Abstract
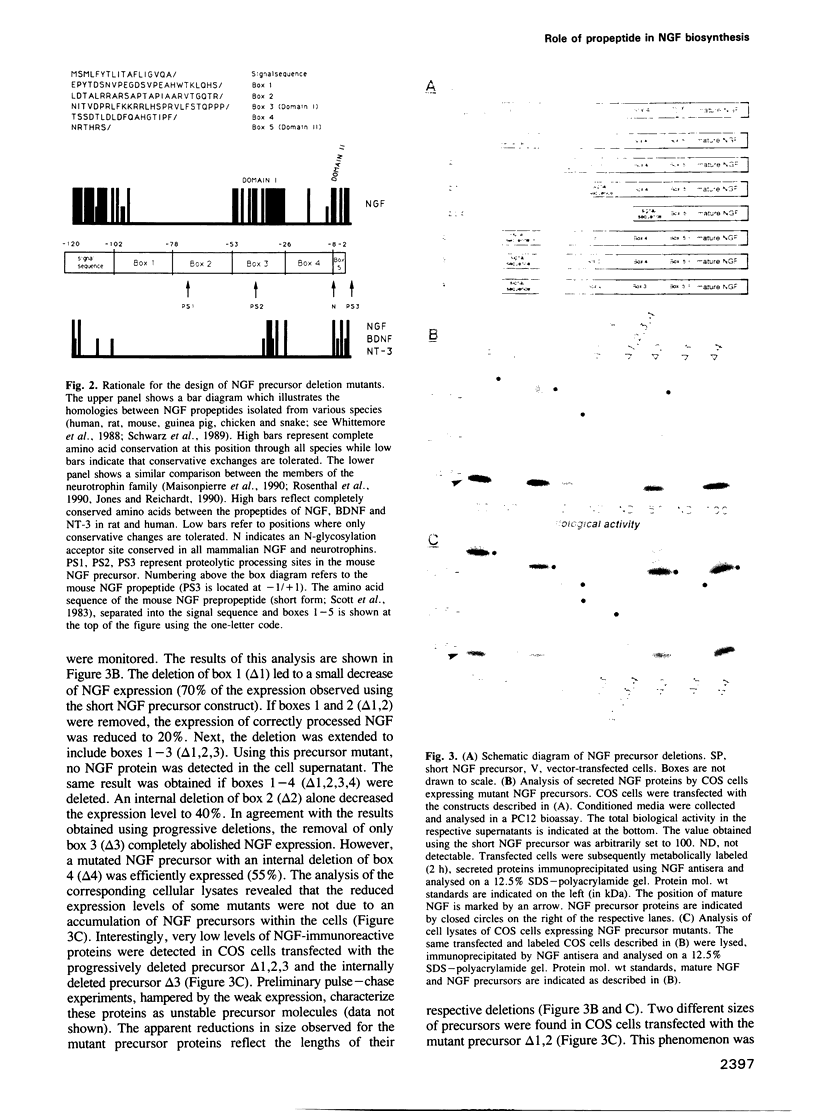
The three members of the neurotrophin family (NGF, BDNF and NT-3) are synthesized as large precursor proteins which undergo proteolytic processing to yield biologically active, mature neurotrophic factors. We have used in vitro mutagenesis to examine the pro-region in the NGF precursor protein as a first step towards a general understanding of the role of propeptides in the biosynthesis of neurotrophins. Our results demonstrate that only two small domains within the NGF propeptide are required for the expression and secretion of properly processed and biologically active, recombinant mouse NGF in COS-7 cells. Domain I plays an important role in the expression of active NGF while domain II is involved in proteolytic processing. Both domains are partially conserved between the propeptides of NGF proteins isolated from different species as well as BDNF and NT-3.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coll M., Guasch A., Avilés F. X., Huber R. Three-dimensional structure of porcine procarboxypeptidase B: a structural basis of its inactivity. EMBO J. 1991 Jan;10(1):1–9. doi: 10.1002/j.1460-2075.1991.tb07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling T. L., Petrides P. E., Beguin P., Frey P., Shooter E. M., Selby M., Rutter W. J. The biosynthesis and processing of proteins in the mouse 7S nerve growth factor complex. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):427–434. doi: 10.1101/sqb.1983.048.01.047. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Persson H., Larhammar D., Lundströmer K., Olson L. Characterization of antibodies to synthetic nerve growth factor (NGF) and proNGF peptides. J Neurosci Res. 1989 Mar;22(3):223–240. doi: 10.1002/jnr.490220302. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Garcia P. D., Rutter W. J. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988 May 15;263(14):6810–6815. [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Mobley W. C., Weinrich S. L., Hruby D. E., Rutter W. J. Processing and secretion of nerve growth factor: expression in mammalian cells with a vaccinia virus vector. Mol Cell Biol. 1988 Jun;8(6):2456–2464. doi: 10.1128/mcb.8.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Rutter W. J. Differential RNA splicing predicts two distinct nerve growth factor precursors. 1986 Feb 27-Mar 5Nature. 319(6056):784–787. doi: 10.1038/319784a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S., Boileau G., Zollinger L., Nault C., Rholam M., Cohen P. Site-specific mutagenesis identifies amino acid residues critical in prohormone processing. EMBO J. 1989 Oct;8(10):2911–2916. doi: 10.1002/j.1460-2075.1989.tb08440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A. M., Mason A. J. Requirement for activin A and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990 Mar 16;247(4948):1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- Hallbök F., Ebendal T., Persson H. Production and characterization of biologically active recombinant beta nerve growth factor. Mol Cell Biol. 1988 Jan;8(1):452–456. doi: 10.1128/mcb.8.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Ibáez C. F., Hallbök F., Ebendal T., Persson H. Structure-function studies of nerve growth factor: functional importance of highly conserved amino acid residues. EMBO J. 1990 May;9(5):1477–1483. doi: 10.1002/j.1460-2075.1990.tb08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Molecular structure of an aspartic proteinase zymogen, porcine pepsinogen, at 1.8 A resolution. Nature. 1986 Jan 2;319(6048):33–38. doi: 10.1038/319033a0. [DOI] [PubMed] [Google Scholar]
- Jones K. R., Reichardt L. F. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongstra-Bilen J., Coblentz L., Shooter E. M. The in vitro processing of the NGF precursors by the gamma-subunit of the 7S NGF complex. Brain Res Mol Brain Res. 1989 Mar;5(2):159–169. doi: 10.1016/0169-328x(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen M. J., Cantor A. B., Furie B. C., Brown C. L., Shoemaker C. B., Furie B. Recognition site directing vitamin K-dependent gamma-carboxylation resides on the propeptide of factor IX. Cell. 1987 Jan 30;48(2):185–191. doi: 10.1016/0092-8674(87)90422-3. [DOI] [PubMed] [Google Scholar]
- Jung L. J., Scheller R. H. Peptide processing and targeting in the neuronal secretory pathway. Science. 1991 Mar 15;251(4999):1330–1335. doi: 10.1126/science.2003219. [DOI] [PubMed] [Google Scholar]
- Kaisho Y., Yoshimura K., Nakahama K. Cloning and expression of a cDNA encoding a novel human neurotrophic factor. FEBS Lett. 1990 Jun 18;266(1-2):187–191. doi: 10.1016/0014-5793(90)81536-w. [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- McCracken A. A., Kruse K. B. Intracellular transport of rat serum albumin is altered by a genetically engineered deletion of the propeptide. J Biol Chem. 1989 Dec 15;264(35):20843–20846. [PubMed] [Google Scholar]
- Rosenthal A., Goeddel D. V., Nguyen T., Lewis M., Shih A., Laramee G. R., Nikolics K., Winslow J. W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990 May;4(5):767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M. A., Fisher D., Bradshaw R. A., Isackson P. J. Isolation and sequence of a cDNA clone of beta-nerve growth factor from the guinea pig prostate gland. J Neurochem. 1989 Apr;52(4):1203–1209. doi: 10.1111/j.1471-4159.1989.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Scott J., Selby M., Urdea M., Quiroga M., Bell G. I., Rutter W. J. Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983 Apr 7;302(5908):538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Edwards R., Sharp F., Rutter W. J. Mouse nerve growth factor gene: structure and expression. Mol Cell Biol. 1987 Sep;7(9):3057–3064. doi: 10.1128/mcb.7.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Silen J. L., Agard D. A. The alpha-lytic protease pro-region does not require a physical linkage to activate the protease domain in vivo. Nature. 1989 Oct 5;341(6241):462–464. doi: 10.1038/341462a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller T. J., Shields D. The propeptide of preprosomatostatin mediates intracellular transport and secretion of alpha-globin from mammalian cells. J Cell Biol. 1989 May;108(5):1647–1655. doi: 10.1083/jcb.108.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell J., Billeter M., Wider G., Avilés F. X., Wüthrich K. The NMR structure of the activation domain isolated from porcine procarboxypeptidase B. EMBO J. 1991 Jan;10(1):11–15. doi: 10.1002/j.1460-2075.1991.tb07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcher A. A., Bitler C. M., Radeke M. J., Shooter E. M. Nerve growth factor binding domain of the nerve growth factor receptor. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):159–163. doi: 10.1073/pnas.88.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren K. M., Potts J. T., Jr, Kronenberg H. M. Importance of the propeptide sequence of human preproparathyroid hormone for signal sequence function. J Biol Chem. 1988 Dec 25;263(36):19771–19777. [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- Zhu X. L., Ohta Y., Jordan F., Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989 Jun 8;339(6224):483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]