Abstract
How the brain processes natural sensory input remains an important and poorly understood problem in neuroscience. The efficient coding hypothesis asserts that the brain’s coding strategies are adapted to the statistics of natural stimuli in order to efficiently process them, thereby optimizing their perception by the organism. Here we examined whether gymnotiform weakly electric fish displayed behavioral responses that are adapted to the statistics of the natural electrosensory envelopes. Previous studies have shown that the envelopes resulting from movement tend to consist of low (<1 Hz) temporal frequencies and are behaviorally relevant whereas those resulting from social interactions consist of higher (>1 Hz) temporal frequencies that can thus mask more behaviorally relevant signals. We found that the self-generated electric organ discharge frequency follows the detailed time course of the envelope around a mean value that is positively offset with respect to its baseline value for temporal frequencies between 0.001 Hz and 1 Hz. The frequency-following component of this behavioral response decreased in magnitude as a power law as a function of the envelope frequency and was negligible for envelope frequencies above 1 Hz. In contrast, the offset component was relatively constant and somewhat increased for envelope frequencies above 1 Hz. Thus, our results show that weakly electric fish display behavioral responses that track the detailed time course of low but not high frequency envelope stimuli. Furthermore, we found that the magnitude of the frequency-following behavioral response matches, in a one-to-one fashion, the spectral power of natural second-order stimulus attributes observed during movement. Indeed, both decayed as a power law with the same exponent for temporal frequencies spanning three orders of magnitude. Thus, our findings suggest that the neural coding strategies used by weakly electric fish perceive the detailed time course of movement envelopes and are adapted to their statistics as found in the natural environment. They also suggest that weakly electric fish might take advantage of the differential frequency content of movement and social envelopes in order to give appropriate behavioral responses during encounters between two or more conspecifics.
Keywords: Envelope, Weakly electric fish, Neural coding, Natural stimulus, Statistics
INTRODUCTION
Gymnotiform weakly electric fish generate a quasi-sinusoidal electric field through an electric organ discharge (EOD) and constitute an attractive model system for studying how the brain processes second-order stimulus attributes because of well-characterized anatomy and behavior (Chacron et al., 2003; Chacron et al., 2011; Krahe and Maler, 2014; Márquez et al., 2013; Marsat et al., 2012; Stamper et al., 2013). Specific electroreceptors scattered on the skin surface monitor changes in the amplitude of the field (i.e. the stimulus) caused by objects with conductivity different than that of the surrounding water and relay this information to pyramidal neurons within the hindbrain electrosensory lateral line lobe (ELL), which then project to the midbrain torus semicircularis.
Natural electrosensory stimuli comprise the sinusoidal variations in the amplitude of each fish’s EOD (i.e. beats, whose waveform is considered a first order stimulus attribute) that occur when two fish are in close proximity to each other and whose frequency is equal to the difference between both fish’s EOD frequencies. Perhaps the best known electrosensory behavior in response to beats is the jamming avoidance response (JAR), in which two fish whose EOD frequencies are within a few hertz of another (thereby causing a low frequency beat) each shift their EOD frequencies in order to increase the beat frequency, thereby moving it away from the frequency range of more behaviorally relevant stimuli such as prey (Heiligenberg, 1991). Recent studies have shown that the beat amplitude (i.e. the envelope, which is considered a second-order attribute) can vary in time during two different behavioral contexts. In the first context, a time-varying envelope is created during movement when two or more fish interact. These ‘movement’ envelopes carry important information about the sensory environment such as the distance between both animals and primarily contain low (<1 Hz) temporal frequencies (Fotowat et al., 2013; Yu et al., 2012). In the second context, interference between the electric fields of three or more stationary fish creates an envelope that varies sinusoidally at a frequency given by the difference between the two beat frequencies. These ‘social’ envelopes generally contain higher (>1 Hz) frequencies (Fotowat et al., 2013; Stamper et al., 2010). It is important to understand that, unlike movement envelopes, the animal can actually modify the frequency content of social envelopes by changing its EOD frequency (Stamper et al., 2013; Stamper et al., 2012). In fact, a recent study has shown that the weakly electric fish species Eigenmannia viriscens displays an avoidance response to social envelopes by changing its EOD frequency in order to increase the envelope frequency, higher than the frequency range of more behaviorally relevant stimuli such as prey (Stamper et al., 2012). This suggests that social envelopes are more of a jamming signal that, similar to low frequency beats, impair the animal’s ability to electrolocate. Previous studies have shown that, whereas both electroreceptors and pyramidal neurons respond to both first and second-order attributes of electrosensory stimuli (McGillivray et al., 2012; Middleton et al., 2006; Savard et al., 2011; Vonderschen and Chacron, 2011), different subsets of torus semicircularis neurons each respond selectively to first- and second-order attributes (McGillivray et al., 2012). However, whether weakly electric fish display behavioral responses to movement envelopes such as being able to extract the behaviorally relevant information that they contain remains unknown to this day.
In this study we examined whether gymnotiform weakly electric fish display behavioral responses to mimics of the low frequency second-order stimulus attributes occurring during movement. We found that the animals’ EOD frequency actively followed the detailed time course of these stimuli for frequencies below 1 Hz, indicating that the detailed information contained in the signal reaches higher order brain areas. This behavior occurred in both restrained and freely moving animals and showed habituation during repeated presentations of the stimulus. For higher (>1 Hz) frequencies, we instead observed that the animals increased their EOD frequency, presumably to minimize interference with other more behaviorally relevant signals. Finally, we showed that the frequency-following behavior is adapted to the scale invariant natural statistics of natural movement envelopes in a manner consistent with these signals being optimally processed in the brain. Thus, our results strongly suggest that weakly electric fish display behavioral responses that are adapted to the natural frequency content of envelopes.
RESULTS
Weakly electric fish display behavioral responses to mimics of movement envelopes
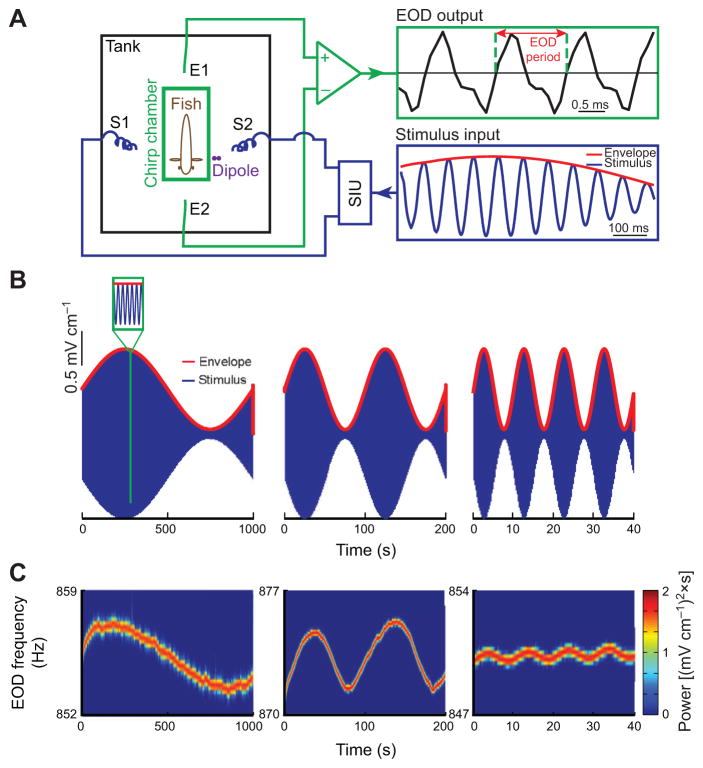
To determine whether weakly electric fish show behavioral responses to movement envelopes, we recorded (Fig. 1A) the EODs of three species (Apteronotus leptorhynchus, Apteronotus albifrons and Sternopygus sp.) in response to such stimuli. These consisted of a sinusoidal waveform (i.e. the carrier) whose amplitude (i.e. the envelope) also varied sinusoidally but at a lower frequency (Fig. 1B). This stimulus was initially given as an amplitude modulation of the animal’s EOD in order to mimic the beat that occurs when two conspecifics come into close proximity. We found that the animal’s EOD frequency varied in response to these stimuli and thus followed the detailed time course of the envelope waveform (Fig. 1C). Higher envelope frequencies gave rise to weaker behavioral responses (compare left, middle and right panels of Fig. 1C). Closer visual inspection of the EOD frequency revealed a quasi-sinusoidal variation around a mean value that was positively offset with respect to baseline (i.e. in the absence of stimulation; Fig. 2A).
Fig. 1. Behavioral responses of weakly electric fish to second-order stimuli.
(A) Schematic of the experimental setup in which a fish is restrained in a tube (‘chirp chamber’) placed in an otherwise empty tank. The animal’s electric field is monitored by a pair of electrodes located in front and behind the animal (straight lines; E1, E2) while the stimulus is delivered using a separate set of electrodes positioned on each side (spirals; S1, S2). SIU, stimulus isolation unit. (B) Example stimuli showing the carrier (blue) and the envelope (red) for three different envelope frequencies using a carrier of 15 Hz. Left: 0.001 Hz; middle: 0.01 Hz; right: 0.1 Hz. The inset shows a magnification of the stimulus. (C) EOD spectrograms (i.e. EOD power spectrum as a function of time) showing behavioral responses to the stimuli shown in B from an example specimen of Apteronotus leptorhynchus.
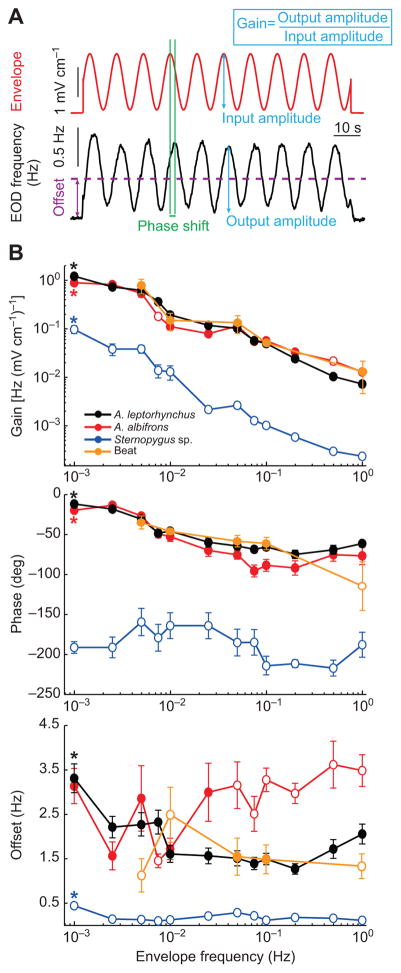
Fig. 2. Behavioral responses to second-order stimuli are species dependent.
(A) Example envelope stimulus (top, red) and EOD frequency response (bottom, black). Also shown are the parameters used to characterize the behavior of the fish: the gain (blue arrows and inset), which is the ratio of the response amplitude to the envelope amplitude; the phase, which is expressed as the time difference between the peak of the envelope and the peak of the response (green vertical lines) normalized to the stimulus period; the offset (purple arrow and dashed line), which is computed as the mean EOD frequency during stimulation minus the baseline EOD frequency (i.e. in the absence of stimulation). (B) Gain (top), phase (middle) and offset (bottom) as a function of envelope frequency for all three species tested (A. leptorhynchus, black; A. albifrons, red; Sternopygus sp., blue) as well as for the beat configuration (orange) for A. leptorhynchus. Asterisks indicate a significant change in response across envelope frequencies at the P=0.01 level using a one-way ANOVA. Open circles indicate statistically significant differences to the values obtained for A. leptorhynchus at the corresponding envelope frequencies at the P=0.05 level using a one-way ANOVA with Bonferroni post hoc correction. Errors bars show the s.e.m.
We characterized the relationship between EOD frequency and envelope by using linear system identification techniques. Specifically, we measured the gain (i.e. the ratio of output peak-to-peak amplitude to input peak-to-peak amplitude), the phase shift (i.e. the amount by which the input must be shifted in time relative to input period in order to be in phase with the output), and the offset (i.e. the difference between the mean EOD frequency during stimulation and that obtained in the absence of stimulation) for different carrier and envelope frequencies (Fig. 2A). We found that the gain for all three species decreased significantly as a function of increasing envelope frequency (Fig. 2B, top; one-way ANOVA, P<0.001) although the gain obtained for Sternopygus sp. was consistently lower by about an order of magnitude for all tested envelope frequencies than the gain obtained for either A. leptorhynchus or A. albifrons (one-way ANOVA with Bonferroni post hoc correction, P<0.05). However, the gain values obtained for A. leptorhynchus and A. albifrons did not differ significantly (one-way ANOVA with Bonferroni post hoc correction, P>0.05), except for envelope frequencies of 0.0075, 0.5 and 1 Hz (one-way ANOVA with Bonferroni post hoc correction, P<0.05).
We also observed a phase lag that significantly increased in magnitude as a function of envelope frequency for both A. leptorhynchus and A. albifrons (Fig. 2B, middle; one-way ANOVA, P<0.001), but not for Sternopygus sp. (one-way ANOVA, P=0.1715). The phase lag was considerably larger for all envelope frequencies for Sternopygus sp. than for either of A. leptorhynchus or A. albifrons (Fig. 2B, middle; one-way ANOVA with Bonferroni post hoc correction, P<0.05). Similar to the gain, we found no significant difference between A. leptorhynchus and A. albifrons (one-way ANOVA with Bonferroni post hoc correction, P>0.05).
Finally, offset values were relatively constant for A. albifrons (Fig. 2B, bottom; one-way ANOVA, P=0.0735). However, a significant decrease in offset was observed in A. leptorhynchus and Sternopygus sp. (one-way ANOVA, P<0.01). Furthermore, for higher envelope frequencies values were significantly higher for A. albifrons and significant lower for Sternopygus sp. compared with A. leptorhynchus (Fig. 2B, bottom; one-way ANOVA with Bonferroni post hoc correction, P<0.05).
Importantly, A. leptorhynchus displayed similar behavioral responses when the stimuli were delivered as beats (i.e. contained both amplitude and phase modulations; see Materials and methods; Fig. 2B, orange lines), indicating that the behavioral responses observed are not an artifact resulting from the fact that the stimuli were applied as amplitude modulations of the animal’s own EOD. There were no significant differences observed for the gain between the two conditions (one-way ANOVA, P>0.05). For the phase lag, we also found no significant difference (one-way ANOVA with Bonferroni post hoc correction, P>0.05) except for an envelope frequency of 1 Hz (one-way ANOVA with Bonferroni post hoc correction, P<0.05). Regarding offset, the overall responses were also similar for both stimulation conditions, although we found significant differences for envelope frequencies of 0.005, 0.01 and 1 Hz (one-way ANOVA with Bonferroni post hoc correction, P<0.05).
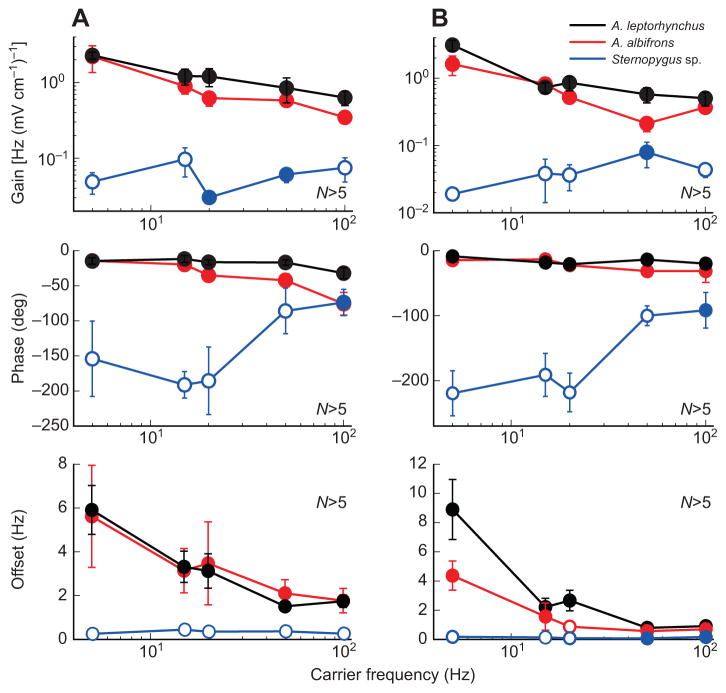
We next varied the carrier frequency while keeping the envelope frequency constant. We found that the gain (Fig. 3A,B, top), the phase (Fig. 3A,B, middle) and the offset (Fig. 3A,B, bottom) did not vary significantly for increasing carrier frequencies up to 100 Hz for all species (one-way ANOVA, P>0.05), indicting that this response is independent of carrier frequency and is distinct from other behavioral responses such as the JAR, which are not elicited in response to such high frequencies (Heiligenberg, 1991). As for varying envelope frequencies, Sternopygus sp. showed significantly different values for each of the measured quantities (one-way ANOVA with Bonferroni post hoc correction, P<0.05).
Fig. 3. Behavioral responses are independent of the carrier frequency.
(A) Gain (top), phase (middle) and offset (bottom) as a function of carrier frequency for a given envelope frequency of 0.001 Hz for all three species tested (A. leptorhynchus, black; A. albifrons, red; Sternopygus sp., blue). (B) Gain (top), phase (middle) and offset (bottom) as a function of carrier frequency for a given envelope frequency of 0.0025 Hz for all three species tested (colors as in A). Open circles indicate statistically significant difference with values obtained for A. leptorhynchus at the P=0.05 level using an ANOVA with Bonferroni correction. Errors bars show s.e.m.
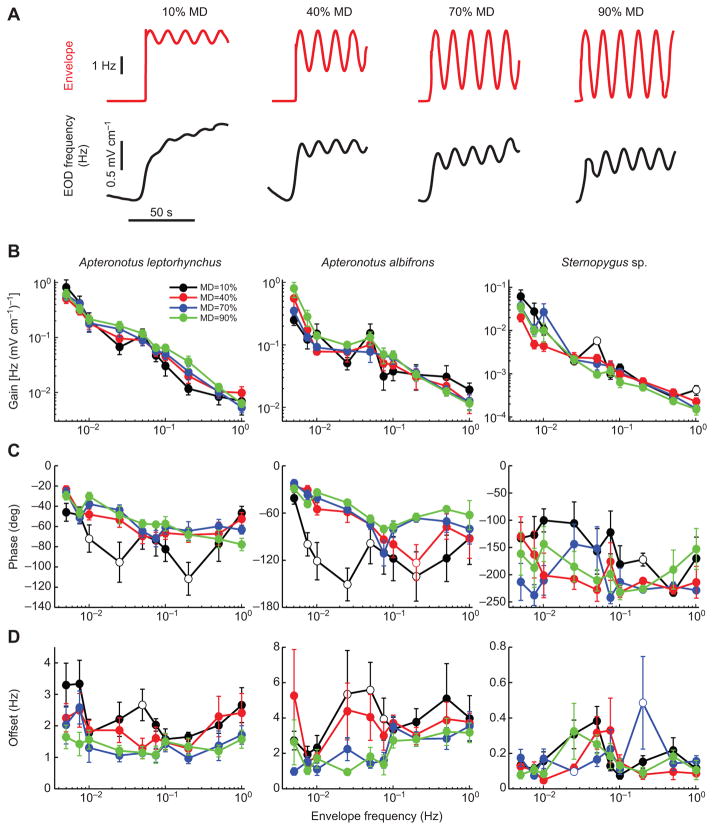
We next used envelope stimuli with different depths of modulation (Fig. 4A) in order to test whether our measures of linear systems identification techniques were appropriate. Our prediction is that similar values of gain, phase and offset will be obtained regardless of the depth of modulation. We found similar gain (Fig. 4B), phase (Fig. 4C) and offset (Fig. 4D) values for all depths of modulation tested for all three species (one-way ANOVA, P>0.05). These results confirm that the behavioral responses observed can indeed be accurately described using linear systems identification techniques.
Fig. 4. Behavioral responses are independent of the modulation depth (MD) across species.
(A) Example envelope stimulus (top, red) and EOD frequency response (bottom, black) for the different depth of modulations used. (B) Gain as a function of envelope frequency for all depths of modulation tested (10%, black; 40%, red; 70%, blue; 90%, green). (C) Phase as a function of envelope frequency for all depths of modulation tested (colors as in B). (D) Offset as a function of envelope frequency for all depths of modulation tested (colors as in B). Open circles indicate significant difference at the P=0.05 level to a modulation depth of 90% using an ANOVA with Bonferroni correction. Errors bars show s.e.m.
Overall, our results have shown that there are clear differences between A. albifrons/leptorhynchus and Sternopygus sp. in terms of behavioral responses to envelopes, which might reflect interesting differences in terms of neural processing as discussed below. However, since much more is known about the neural processing of envelopes in A. leptorhynchus than A. albifrons/Sternopygus sp. (McGillivray et al., 2012; Middleton et al., 2006; Savard et al., 2011; Vonderschen and Chacron, 2011), we focused on the former for the experiments presented below.
Weakly electric fish display habituation in response to repeated presentations of envelope stimuli
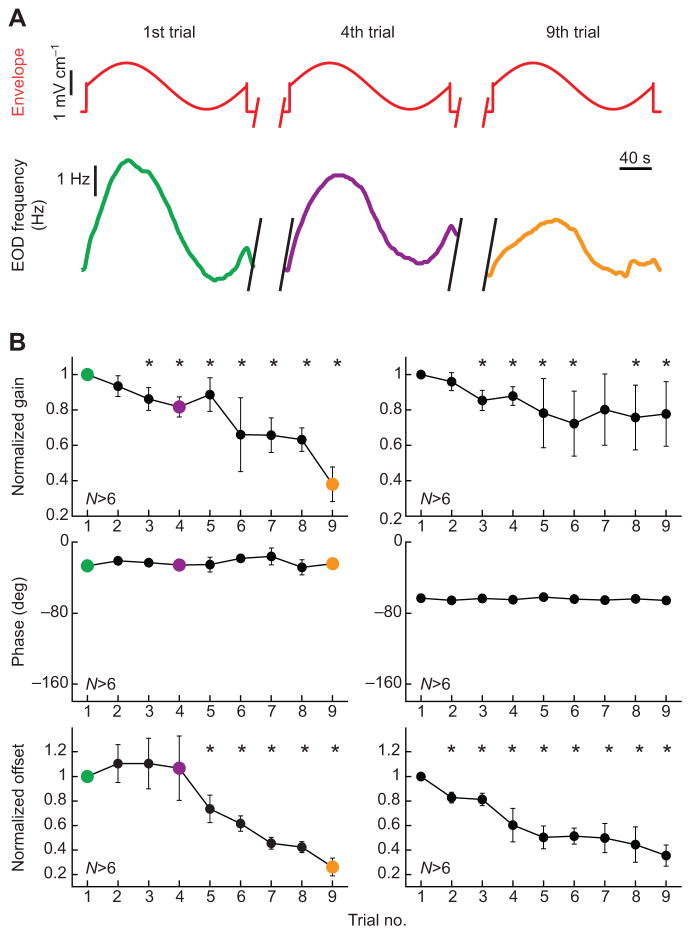
We next tested whether behavioral responses to envelopes were plastic. To do so, we recorded behavioral responses to repeated presentations of the same stimulus (Fig. 5A; see Materials and methods). Our results show that behavioral responses display habituation as the response amplitude decreases for later trials. This decrease was characterized by decreases in gain and offset while the phase lag remained constant (Fig. 5B). These results imply that the behavioral responses observed are plastic as they can be influenced by experience: these changes are most likely caused by top-down neural signals as discussed below.
Fig. 5. Behavioral responses habituate and strongly depend on envelope frequency content.
(A) Envelope stimulus (top) and EOD frequency response (bottom) during the first (left), fourth (center) and ninth (right) trial in A. leptorhynchus.
(B) Normalized gain (top), phase (center) and normalized offset (bottom) as a function of trial number for envelope frequencies of 0.005 Hz (left) and 0.2 Hz (right). Asterisks indicate statistically significant difference to the first trial at the P=0.05 level, using a paired t-test.
Behavioral responses are adapted to the frequency content of envelopes resulting from movement and social interaction
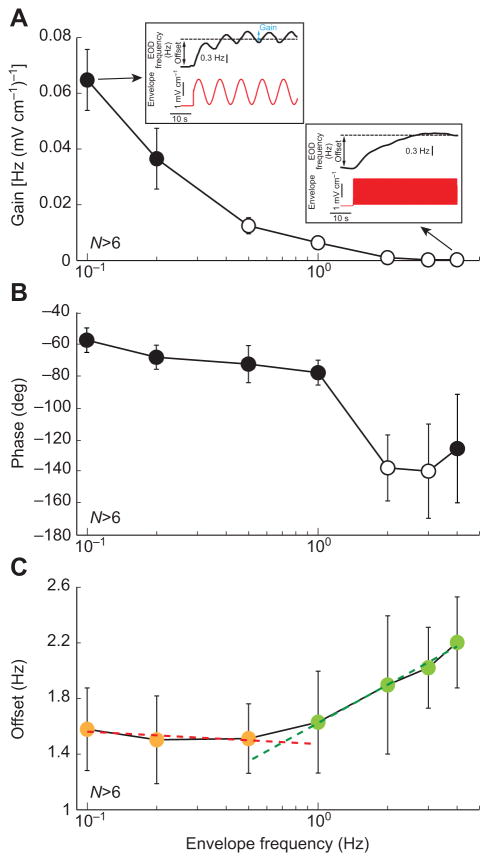
So far, the range of envelope frequencies tested concentrated mainly on envelopes caused by movement. However, as mentioned above, a recent study has shown that another species of weakly electric fish, Eigenmannia viriscens, actively shifts its EOD frequency in response to envelope stimuli resulting from social interaction (Stamper et al., 2012). Thus, we next tested whether behavioral responses might strongly differ when we considered instead envelopes with higher frequency content such as those resulting from social interactions in A. leptorhynchus. To do so, we used higher envelope frequencies (>1 Hz; Fig. 6). Our results show that, for frequencies >1 Hz, behavioral responses consisted almost exclusively of an increase in EOD frequency (i.e. an offset response; Fig. 6C). The frequency-following response was negligible (Fig. 6A) as the gain was not significantly different from zero for envelope frequencies higher than 1 Hz (one-way ANOVA with Bonferroni post hoc correction, P>0.05). The phase lag also increased for higher envelope frequencies (Fig. 6B) and became significant for envelope frequencies higher than 1 Hz. To quantify changes in offset response, we computed the slopes of the best fit straight lines through the offset responses for the first three envelope frequencies (0.1–0.5 Hz; Fig. 6C, red dashed line) and the last four envelope frequencies (1–4 Hz; Fig. 6C, green dashed line). We observed that the offset response tends to increase for the higher envelope frequencies as the former but not latter slope was significantly greater than zero at the P=0.05 level. These results show that A. leptorhynchus responds to higher envelope frequencies only by shifting its EOD frequency.
Fig. 6. Behavioral responses depend on envelope frequency.
(A) Gain as a function of envelope frequency. The insets show the envelope waveform (bottom, red) and the EOD frequency (top, black) for 0.1 (top left) and 4 Hz (top right). Note the significant decrease in gain for increasing envelope frequencies. In particular, gain estimates were not significantly different from zero for frequencies above 1 Hz (one-way ANOVA with Bonferroni post hoc correction, P>0.05). (B) Phase as a function of envelope frequency.
(C) Offset as a function of envelope frequency. Shown are best linear fits to the portion of the response curve below (red dashed line) and above (green dashed line) 1 Hz. The slope of the latter (0.4±0.035) but not the former (−0.04±0.04) line was significantly different from zero at the P=0.05 level. Open circles indicate statistically significant difference to the values obtained at an envelope frequency 0.1 Hz at the P=0.05 level using a one-way ANOVA with Bonferroni post hoc correction. Errors bars show s.e.m.
Behavioral responses to second-order stimuli occur in freely moving fish
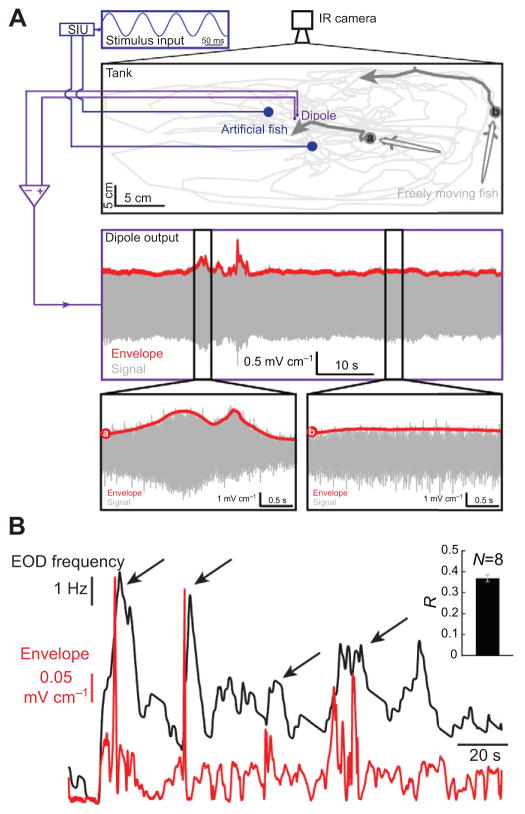
We next wanted to confirm that the behavioral responses were not an artifact of having the animal restrained. To do so, we measured the behavioral responses of freely moving fish to the envelopes caused by their own movements. It is important to note that other known behaviors (e.g. JAR, abrupt frequency rises), which are not due to changes in the envelope, can give rise to changes in EOD frequency similar to those reported here (Zakon et al., 2002). Thus, it is at best difficult to determine whether changes in EOD frequency are indeed due to an increase in the envelope when two or more freely moving fish are interacting. Hence, to ensure that any change in the animal’s EOD frequency was truly due to changes in the movement envelope stimulus, it was necessary to use a stationary dipole mimicking the EOD created by another fish (Fig. 7A, top panel). The dipole emitted a sinusoidal waveform whose frequency was at least 15 Hz above the fish’s own EOD frequency in order to ensure that any changes in EOD frequency were not due to other behaviors such as the JAR (see Materials and methods). Another dipole placed perpendicularly to the EOD-emitting dipole measured the stimuli (Fig. 7A, top panel) while an infrared camera positioned above the tank tracked the animal’s movement. Our results show that the freely moving fish explored the tank. Looming motion towards the dipole gave rise to an increase in the envelope stimulus, whereas lateral movement instead gave rise to a constant envelope (Fig. 7A, compare left and right bottom panels). This is consistent with previous reports (Fotowat et al., 2013; Middleton et al., 2006; Yu et al., 2012). We then extracted the moving fish’s time-varying EOD frequency and compared it with the time-varying envelope stimulus measured by the dipole (Fig. 7B). We observed that the animal’s EOD frequency tended to increase after an increase in the envelope stimulus (see arrows in Fig. 7B) and that there was a significant positive correlation between both signals (Fig. 7B, inset). These results thus demonstrate that freely moving weakly electric fish display behavioral responses to the envelope stimuli caused by their own movements.
Fig. 7. Behavioral responses occur in freely moving fish.
(A) Top: behavioral setup in which a fish is free to move in a tank containing two dipoles. One dipole is meant to mimic a fish (‘artificial fish’) while the other records the signals close to it. The inset shows the signal that is applied to the emitting dipole. The freely moving fish’s trajectory during an example session is shown in light gray and was filmed using an infrared camera. The dark gray arrows depict two different movement patterns of the same freely moving fish: one where the fish showed a looming motion towards the restrained fish (a) and a second situation where the fish was swimming far away from the restrained fish (b). Center: signal (gray) and envelope (red) recorded from the dipole during a typical trial. Increases in the envelope were seen when the animal approached the dipole. Bottom: Signal (gray) and envelope (red) corresponding to the trajectories (a and b) are shown in the left and right panels, respectively. Note that we used the artificial fish because it is not possible to reliably ascertain whether increases in EOD frequency are actually due to increases in the envelope rather than other behaviors (see Results and Materials and methods). (B) Envelope stimulus (red) and EOD frequency (black) of the freely moving fish. The inset shows the correlation coefficient between envelope and EOD frequency averaged over eight A. leptorhynchus specimens.
Behavioral responses are adapted to the statistics of natural movement envelopes
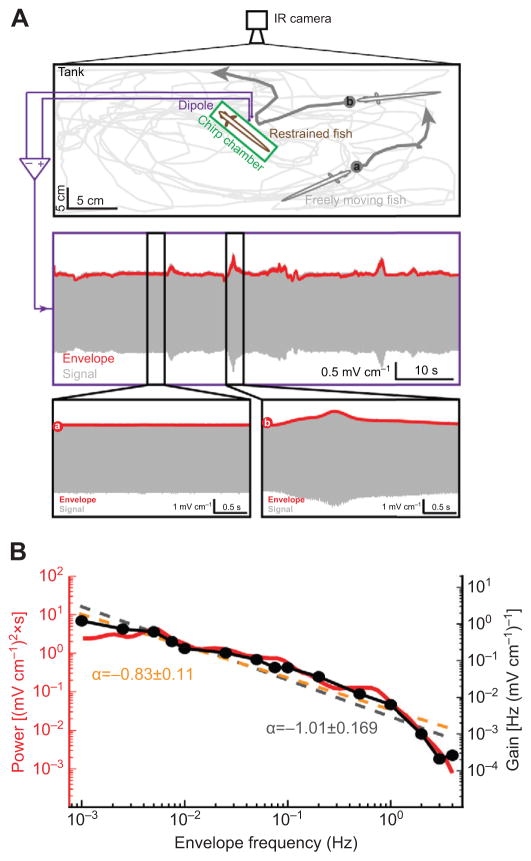
Finally, we tested whether the behavioral responses reported here were adapted to the natural statistics of movement envelopes. Although previous studies have reported that movement envelopes primarily contain low temporal frequencies (Yu et al., 2012) because their power spectra decays as a power law (Fotowat et al., 2013), these only measured the frequency content for frequencies higher than 0.05 Hz, whereas our results show that behavioral responses were obtained for frequencies as low as 0.001 Hz (see Fig. 2). Thus, we took long movies of a freely moving weakly electric fish interacting with another one that is restrained in a chirp chamber with a small dipole (Fig. 8A, see Materials and methods). This was done because field studies have shown that A. leptorhynchus were five times more likely to be found in pairs than in larger groups (Stamper et al., 2010). We found that the resulting envelope signal was almost constant for significant (10 s) amounts of time (Fig. 8A, middle and left bottom panels) which corresponded to periods when the freely moving fish was either immobile or performed lateral motion far from the restrained fish. In contrast, periods when the freely moving fish performed looming motion gave rise to increases in the envelope (Fig. 8A, middle and bottom right panels). We then measured the frequency content of the envelope signal and found that spectral power decayed as a power law with exponent −0.83±0.11 (R2=0.86) as a function of temporal frequency (Fig. 8B). Thus, our results confirm that natural movement envelopes indeed include scale invariance (Fotowat et al., 2013) but extend them to frequencies as low as 0.001 Hz. It is important to note that previous measurements using one freely moving fish and one restrained fish (Yu et al., 2012) and using two freely moving fish with one wearing a wireless transmitter (Fotowat et al., 2013) only measured frequencies as low as 0.05 Hz. If we restrict the fitting range to frequencies considered in these previous studies, then we find an exponent α= −1.86±0.16 (R2=0.91), which is not significantly different from the exponent value of −1.73 reported by Fotowat et al. (Fotowat et al., 2013). It is thus unlikely that our measurements were an artifact of having one fish restrained. Importantly, we found a one-to-one match between the gain of the frequency-following behavioral response and the frequency content of natural movement envelopes (Fig. 8B). Indeed, we found that the gain curve was well fitted by a power law with exponent α= −1.01±0.169 (R2=0.93). We found no significant difference between the power exponents for the gain and power spectra (P>0.05, paired t-test), indicating that both curves obey the same power law up to a proportionality constant. These results strongly suggest that the frequency components of natural movement envelopes with the highest power give rise to the strongest behavioral responses, as measured by gain (discussed below).
Fig. 8. Behavioral responses are adapted to natural statistics of movement envelopes.
(A) Top: behavioral setup in which a freely moving fish interacts with a restrained fish. The freely moving fish’s trajectory during an example session is shown in light gray and was filmed using an IR camera. The dark gray arrows depict two different movement patterns of the freely moving fish: one where the fish was swimming far away from the restrained fish (a) and a second situation where the fish showed a looming motion towards the restrained fish (b). Center: signal (gray) and envelope (red) recorded by the dipole. Bottom: signal (gray) and envelope (red) corresponding to the trajectories a and b are shown in the left and right panels, respectively. (B) Power spectrum of the envelope signal and best power law fit (orange dashed line) with power law exponent α=−0.83±0.11 (R2=0.86). Superimposed is the gain measured for A. leptorhynchus from Fig. 2B whose best power law fit (gray dashed line) had an exponent α =−1.01±0.169 (R2=0.93). Note that the ranges are the same for both y-axes. Therefore, because exponents did not differ significantly from one another (P>0.05, paired t-test) both curves can be described by the same power law up to a proportionality constant.
DISCUSSION
We investigated whether weakly electric fish responded behaviorally to mimics of the second-order stimulus features that occur in their natural environment. We found that the EOD frequency of A. leptorhynchus, A. albifrons and Sternopygus sp. followed the detailed time course of low frequency envelopes around a mean value that was positively offset with respect to baseline. Frequency following was strongest for low (<1 Hz) whereas the offset was strongest for higher (>1 Hz) envelope frequencies. Both frequency-following and offset responses displayed habituation in response to repeated presentations of the stimulus. Furthermore, we found that freely moving fish increased the EOD frequency during looming motion, which also causes increases in the envelope. Finally, we found a one-to-one match between the gain of the frequency-following response and the power content of natural movement envelopes for over three orders of magnitude, strongly suggesting that behavioral responses are adapted to the statistics of natural stimuli consistent with optimal processing by the brain.
Indeed, it has long been assumed that neurons are adapted on evolutionary, developmental and behavioral time scales, to the signals to which they are exposed. However, because not all signals are equally likely, it is natural to assume that perceptual systems should be able to best process those signals that occur most frequently (Simoncelli and Olshausen, 2001). Thus, optimal coding theory predicts that sensory neurons should be best tuned to stimuli that occur most frequently in the natural environment (Laughlin, 1981; Wark et al., 2007), which in turn should give rise to the strongest behavioral responses. Thus, our results show that the frequency-following response is matched to the frequency content of natural movement envelopes if we assume that signal power is proportional to the probability of occurrence in the natural environment. Our results therefore strongly suggest that weakly electric fish display behavioral responses that have adapted to the statistics of movement envelopes in their natural environment in a manner that is consistent with them being efficiently processed by the brain.
Our results have shown that Sternopygus sp. gave behavioral responses to envelope stimuli but that these are considerably weaker than responses of either A. leptorhynchus or A. albifrons, by approximately an order of magnitude. The large phase lags observed for this species is likely to result in part from this weak response. This difference is interesting given that the former does not display a JAR whereas the latter two do (Bullock et al., 1975). We thus propose that the neural circuits that mediate the frequency-following response are at least in part different from those mediating the JAR. However, it is probable that the neural circuits that mediate the offset response are similar to those that mediate the JAR, given the relative similarity between the former and the social envelope response observed in Eigenmannia (Stamper et al., 2012). Further support for this hypothesis comes from the fact that larger offset responses were observed for low frequency carriers.
Our results strongly suggest that the frequency-following behavioral response is adapted to the natural statistics of envelopes in A. leptorhynchus that occur during movement, thereby indicating that this response serves an important function. As EOD frequency has been associated with social status in A. leptorhynchus (Dunlap and Oliveri, 2002), we propose that increases in EOD frequency when the distance between two fish lessens (e.g. during looming motion) serve to make each fish appear more dominant and thus discourage further aggressive behavior (e.g. fighting). Thus, it would then be necessary for the brain to keep track of second-order stimulus features that carry information about the relative position of other fish at all times (Fotowat et al., 2013). It is therefore probable that the very low frequency components of natural movement envelopes, which arise either because fish remain stationary for extended periods of time or because of lateral motion, carry important behaviorally relevant information and must be processed by the brain. We nevertheless point out that the relationship between EOD frequency and sex and/or social status is actually reversed in both A. albifrons (Dunlap et al., 1998; Kolodziejski et al., 2005) and Sternopygus (Zakon et al., 1991). It is thus very probable that the observed behavioral responses to envelopes in these species will have different functions than the one proposed above for A. leptorhynchus.
Processing stimuli varying over large timescales poses a serious challenge because many neurons display sensory adaptation (i.e. the capacity of neural circuits to alter their tuning in response to long-term changes in stimulus statistics): which optimizes information transmission at the expense of creating ambiguity (Wark et al., 2007) by attenuating, if not removing, neural responses to stimuli varying over large timescales (Benda and Hennig, 2008). We argue that at least a subpopulation of electrosensory neurons should not adapt to second-order stimulus features in order to keep track of second-order stimulus features resulting from movement. Although we previously found that a subgroup of neurons within the electrosensory midbrain responded selectively to second-order features (McGillivray et al., 2012; Vonderschen and Chacron, 2011), further studies are needed to establish whether these actually respond to very low frequencies and whether they constitute the neural correlate of behavioral responses observed in this study. Importantly, all neurophysiological studies looking at how electrosensory neurons respond to envelopes were done using artificial (e.g. noise) stimuli (McGillivray et al., 2012; Middleton et al., 2006; Savard et al., 2011). Recent studies have quantified the statistics of natural movement (Fotowat et al., 2013; Yu et al., 2012) as well as social (Stamper et al., 2010) envelopes, thereby opening new avenues of research investigating whether and, if so, how electrosensory neurons are optimized to the statistics of natural envelopes as has been done in other sensory modalities (Laughlin, 1981; Lewen et al., 2001; Lewicki, 2002; Rodríguez et al., 2010; Woolley et al., 2005). In particular, these should focus on understanding the nature of the neural circuits that mediate the frequency-following response observed here. Moreover, our results have shown that behavioral responses to envelopes decreased in response to repeated presentations of the same stimulus. This habituation probably originates from feedback onto electrosensory neurons. Further studies should therefore focus on neural correlates of the habituation and the mechanisms responsible.
Our results show that the EOD frequency of the weakly electric fish A. leptorhynchus can follow the time course of low frequency (<1 Hz) envelopes, demonstrating that detailed information on these stimuli is retained in the brain. Although the one-to-one match between the gain of the frequency-following response and the spectral content of envelope signals measured during movement supports the interesting hypothesis that behavioral responses to movement envelopes have adapted to their statistics in the natural environment, it is important to realize that our measurements of envelope signals were not performed in natural settings as one fish was restrained. Nevertheless, the concordance between our results and those of Fotowat et al. (Fotowat et al., 2013), which were obtained from two or more freely moving animals with wireless transmitters, when looking at similar frequency ranges suggests that the fact that our measurements were made with one fish restrained does not significantly alter the obtained power spectrum. Together with the fact that A. leptorhynchus are primarily found alone or in pairs in the wild (Stamper et al., 2010), this suggests that our measurements of envelope signals resulting from movement accurately represent those found in the natural environment. Nevertheless, further studies measuring the envelope signals occurring in the natural electrosensory environment are necessary to validate our hypothesis.
Finally, our results have shown that A. leptorhynchus responds to higher frequency envelope signals through an increase in EOD frequency that is greater for higher (i.e. >1 Hz) frequencies. Previous studies have also shown that envelopes resulting from movement predominantly contain low (<1 Hz) temporal frequencies (Fotowat et al., 2013; Yu et al., 2012), whereas those resulting from social interactions predominantly contain higher (>1 Hz) frequencies (Stamper et al., 2010). Thus, our results suggest that A. leptorhynchus displays differential behavioral responses to envelopes arising from differential contexts. Further, we suggest that the increases in EOD frequency seen for higher envelope frequencies would serve to increase the frequency content of the envelope signal, thereby shifting it away from the frequency range of more interesting signals such as those caused by prey (Nelson and MacIver, 1999), as was recently observed in the weakly electric fish species Eigenmannia viriscens (Stamper et al., 2012). Although our results support this hypothesis, it is important to realize that, for the stimuli considered in this study, the animal cannot actually change the frequency content of the envelope signal as these were designed to mimic the envelope signals that occur in response to movement rather than those occurring during social interactions between three or more animals (Stamper et al., 2013; Stamper et al., 2012). It is thus probable that we underestimated the actual offset responses that might occur in the presence of stimuli actually mimicking those encountered during social interactions, such as those used by Stamper et al. (Stamper et al., 2012). Further studies are needed to test this. If true, these results would support the hypothesis that A. leptorhynchus evolved behavioral responses that rely on these two stimulus categories differing in their frequency content in order to take advantage of the fact that the former carries behaviorally relevant information whereas the latter is a jamming signal preventing electrolocation.
MATERIALS AND METHODS
We used three species of gymnotiform weakly electric fish: Apteronotus leptorhynchus (Ellis 1912) (N=48), Apteronotus albifrons (Linnaeus 1766) (N=38) and Sternopygus sp. (N=23). Average electric organ discharge (EODs) frequencies were 834±98 Hz (A. leptorhynchus), 1051±113 Hz (A. albifrons) and 129±25 Hz (Sternopygus sp.). Fish were obtained from tropical fish dealers and acclimated to the laboratory as per published guidelines (Hitschfeld et al., 2009). All animal care and experimental procedures were approved by McGill University’s animal care committee. Each fish was placed in an enclosure (referred to as a ‘chirp chamber’) in an otherwise empty experimental tank (30×30×10 cm) as described previously (Cuddy et al., 2012; Deemyad et al., 2013) (Fig. 1A). The fish’s EOD was recorded using two metal electrodes placed in the head to tail orientation (Fig. 1A, E1 and E2). The EOD was amplified with a DAM 50 differential amplifier (World Precision Instruments, Sarasota, FL, USA) and digitized with CED 1401plus hardware and Spike2 software (Cambridge Electronic Design, Cambridge, UK) at a sampling rate of 10 kHz.
Under natural conditions, electric fish will experience both amplitude modulations (AMs) as well as phase modulations of their own EOD. The stimuli used here consisted of amplitude modulations (AMs) of the animals own EOD that were obtained by multiplying the AM waveform with a sinusoidal carrier wave that was phase-locked to the animal’s own EOD (MT3 multiplier; Tucker-Davis Technologies, Alachua, FL, USA). The resulting signal was then attenuated (Leader, LAT-45, Leader Electronics, Japan), isolated from ground (World Precision Instruments A395 linear stimulus isolator, Sarasota, FL, USA) and delivered to the experimental tank through a pair of metal electrodes positioned ca. 15 cm away of each side of the chirp chamber (Fig. 1A). It is important to note that the EOD waveform itself, a quasi-sinusoidal signal, can be considered as a carrier signal and that the relevant stimulus here is the AM of this carrier signal. Therefore, we will henceforth refer to the AM as the stimulus. To obtain contrast modulated AMs each stimulus was further modulated with a low frequency sine wave (herein referred to as the envelope) with different depths of modulation (Fig. 1B). The envelope frequencies used were 0.001, 0.0025, 0.005, 0.0075, 0.01, 0.025, 0.05, 0.075, 0.1, 0.2, 0.5, 1, 2 and 4 Hz. The depths of modulation used were 10, 40, 70 and 90% and the stimulus frequency was set to 15 Hz (Fig. 4). Each stimulus lasted 200 s except for envelope frequencies 0.001 and 0.0025 Hz, which lasted 1000 and 400 s, respectively, in order to obtain one full cycle. We measured all stimuli presented to the animal using a small dipole positioned ~5 mm lateral to the chirp chamber (Fig. 1A, purple dots). The stimulus intensity was calibrated to be 2 mV cm−1. In addition to different envelope frequencies at a constant stimulus frequency, we also tested the behavioral responses of A. leptorhynchus to a given envelope frequency (0.001 and 0.0025 Hz, respectively; Fig. 3) with different stimulus frequencies (5, 15, 20, 50 and 100 Hz). The depth of modulation here was set to 90%. In order to compare the behavioral responses obtained for the contrast modulated AMs with responses to a more naturalistic context, we presented a subset of our stimuli (envelope frequencies: 0.005, 0.01, 0.05, 0.1 and 1 Hz; depth of modulation: 90%) in the ‘beat’ configuration (Fig. 2B). Beats occur when two fish come into close contact. Each fish will experience a beat (or sinusoidal AM) of their field the frequency of which is equal to the difference frequency between the fields and ranges between 0 and 400 Hz (Benda et al., 2005; Akon et al., 2002). Therefore, the desired signal waveform was multiplied (MT3 multiplier; Tucker-Davis Technologies) with a sinusoidal carrier wave whose frequency was 15 Hz higher than the animal’s own EOD frequency.
In order to elucidate whether the behavioral responses habituate to repetitive trials using the same stimulus, we used a 15 Hz AM that was modulated with either one of two different envelope frequencies (0.005 and 0.2 Hz) with 90% depth of modulation. Each of these stimuli were presented nine times to A. leptorhynchus with an inter-stimulus interval of 1 min. Additionally, we tested for offset responses of A. leptorhynchus to envelope frequencies up to 4 Hz.
To calculate the frequency content of movement-generated envelopes, we took 3 h recordings in different pairs of fish (N=6). Trials were performed in an experimental tank (45×35×15 cm) in the absence of light. One of the two fish was placed in a chirp chamber and the other fish was freely moving around. The EOD of the freely moving fish was recorded using two pairs of electrodes positioned in opposite corners of the tank. The chirp chamber was made of electrical transparent clay with small holes at the top and bottom to guarantee fresh water circulation. The clay was used to minimize mechanosensory or visual perception of the other fish outside the chirp chamber. The chirp chamber was further equipped with a pair of electrodes at each end of it to determine the baseline EOD frequency of the fish in it. The EOD electrodes were connected to an AM Systems (Carlsborg, WA, USA) amplifier, model 1700 (amplified 10×, low frequency cut-off of 100 Hz, high frequency cut-off of 5 kHz). Additionally, a 2 mm transverse dipole (Fig. 7A; Fig. 8A, upper panels, purple electrodes) adjacent to midpoint of the chirp chamber was used to measure the local electric field.
The circuitry of the dipole was the same as for the EOD electrodes. The EOD electrodes were used to determine the baseline frequencies of the two fish at the beginning of each trial, whereas the dipole signal was used to extract the envelope as well as the time course of the EOD frequencies of both fish. The EODs of the restrained fish and the freely moving fish were digitized at 10 kHz sampling rate each and the local electric field was digitized at 50 kHz sampling rate using CED 1401plus hardware and Spike2 software (Cambridge Electronic Design, Cambridge, UK), and stored on a computer hard disk for offline analysis. The trials were recorded from above using a camera (model UV-5803, Unique Vision, Longhua Town, Shenzhen, China) equipped with infrared illumination. In some experiments (N=8), we replaced the fish in the chirp chamber with an ‘artificial fish’ that consisted of a dipole that was used to emit a sine wave with a frequency at least 50 Hz higher or lower than that of the freely moving fish (Fig. 7A). This was necessary because it is currently not possible to unambiguously relate changes in the fish’s EOD frequency to changes in the envelope because of previously described behaviors occurring during social interactions (Tallarovic and Zakon, 2005; Triefenbach and Zakon, 2008).
Analysis was performed using custom-written scripts in MATLAB (MathWorks, Natick MA, USA). We first extracted the fishes’ EOD frequencies by computing a spectrogram of the recorded signal. The EOD frequency was then determined as the frequency with the highest power near the fourth harmonic of the fish’s baseline EOD frequency in order to minimize the effects of chirps. The extracted EOD frequency was then divided by four in order to get the true EOD frequency of the fish. We then extracted the envelope E(t) from the stimulus S(t) as described previously (McGillivray et al., 2012). The envelope E(t) can be regarded as the instantaneous amplitude of the stimulus S(t), or its time-varying contrast. It can be obtained from the stimulus S(t) by the following non-linear transformation:
| (1) |
where Ŝ(t) is the Hilbert transform of S(t) (Middleton et al., 2006; Myers et al., 2003; Savard et al., 2011):
| (2) |
where C is the Cauchy principal value, t is time and τ is the integration variable.
We quantified the behavioral response of each fish by calculating the parameters gain, phase and offset to each particular envelope frequency. Therefore we extracted the time-dependent EOD frequency from the recording as described above in order to obtain the response R(t). To calculate the gain and the phase, we next averaged the extracted EOD frequency over the number of envelope cycles present in the stimulus in order to get the response, R. We further computed the power spectrum of the envelope E(t) extracted from the dipole. The gain is defined as the ratio between the response R and the envelope E (Fig. 2A, inset):
| (3) |
where Aresponse is the amplitude of the averaged response R and Aenvelope the amplitude of the envelope E, calculated as:
| (4) |
where max(…) and min(…) are the maximum and minimum values and |…| denotes the absolute value. Aenvelope is defined as:
| (5) |
Additionally, we determined the phase shift between the response R and the envelope E by determining the difference between the maximum peak of the envelope E and the maximum peak of the response R (Fig. 2A):
| (6) |
where T is the timing of the maximum peak of the envelope E and the response R, TE is the envelope period and int(…) denotes the integer part.
The offset was determined by calculating the mean EOD frequency during the last cycle of the stimulation and subtracting the baseline EOD frequency (Fig. 2A):
| (7) |
where <…> is the average.
We further computed the power spectrum of the envelope E(t) extracted from the dipole.
We extracted the x–y position from each trial using Videopoint software (Lenox, MA, USA) as described previously (Hupé and Lewis, 2008). Frames of every 200 ms were analyzed. The distance from the tip of the snout of the freely moving fish to the dipole was used as a functional indication of the distance between the two fish.
Acknowledgments
The authors would like to thank M. Kimyaci and Z. Li for help in gathering data.
Funding
This work was funded by Canadian Institutes of Health Research [to M.J.C.]; and Fonds de recherche du Québec – Nature et technologies [to M.J.C.].
List of abbreviations
- EOD
electric organ discharge
- ELL
electrosensory lateral line lobe
- JAR
jamming avoidance response
- SIU
stimulus isolation unit
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M.G.M. executed the experiments and analysis. M.G.M. and M.J.C. conceived and designed the experiments. M.G.M. and M.J.C. interpreted the findings as well as drafted and revised the manuscript.
References
- Benda J, Hennig RM. Spike-frequency adaptation generates intensity invariance in a primary auditory interneuron. J Comput Neurosci. 2008;24:113–136. doi: 10.1007/s10827-007-0044-8. [DOI] [PubMed] [Google Scholar]
- Benda J, Longtin A, Maler L. Spike-frequency adaptation separates transient communication signals from background oscillations. J Neurosci. 2005;25:2312–2321. doi: 10.1523/JNEUROSCI.4795-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, Behrend K, Heiligenberg W. Comparison of the jamming avoidance responses of gymnotoid and gymnarchid electric fish: a case of convergent evolution of behavior and its sensory basis. J Comp Physiol. 1975;103:97–121. [Google Scholar]
- Chacron MJ, Doiron B, Maler L, Longtin A, Bastian J. Non-classical receptive field mediates switch in a sensory neuron’s frequency tuning. Nature. 2003;423:77–81. doi: 10.1038/nature01590. [DOI] [PubMed] [Google Scholar]
- Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Curr Opin Neurobiol. 2011;21:752–760. doi: 10.1016/j.conb.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddy M, Aubin-Horth N, Krahe R. Electrocommunication behaviour and non invasively-measured androgen changes following induced seasonal breeding in the weakly electric fish, Apteronotus leptorhynchus. Horm Behav. 2012;61:4–11. doi: 10.1016/j.yhbeh.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Deemyad T, Metzen MG, Pan Y, Chacron MJ. Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics. Proc Natl Acad Sci USA. 2013;110:19609–19614. doi: 10.1073/pnas.1314008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap KD, Oliveri LM. Retreat site selection and social organization in captive electric fish, Apteronotus leptorhynchus. J Comp Physiol A. 2002;188:469–477. doi: 10.1007/s00359-002-0319-5. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Thomas P, Zakon HH. Diversity of sexual dimorphism in electrocommunication signals and its androgen regulation in a genus of electric fish, Apteronotus. J Comp Physiol A. 1998;183:77–86. doi: 10.1007/s003590050236. [DOI] [PubMed] [Google Scholar]
- Fotowat H, Harrison RR, Krahe R. Statistics of the electrosensory input in the freely swimming weakly electric fish Apteronotus leptorhynchus. J Neurosci. 2013;33:13758–13772. doi: 10.1523/JNEUROSCI.0998-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiligenberg W. Neural Nets in Electric Fish. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- Hitschfeld ÉM, Stamper SA, Vonderschen K, Fortune ES, Chacron MJ. Effects of restraint and immobilization on electrosensory behaviors of weakly electric fish. ILAR J. 2009;50:361–372. doi: 10.1093/ilar.50.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupé GJ, Lewis JE. Electrocommunication signals in free swimming brown ghost knifefish, Apteronotus leptorhynchus. J Exp Biol. 2008;211:1657–1667. doi: 10.1242/jeb.013516. [DOI] [PubMed] [Google Scholar]
- Kolodziejski JA, Nelson BS, Smith GT. Sex and species differences in neuromodulatory input to a premotor nucleus: a comparative study of substance P and communication behavior in weakly electric fish. J Neurobiol. 2005;62:299–315. doi: 10.1002/neu.20095. [DOI] [PubMed] [Google Scholar]
- Krahe R, Maler L. Neural maps in the electrosensory system of weakly electric fish. Curr Opin Neurobiol. 2014;24:13–21. doi: 10.1016/j.conb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Laughlin S. A simple coding procedure enhances a neuron’s information capacity. Z Naturforsch (Sect C) 1981;36:910–912. [PubMed] [Google Scholar]
- Lewen GD, Bialek W, de Ruyter van Steveninck RR. Neural coding of naturalistic motion stimuli. Network. 2001;12:317–329. [PubMed] [Google Scholar]
- Lewicki MS. Efficient coding of natural sounds. Nat Neurosci. 2002;5:356–363. doi: 10.1038/nn831. [DOI] [PubMed] [Google Scholar]
- Márquez BT, Krahe R, Chacron MJ. Neuromodulation of early electrosensory processing in gymnotiform weakly electric fish. J Exp Biol. 2013;216:2442–2450. doi: 10.1242/jeb.082370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsat G, Longtin A, Maler L. Cellular and circuit properties supporting different sensory coding strategies in electric fish and other systems. Curr Opin Neurobiol. 2012;22:686–692. doi: 10.1016/j.conb.2012.01.009. [DOI] [PubMed] [Google Scholar]
- McGillivray P, Vonderschen K, Fortune ES, Chacron MJ. Parallel coding of first- and second-order stimulus attributes by midbrain electrosensory neurons. J Neurosci. 2012;32:5510–5524. doi: 10.1523/JNEUROSCI.0478-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Longtin A, Benda J, Maler L. The cellular basis for parallel neural transmission of a high-frequency stimulus and its low-frequency envelope. Proc Natl Acad Sci USA. 2006;103:14596–14601. doi: 10.1073/pnas.0604103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O’Malley M, Vaughan CL, Heneghan C, St Clair Gibson A, Harley YX, Sreenivasan R. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods. 2003;124:157–165. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Nelson ME, MacIver MA. Prey capture in the weakly electric fish Apteronotus albifrons: sensory acquisition strategies and electrosensory consequences. J Exp Biol. 1999;202:1195–1203. doi: 10.1242/jeb.202.10.1195. [DOI] [PubMed] [Google Scholar]
- Rodríguez FA, Chen C, Read HL, Escabí MA. Neural modulation tuning characteristics scale to efficiently encode natural sound statistics. J Neurosci. 2010;30:15969–15980. doi: 10.1523/JNEUROSCI.0966-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard M, Krahe R, Chacron MJ. Neural heterogeneities influence envelope and temporal coding at the sensory periphery. Neuroscience. 2011;172:270–284. doi: 10.1016/j.neuroscience.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncelli EP, Olshausen BA. Natural image statistics and neural representation. Annu Rev Neurosci. 2001;24:1193–1216. doi: 10.1146/annurev.neuro.24.1.1193. [DOI] [PubMed] [Google Scholar]
- Stamper SA, Carrera-G E, Tan EW, Fugère V, Krahe R, Fortune ES. Species differences in group size and electrosensory interference in weakly electric fishes: implications for electrosensory processing. Behav Brain Res. 2010;207:368–376. doi: 10.1016/j.bbr.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Stamper SA, Madhav MS, Cowan NJ, Fortune ES. Beyond the Jamming Avoidance Response: weakly electric fish respond to the envelope of social electrosensory signals. J Exp Biol. 2012;215:4196–4207. doi: 10.1242/jeb.076513. [DOI] [PubMed] [Google Scholar]
- Stamper SA, Fortune ES, Chacron MJ. Perception and coding of envelopes in weakly electric fishes. J Exp Biol. 2013;216:2393–2402. doi: 10.1242/jeb.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarovic SK, Zakon HH. Electric organ discharge frequency jamming during social interactions in brown ghost knifefish, Apteronotus leptorhynchus. Anim Behav. 2005;70:1355–1365. [Google Scholar]
- Triefenbach F, Zakon H. Changes in signalling during agonistic interactions between male weakly electric knifefish, Apteronotus leptorhynchus. Anim Behav. 2008;75:1263–1272. [Google Scholar]
- Vonderschen K, Chacron MJ. Sparse and dense coding of natural stimuli by distinct midbrain neuron subpopulations in weakly electric fish. J Neurophysiol. 2011;106:3102–3118. doi: 10.1152/jn.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Yu N, Hupé G, Garfinkle C, Lewis JE, Longtin A. Coding conspecific identity and motion in the electric sense. PLOS Comput Biol. 2012;8:e1002564. doi: 10.1371/journal.pcbi.1002564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon HH, Thomas P, Yan HY. Electric organ discharge frequency and plasma sex steroid levels during gonadal recrudescence in a natural population of the weakly electric fish Sternopygus macrurus. J Comp Physiol A. 1991;169:493–499. doi: 10.1007/BF00197661. [DOI] [PubMed] [Google Scholar]
- Zakon H, Oestreich J, Tallarovic S, Triefenbach F. EOD modulations of brown ghost electric fish: JARs, chirps, rises, and dips. J Physiol Paris. 2002;96:451–458. doi: 10.1016/S0928-4257(03)00012-3. [DOI] [PubMed] [Google Scholar]