.22120. [DOI] [PubMed] [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Behav Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Maler L, Collins M, Mathieson WB. The distribution of acetylcholinesterase and choline acetyl transferase in the cerebellum and posterior lateral line lobe of weakly electric fish (Gymnotidae) Brain Res. 1981;226:320–325. doi: 10.1016/0006-8993(81)91106-9. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Marsat G, Maler L. Neural heterogeneity and efficient population codes for communication signals. J Neurophysiol. 2010;104:2543–2555. doi: 10.1152/jn.00256.2010. [DOI] [PubMed] [Google Scholar]
- Marsat G, Maler L. Preparing for the unpredictable: adaptive feedback enhances the response to unexpected communication signals. J Neurophysiol. 2012;107:1241–1246. doi: 10.1152/jn.00982.2011. [DOI] [PubMed] [Google Scholar]
- Marsat G, Proville RD, Maler L. Transient signals trigger synchronous bursts in an identified population of neurons. J Neurophysiol. 2009;102:714–723. doi: 10.1152/jn.91366.2008. [DOI] [PubMed] [Google Scholar]
- Marsat G, Longtin A, Maler L. Cellular and circuit properties supporting different sensory coding strategies in electric fish and other systems. Curr Opin Neurobiol. 2012;22:686–692. doi: 10.1016/j.conb.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Mathieson WB, Maler L. Morphological and electrophysiological properties of a novel in vitro preparation: the electrosensory lateral line lobe brain slice. J Comp Physiol A. 1988;163:489–506. doi: 10.1007/BF00604903. [DOI] [PubMed] [Google Scholar]
- McMahon A, Fowler SC, Perney TM, Akemann W, Knöpfel T, Joho RH. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur J Neurosci. 2004;19:3317–3327. doi: 10.1111/j.0953-816X.2004.03385.x. [DOI] [PubMed] [Google Scholar]
- Mehaffey WH, Doiron B, Maler L, Turner RW. Deterministic multiplicative gain control with active dendrites. J Neurosci. 2005;25:9968–9977. doi: 10.1523/JNEUROSCI.2682-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaffey WH, Fernandez FR, Rashid AJ, Dunn RJ, Turner RW. Distribution and function of potassium channels in the electrosensory lateral line lobe of weakly electric apteronotid fish. J Comp Physiol A. 2006;192:637–648. doi: 10.1007/s00359-006-0103-z. [DOI] [PubMed] [Google Scholar]
- Mehaffey WH, Maler L, Turner RW. Intrinsic frequency tuning in ELL pyramidal cells varies across electrosensory maps. J Neurophysiol. 2008a;99:2641–2655. doi: 10.1152/jn.00028.2008. [DOI] [PubMed] [Google Scholar]
- Mehaffey WH, Ellis LD, Krahe R, Dunn RJ, Chacron MJ. Ionic and neuromodulatory regulation of burst discharge controls frequency tuning. J Physiol Paris. 2008b;102:195–208. doi: 10.1016/j.jphysparis.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner W, Juranek J. A sensory brain map for each behavior? Proc Natl Acad Sci USA. 1997;94:14798–14803. doi: 10.1073/pnas.94.26.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner W, Koch C, Wessel R, Gabbiani F. Feature extraction by burst-like spike patterns in multiple sensory maps. J Neurosci. 1998;18:2283–2300. doi: 10.1523/JNEUROSCI.18-06-02283.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Bemis WE, Northcutt RG, Davis MC, Baker CVH. Electrosensory ampullary organs are derived from lateral line placodes in bony fishes. Nat Commun. 2011;2:496. doi: 10.1038/ncomms1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Nakajima S, Leonard RJ, Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1986;83:3022–3026. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Maciver MA. Prey capture in the weakly electric fish Apteronotus albifrons: sensory acquisition strategies and electrosensory consequences. J Exp Biol. 1999;202:1195–1203. doi: 10.1242/jeb.202.10.1195. [DOI] [PubMed] [Google Scholar]
- Oswald AMM, Chacron MJ, Doiron B, Bastian J, Maler L. Parallel processing of sensory input by bursts and isolated spikes. J Neurosci. 2004;24:4351–4362. doi: 10.1523/JNEUROSCI.0459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan M, Maler L. Distribution of muscarinic receptors in the caudal cerebellum and electrosensory lateral line lobe of gymnotiform fish. Neurosci Lett. 1983;42:137–143. doi: 10.1016/0304-3940(83)90396-8. [DOI] [PubMed] [Google Scholar]
- Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci. 2008;28:3209–3220. doi: 10.1523/JNEUROSCI.4310-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, Dunn RJ, Turner RW. A prominent soma-dendritic distribution of Kv3.3 K+ channels in electrosensory and cerebellar neurons. J Comp Neurol. 2001a;441:234–247. doi: 10.1002/cne.1409. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, Morales E, Turner RW, Dunn RJ. The contribution of dendritic Kv3 K+ channels to burst threshold in a sensory neuron. J Neurosci. 2001b;21:125–135. doi: 10.1523/JNEUROSCI.21-01-00125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Saunders J, Bastian J. The physiology and morphology of two types of electrosensory neurons in the weakly electric fish Apteronotus leptorhynchus. J Comp Physiol A. 1984;154:199–209. [Google Scholar]
- Shapiro MS, Gomeza J, Hamilton SE, Hille B, Loose MD, Nathanson NM, Roche JP, Wess J. Identification of subtypes of muscarinic receptors that regulate Ca2+ and K+ channel activity in sympathetic neurons. Life Sci. 2001;68:2481–2487. doi: 10.1016/s0024-3205(01)01042-6. [DOI] [PubMed] [Google Scholar]
- Shumway CA. Multiple electrosensory maps in the medulla of weakly electric gymnotiform fish. II. Anatomical differences. J Neurosci. 1989;9:4400–4415. doi: 10.1523/JNEUROSCI.09-12-04400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Combs N. Serotonergic activation of 5HT1A and 5HT2 receptors modulates sexually dimorphic communication signals in the weakly electric fish Apteronotus leptorhynchus. Horm Behav. 2008;54:69–82. doi: 10.1016/j.yhbeh.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Zakon HH, Markham MR, McAnelly L. Regulation and modulation of electric waveforms in gymnotiform electric fish. J Comp Physiol A. 2006;192:613–624. doi: 10.1007/s00359-006-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Exp Biol. 2006;209:4581–4589. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- Toporikova N, Chacron MJ. SK channels gate information processing in vivo by regulating an intrinsic bursting mechanism seen in vitro. J Neurophysiol. 2009;102:2273–2287. doi: 10.1152/jn.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano-Márquez B, Dunn RJ, Krahe R. Distribution of muscarinic acetylcholine receptor mRNA in the brain of the weakly electric fish Apteronotus leptorhynchus. J Comp Neurol. 2013;521:1054–1072. doi: 10.1002/cne.23218. [DOI] [PubMed] [Google Scholar]
- Turner RW, Lemon N, Doiron B, Rashid AJ, Morales E, Longtin A, Maler L, Dunn RJ. Oscillatory burst discharge generated through conditional backpropagation of dendritic spikes. J Physiol Paris. 2002;96:517–530. doi: 10.1016/S0928-4257(03)00007-X. [DOI] [PubMed] [Google Scholar]
- Vonderschen K, Chacron MJ. Sparse and dense coding of natural stimuli by distinct midbrain neuron subpopulations in weakly electric fish. J Neurophysiol. 2011;106:3102–3118. doi: 10.1152/jn.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wong CJ. Connections of the basal forebrain of the weakly electric fish, Eigenmannia virescens. J Comp Neurol. 1997;389:49–64. [PubMed] [Google Scholar]
- Zakon H, Oestreich J, Tallarovic S, Triefenbach F. EOD modulations of brown ghost electric fish: JARs, chirps, rises, and dips. J Physiol Paris. 2002;96:451–458. doi: 10.1016/S0928-4257(03)00012-3. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Jacoby R, Wu SM. Light- and dopamine-regulated receptive field plasticity in primate horizontal cells. J Comp Neurol. 2011;519:2125–2134. doi: 10.1002/cne.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GKH, Maler L. Evoked chirping in the weakly electric fish Apteronotus leptorhynchus: a quantitative biophysical analysis. Can J Zool. 1993;71:2301–2310. [Google Scholar]