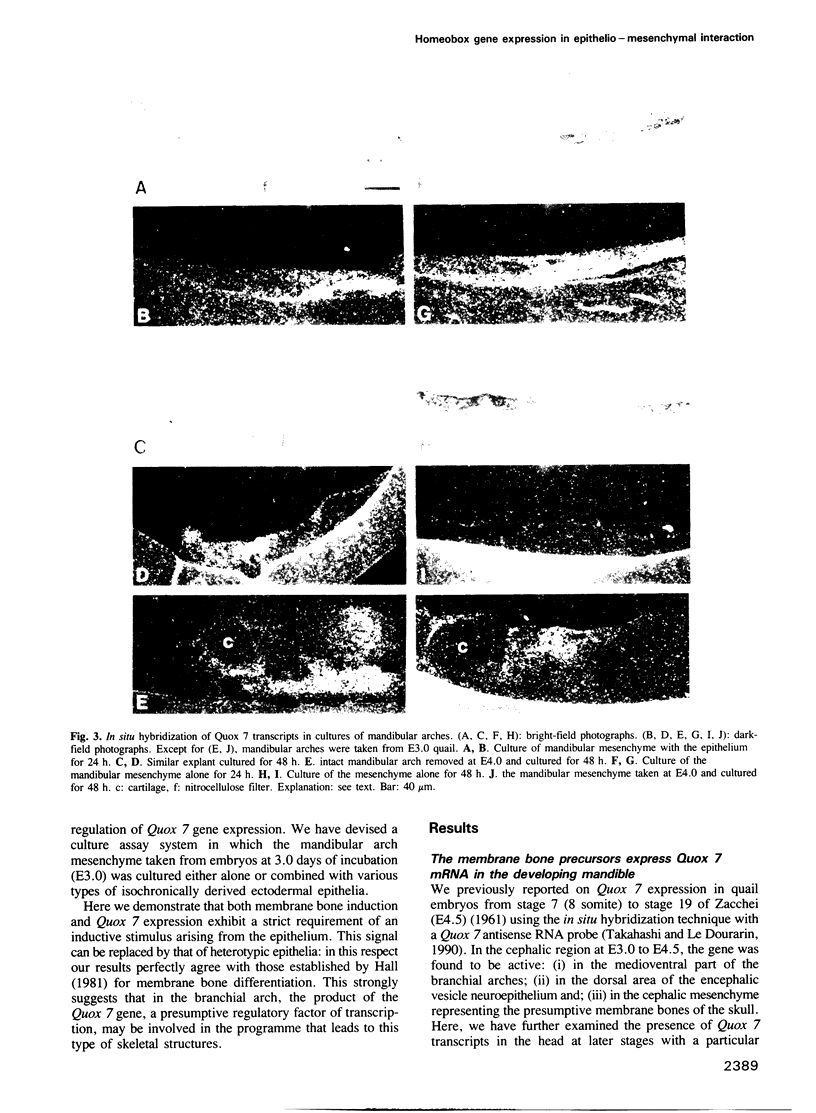
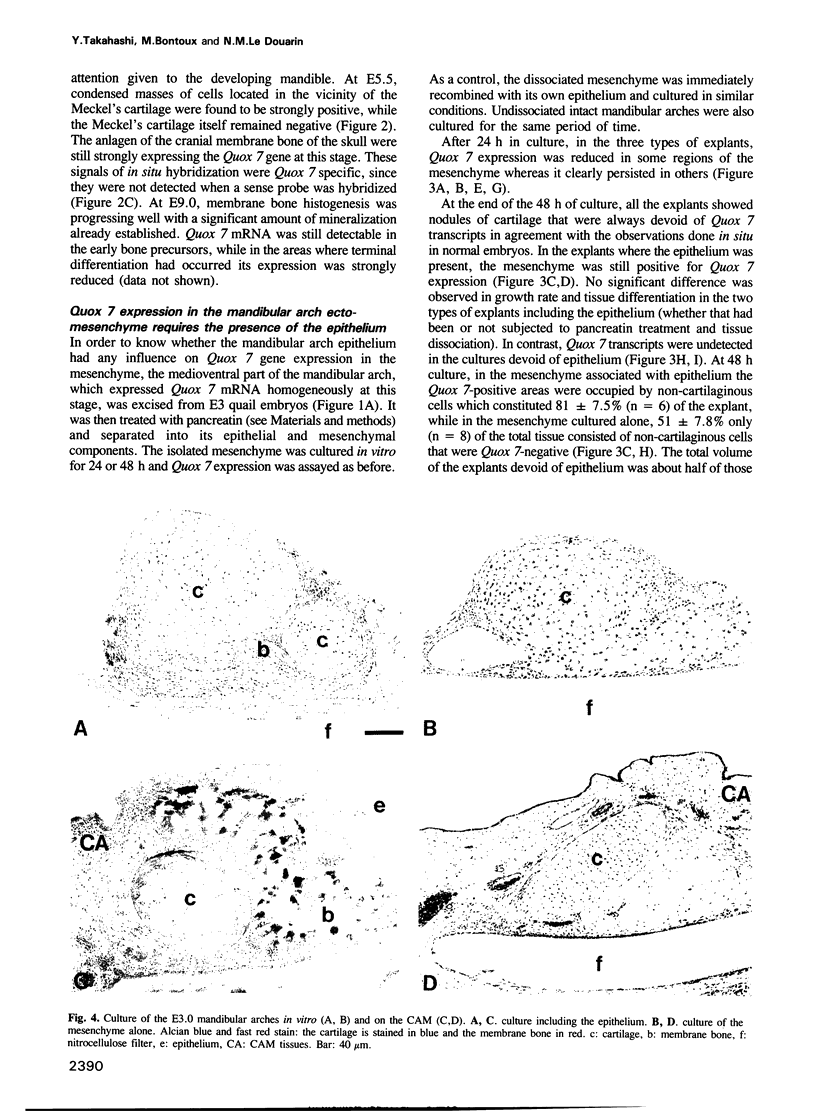
Abstract
In higher vertebrates, branchial arch mesenchyme (ectomesenchyme) is derived from the cephalic neural crest. The ectomesenchyme of the mandibular arch yields the Meckel's cartilage and several membrane bones. We previously reported the isolation of a quail homeobox gene, Quox 7. In common with its mouse counterpart Hox 7, Quox 7 is highly expressed in the medioventral part of the mandibular arch and later in the precursor cells of the membrane bones. Since bone differentiation from ectomesenchyme is strictly dependent upon a signal provided by the mandibular epithelium, we decided to see whether the regulation of Quox 7 gene activity might be correlated with epithelio--mesenchymal interactions. Quox 7 expression was studied in E3 mandibular ectomesenchyme cultured in vitro or grafted on the chick chorioallantoic membrane either alone or recombined with the homotopic and heterotopic epithelia. We found that Quox 7 mRNA was undetectable after 48 h in cultures of mesenchyme alone while it remained abundant in non-cartilaginous tissue of the mandibular arch ectomesenchyme recombined with its own epithelium. The signal provided by the mandibular epithelium for Quox 7 expression can also arise from various heterotopic epithelia, e.g. of dorsal or ventral body wall and of limb bud. Thus the effect of the epithelium on Quox 7 expression in mesenchymal cells strictly parallels that on bone formation. These results strongly suggest that the epithelio-mesenchymal interactions have an essential role on the regulation of Quox 7 gene, the product of which seems to be, in turn, necessary for the execution of the skeletal developmental program in the facial area.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bee J., Thorogood P. The role of tissue interactions in the skeletogenic differentiation of avian neural crest cells. Dev Biol. 1980 Jul;78(1):47–62. doi: 10.1016/0012-1606(80)90317-6. [DOI] [PubMed] [Google Scholar]
- Couly G. Concepts nouveaux de la biologie du développement céphalique humain. Observations et hypothèses. Ann Genet. 1982;25(4):201–206. [PubMed] [Google Scholar]
- Epperlein H. H. The ectomesenchymal-endodermal interaction-system (EEIS) of Triturus alpestris in tissue culture. I. Observations on attachment, migration and differentiation of neural crest cells. Differentiation. 1974 Jun;2(3):151–168. doi: 10.1111/j.1432-0436.1974.tb00349.x. [DOI] [PubMed] [Google Scholar]
- GIROUD A. Phénomènes d'induction et leurs perturbations chez les mammifères. Acta Anat (Basel) 1957;30(1-4):297–306. [PubMed] [Google Scholar]
- Gregg B. C., Rowe A., Brickell P. M., Wolpert L. Ectodermal inhibition of cartilage differentiation in micromass culture of chick limb bud mesenchyme in relation to gene expression and cell shape. Development. 1989 Apr;105(4):769–777. doi: 10.1242/dev.105.4.769. [DOI] [PubMed] [Google Scholar]
- Hall B. K., Coffin-Collins P. A. Reciprocal interactions between epithelium, mesenchyme, and epidermal growth factor (EGF) in the regulation of mandibular mitotic activity in the embryonic chick. J Craniofac Genet Dev Biol. 1990;10(3):241–261. [PubMed] [Google Scholar]
- Hall B. K. The induction of neural crest-derived cartilage and bone by embryonic epithelia: an analysis of the mode of action of an epithelial-mesenchymal interaction. J Embryol Exp Morphol. 1981 Aug;64:305–320. [PubMed] [Google Scholar]
- Hall B. K., Tremaine R. Ability of neural crest cells from the embryonic chick to differentiate into cartilage before their migration away from the neural tube. Anat Rec. 1979 Jul;194(3):469–475. doi: 10.1002/ar.1091940312. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Jones P. F., Rees A. R., Sime C. M., Justice M. J., Copeland N. G., Jenkins N. A., Graham E., Davidson D. R. A new family of mouse homeo box-containing genes: molecular structure, chromosomal location, and developmental expression of Hox-7.1. Genes Dev. 1989 Jan;3(1):26–37. doi: 10.1101/gad.3.1.26. [DOI] [PubMed] [Google Scholar]
- Ivens A., Flavin N., Williamson R., Dixon M., Bates G., Buckingham M., Robert B. The human homeobox gene HOX7 maps to chromosome 4p16.1 and may be implicated in Wolf-Hirschhorn syndrome. Hum Genet. 1990 Apr;84(5):473–476. doi: 10.1007/BF00195823. [DOI] [PubMed] [Google Scholar]
- Le Lièvre C. S., Le Douarin N. M. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975 Aug;34(1):125–154. [PubMed] [Google Scholar]
- Le Lièvre C. S. Participation of neural crest-derived cells in the genesis of the skull in birds. J Embryol Exp Morphol. 1978 Oct;47:17–37. [PubMed] [Google Scholar]
- Le Liévre C., Le Douarin N. Origine ectodermique du derme de la face et du cou, montrée par des combinaisons interspécifiques chez l'embryon d'oiseau. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jan 21;278(4):517–520. [PubMed] [Google Scholar]
- Lievre C. L. Rôle des cellules mésectodermiques issues des crêtes neurales céphaliques dans la formation des arcs branchiaux et du squelette viscéral. J Embryol Exp Morphol. 1974 Apr;31(2):453–477. [PubMed] [Google Scholar]
- Mackenzie A., Leeming G. L., Jowett A. K., Ferguson M. W., Sharpe P. T. The homeobox gene Hox 7.1 has specific regional and temporal expression patterns during early murine craniofacial embryogenesis, especially tooth development in vivo and in vitro. Development. 1991 Feb;111(2):269–285. doi: 10.1242/dev.111.2.269. [DOI] [PubMed] [Google Scholar]
- Noden D. M. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol. 1978 Dec;67(2):296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- Robert B., Sassoon D., Jacq B., Gehring W., Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989 Jan;8(1):91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solursh M., Singley C. T., Reiter R. S. The influence of epithelia on cartilage and loose connective tissue formation by limb mesenchyme cultures. Dev Biol. 1981 Sep;86(2):471–482. doi: 10.1016/0012-1606(81)90205-0. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Le Douarin N. cDNA cloning of a quail homeobox gene and its expression in neural crest-derived mesenchyme and lateral plate mesoderm. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7482–7486. doi: 10.1073/pnas.87.19.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler M. S. Epithelial influences on membrane bone formation in the maxilla of the embryonic chick. Anat Rec. 1978 Oct;192(2):225–233. doi: 10.1002/ar.1091920203. [DOI] [PubMed] [Google Scholar]
- Tyler M. S., Hall B. K. Epithelial influences on skeletogenesis in the mandible of the embryonic chick. Anat Rec. 1977 Jun;188(2):229–239. doi: 10.1002/ar.1091880208. [DOI] [PubMed] [Google Scholar]
- Tyler M. S., McCobb D. P. The genesis of membrane bone in the embryonic chick maxilla: epithelial-mesenchymal tissue recombination studies. J Embryol Exp Morphol. 1980 Apr;56:269–281. [PubMed] [Google Scholar]
- ZACCHEI A. M. [The embryonal development of the Japanese quail (Coturnix coturnix japonica T. and S.)]. Arch Ital Anat Embriol. 1961;66:36–62. [PubMed] [Google Scholar]