Abstract
Adrenocortical carcinoma (ACC) is a rare but lethal malignancy without effective current therapy for metastatic disease. IL-13-PE is a recombinant cytotoxin consisting of human interleukin-13 (IL-13) and a truncated form of Pseudomonas exotoxin A (PE). The main objectives of this Phase I dose-escalation trial were to assess the maximum-tolerated dose (MTD), safety, and pharmacokinetics (PK) of IL-13-PE in patients with metastatic ACC. Eligible patients had confirmed IL-13 receptor alpha 2 (IL-13Rα2) expressions in their tumors. IL-13-PE at dose of 1–2 μg/kg was administered intravenously (IV) on day 1, 3, and 5 in a 4-week cycle. Six patients received 1 μg/kg and two patients received 2 μg/kg of IL-13-PE. Dose-limiting toxicity was observed at 2 μg/kg, at which patients exhibited thrombocytopenia and renal insufficiency without requiring dialysis. PK analysis demonstrated that at MTD, the mean maximum serum concentration (Cmax) of IL-13-PE was 21.0 ng/mL, and the terminal half-life of IL-13-PE was 30–39 min. Two (25%) of the eight patients had baseline neutralizing antibodies against PE. Three (75%) of the remaining four tested patients developed neutralizing antibodies against IL-13-PE within 14–28 days of initial treatment. Of the five patients treated at MTD and assessed for response, one patient had stable disease for 5.5 months before disease progression; the others progressed within 1–2 months. In conclusion, systemic IV administration of IL-13-PE is safe at 1 μg/kg. All tested patients developed high levels of neutralizing antibodies during IL-13-PE treatment. Use of strategies for immunodepletion before IL-13-PE treatment should be considered in future trials.
Keywords: IL-13-PE, maximum-tolerated dose, metastatic adrenocortical carcinoma, pharmacokinetics, Phase I, systemic administration
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy with an incidence of 0.7–2.0 per million people per year 1–3. It has an overall 5-year relative survival rate of 32–42%, and a median survival of 17–32 months 1,4. The treatment of choice for a localized primary or recurrent ACC is surgical resection. However, patients with recurrent or metastatic disease are rarely curable by surgery alone. Other therapeutic options such as systemic chemotherapy and locoregional radiotherapy have limited impact on survival 5. Thus, identifying new therapeutic targets and strategies are of great importance to the treatment of this malignancy.
Interleukin-13 receptor α2 (IL-13Rα2) is a high-affinity receptor for the Th2-derived cytokine interleukin-13 (IL-13), and is overexpressed in several types of cancers as compared to low or absent expression in embryonic cells and normal tissues 6–11. The high-affinity binding of IL-13 to IL-13Rα2 signals through a STAT-6-independent AP-1-dependent pathway, which leads to increased transforming growth factor-beta (TGF-β) activity 12. We previously reported that IL-13Rα2 was significantly overexpressed in ACCs as compared to normal adrenocortical tissues and benign adrenocortical tumors 13. Thus, IL-13Rα2 represents a promising therapeutic target for ACC and other solid malignancies such as pancreatic adenocarcinoma and hepatocellular carcinoma 14,15.
IL-13-PE is a chimeric fusion protein consisting of human IL-13 and a truncated form of Pseudomonas exotoxin A (cintredekin besudotox, hIL13-PE38QQR) 16,17. Previous studies have shown that IL-13-PE can bind to IL-13Rα2 positive tumor cells and is highly cytotoxic to these cells in both in vitro and in vivo models of multiple malignancies 16,18,19. It has been demonstrated that IL-13Rα2 positive ACC cell lines were sensitive to IL-13-PE as well 13. Moreover, treatment with IL-13-PE caused tumor regression and prolonged survival in a mouse xenograft model of ACC 13. Several Phase I and II clinical trials and one Phase III clinical trial have been performed to evaluate the safety, tolerability, and efficacy of IL-13-PE using regional delivery of the agent for intracranial malignancies 20–24. Here, we report the first Phase I study of systemic intravenous (IV) administration of IL-13-PE in patients with metastatic ACC.
Materials and Methods
Eligibility
Patients with metastatic ACC who failed standard treatments were enrolled. The main eligibility criteria included: age ≥18 years; pathological confirmation of positive IL-13Rα2 expression in ≥30% of the tumor cells by immunohistochemistry (IHC); measurable disease by response evaluation criteria in solid tumors (RECIST v1.1) at presentation; last dose of chemotherapy or last radiotherapy treatment more than 4 weeks prior to starting IL-13-PE treatment; prior or current mitotane therapy was allowed if patients were on the therapy to control hypercortisolemia, tolerating their dose and did not have a tumor response to treatment; no currently active central nervous system metastasis; Eastern Cooperative Oncology Group (ECOG) performance status at 0–2; good organ function. All patients provided written informed consent. The study (Clinicaltrials.gov: NCT01832974) was approved by our Institutional Review Board and conducted in accordance with Helsinki Declaration and good clinical practice guidelines.
Study design
This was an open-label Phase I study to assess the maximum-tolerated dose (MTD) of IL-13-PE. The dose-escalation strategy involved three cohorts: six patients to receive 1 μg/kg IL-13-PE, three to six patients to receive 2 μg/kg, and three to six patients to receive 3 μg/kg (Table S1). Treatments for all dose levels were intravenously administered over a 1-h infusion on days 1, 3, and 5 of the first week in each 4-week cycle. Patients were planned to receive four cycles of treatment, but additional cycles were allowed if they had stable disease or partial/complete response. In the following text, detailed treatment date will be referred to as C_D_, with C for Cycle and D for Day; specific patient will be referred to as Pt._.
At each dose, only two patients were on treatment until the first cycle was completed. Additional patients at the same dose were enrolled only when no dose-limiting toxicity (DLT) was observed in those patients. Escalation to the next higher dose was permitted if no more than 1/6 of the previous cohort experienced DLT or the first three patients in subsequent cohorts did not experience DLT. MTD was defined as the highest dose that induces DLT in less than two patients in a cohort of six patients. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). No intrapatient dose escalation or dose reductions were allowed.
Evaluation
Blood chemistry and urinalysis were tested within 24 h prior to each treatment and daily during week 1 and then weekly (±2 days). Toxicity evaluation, vital signs, ECOG status, and physical examination were completed within 24 h of each treatment. CT scans of the chest, abdomen and pelvis were used to evaluate the response using RECIST criteria v1.1.
Pharmaceutical information
IL-13-PE was produced in Escherichia coli as described previously under clinical grade drug manufacturing by Insys Therapeutics (Chandler, AZ) 17.
Pharmacokinetics
Blood samples from patients were collected at 30 min before infusion, and at 0, 5, 15, 30, 60, 90, 120, 180, 240 min, and 24 h after infusion completion on C1D1, C1D3, and C2D1 if treatment was continued. Serum samples were obtained by centrifuging the blood sample tubes at 1940 g for 10 min at 4°C. Serum IL-13-PE concentration was measured in duplicates by enzyme-linked immunosorbent assay (ELISA) using Quantikine Human IL-13 Immunoassay kit from R&D systems, Minneapolis, MN. Purified IL-13-PE was used to produce the standard curve. The lower limit of quantification for this assay was 1.7 ng/mL.
IL-13-PE serum concentration data were analyzed to calculate pharmacokinetics (PK) parameters of apparent elimination half-life (t½), area under the concentration–time curve from time 0 to the last time point (AUClast) and extrapolated to infinity (AUC0–∞), clearance (CL), and volume of distribution (Vz) via noncompartmental methods using Phoenix WinNonlin Version 6.4 (Pharsight Corp. Mountainview, CA). AUC was computed using the linear (up)/logarithmic (down) trapezoidal rule with a minimum of four quantifiable concentrations. The only observed values included the Cmax and the corresponding peak times (Tmax). Dose-proportionality of AUC and Cmax PK parameters was assessed by determining the geometric mean ratio (GMR) of the two different dose levels.
Anti-IL-13-PE neutralizing antibody detection
A noncommercial, nonisotopic assay was used to detect neutralizing antibody against IL-13-PE. One thousand U251 cells (Human glioma cell line positive for IL-13Rα2 expression) in 100 μL of complete RPMI 1640 medium were plated on 96-well black-wall clear-bottom plates and allowed to attach in a 37°C incubator with 5% CO2 overnight. Medium composition was as described in Ou, W., et al., 2012 25. On the second day, 50 μL serially diluted patient serum was added in quadruplicates into the U251 cells-coated wells. After 2-h incubation in the cell culture incubator, 50 μL of purified IL-13-PE at 1 ng/mL was added to each well. The plates were then returned to the incubator. Four days later, 20 μL of house-made resazurin was added to each well. Fluorescence of the plates was measured using SpectraMax M5 (VWR Corporate, Altlanta, GA) plate reader (Molecular Devices, Sunnyvale, CA) after 4 h (544 nm for excitation and 590 nm for emission).
Immunohistochemistry
IHC staining for IL-13Rα2 was performed with Dako Autostainer (Carpinteria, CA). Primary antibody used was goat anti-IL-13Rα2 antibody (R&D systems, Minneapolis, MN, 1:1000). The signals were detected by ImmPRESS HRP anti-goat Ig (peroxidase) polymer detection kit (Vector Laboratories, Burlingame, CA), developed by DAB+ (Dako, Carpinteria, CA), and followed by a light hematoxylin counterstain. The staining was semiquantitatively assessed for intensity and percent of positive cells. Negative, weakly positive, and strongly positive controls, selected based on RNA expression data, were included in each experiment. The slides were scanned under an Olympus light microscope (Nikon, Tokyo, Japan) and images were acquired at 20× and 40× magnifications.
Results
Patient characteristics and treatment dose
Eight patients were enrolled in this study (N = 6 at dose 1 μg/kg, N = 2 at dose 2 μg/kg) and were evaluable for toxicity. The clinical characteristics of the patients enrolled are summarized in Table1. All patients had stage IV ACC with metastasis to lung (100%), liver (50%) and bone (12.5%). Seven (87.5%) patients had previous operations, two patients (25%) had radiotherapies and all eight (100%) patients had no response to prior systemic chemotherapies. All patients had positive IL-13Rα2 expression in ≥30% of the tumor cells as determined by IHC (Fig.1).
Table 1.
Patient characteristics
| Demographic and clinical characteristics | N | % |
|---|---|---|
| Sex | ||
| Male | 3 | 37.5 |
| Female | 5 | 62.5 |
| Age at diagnosis, median (range) | 39 (15–64) | |
| Age at enrollment, median (range) | 42 (18–65) | |
| Body weight in kg, median (range) | 82.8 (59.8–125.9) | |
| Performance status | ||
| 0 | 7 | 87.5 |
| 2 | 1 | 12.5 |
| Tumor stage at initial diagnosis | ||
| IV | 8 | 100 |
| Site of tumor at enrollment | ||
| Primary adrenal gland | 1 | 12.5 |
| Adrenal bed (local recurrence) | 7 | 87.5 |
| Regional intra-abdominal recurrence1 | 1 | 12.5 |
| Lung metastases | 8 | 100 |
| Liver metastases | 4 | 50 |
| Bone metastases | 1 | 12.5 |
| Prior treatment | ||
| Surgery2 | 7 | 87.5 |
| Radiotherapy | 2 | 25 |
| Chemotherapy | 8 | 100 |
| Mitotane monotherapy | 7 | 87.5 |
| Etoposide, doxorubicin, cisplatin, and mitotane | 8 | 100 |
| Protease inhibitor3 | 1 | 12.5 |
| Abraxane | 1 | 12.5 |
A single patient was found to have metastatic disease to the intra-abdominal mesentery.
Prior surgeries include primary resection of adrenocortical carcinoma, metastectomy, and debulking procedures.
A single patient underwent treatment with proteasome inhibitors, bortezomib, and carfilzomib.
Figure 1.
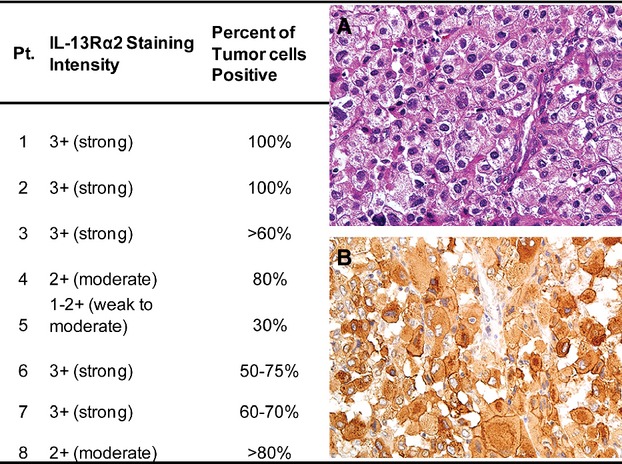
IL-13Rα2 staining intensity and percent positive cells for each enrolled patient with metastatic ACC. (A) Representative hematoxylin and eosin (H&E) staining of patient tumor. (B) Representative IL-13Rα2 staining of patient tumor. IL, interleukin; ACC, adrenocortical carcinoma.
Adverse events
To determine the safety of IL-13-PE, all AEs that were potentially related to the therapy were examined. Of the six patients treated with 1 μg/kg IL-13-PE, the most common AEs included Grade 1 or 2 anemia, proteinuria, increase in alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine and fatigue, all of which were seen in three patients. One patient developed Grade 3 hypertension which resolved within 2 days (Table2). Dose escalation to 2 μg/kg resulted in severe AEs in the first two patients enrolled. Grade 3 anemia was observed in both patients, whereas Grade 3 or 4 proteinuria, creatinine increase, acute kidney injury, hyponatremia, neutropenia, pain, and thrombocytopenia were observed in one of two patients. Both patients recovered from these toxicities with supportive care not requiring hemodialysis. Thus, it was determined that 1 μg/kg was the MTD.
Table 2.
Adverse events of IL-13-PE in patients treated at 1 and 2 μg/kg.
| Common toxicity criteria term | 1 μg/kg (N = 6) | 2 μg/kg (N = 2) | ||
|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | Grade 1 or 2 | Grade 3 or 4 | |
| N (%) | N (%) | N (%) | N (%) | |
| Anemia | 3 (50) | 0 | 2 (100) | 2 (100) |
| Proteinuria | 3 (50) | 0 | 2 (100) | 1 (50) |
| Alanine aminotransferase increased | 2 (33.3) | 0 | 1 (50) | 0 |
| Aspartate aminotransferase increased | 2 (33.3) | 0 | 1 (50) | 0 |
| Creatinine increased | 2 (33.3) | 0 | 2 (100) | 1 (50) |
| Fatigue | 2 (33.3) | 0 | 1 (50) | 0 |
| Alkaline phosphatase increased | 1 (16.7) | 0 | 0 | 0 |
| Chills | 1 (16.7) | 0 | 0 | 0 |
| Cough | 1 (16.7) | 0 | 0 | 0 |
| Dyspnea | 1 (16.7) | 0 | 0 | 0 |
| Edema limbs | 1 (16.7) | 0 | 1 (50) | 0 |
| Electrocardiogram QT corrected interval prolonged | 1 (16.7) | 0 | 0 | 0 |
| Headache | 1 (16.7) | 0 | 1 (50) | 0 |
| Hyperkalemia | 1 (16.7) | 0 | 0 | 0 |
| Hypertension | 1 (16.7) | 1 (16.7) | 0 | 0 |
| Pericardial effusion | 1 (16.7) | 0 | 0 | 0 |
| Weight gain | 1 (16.7) | 0 | 1 (50) | 0 |
| Acute kidney injury | 0 | 0 | 2 (100) | 1 (50) |
| Blood bilirubin increased | 0 | 0 | 1 (50) | 0 |
| Bruising | 0 | 0 | 1 (50) | 0 |
| Epistaxis | 0 | 0 | 1 (50) | 0 |
| Fall | 0 | 0 | 1 (50) | 0 |
| Hematuria | 0 | 0 | 2 (100) | 0 |
| Hypoalbuminemia | 0 | 0 | 2 (100) | 0 |
| Hypocalcemia | 0 | 0 | 1 (50) | 0 |
| Hypokalemia | 0 | 0 | 2 (100) | 0 |
| Hypomagnesemia | 0 | 0 | 1 (50) | 0 |
| Hyponatremia | 0 | 0 | 1 (50) | 1 (50) |
| Neutropenia | 0 | 0 | 1 (50) | 1 (50) |
| Nausea | 0 | 0 | 1 (50) | 0 |
| Pain | 0 | 0 | 0 | 1 (50) |
| Thrombocytopenia | 0 | 0 | 2 (100) | 1 (50) |
IL-13-PE, interleukin-13-Pseudomonas exotoxin.
To determine if the DLT was due to a rapid induction of proinflammatory cytokines, we performed a human 30-plex cytokine array using serum samples within 2 days of the initial treatment. The expression levels of 30 common human pro-/anti-inflammatory cytokines were detected simultaneously within each sample to screen for any candidates whose levels were significantly altered. Among the 30 cytokines tested, eight had expression levels that were below the lowest detection limit in all samples; one (RANTES) had an expression level above the highest detection limit in almost all samples. No significant changes were observed in the expression levels of the remaining 21 cytokines that might have contributed to DLT during the treatment course (Fig. S1).
PK assessment
Serum samples on C1D1 and C1D3 were collected from all eight patients for PK studies, whereas serum samples on C2D1 were collected from Pt.1 and Pt.5. Prior to infusion, none of the patients had a quantifiable level of IL-13-PE in the serum. The time versus serum concentration curves for each patient are shown in Figure2 and the PK parameters related to each dose and treatment cycle are summarized in Table3. After infusion, IL-13-PE serum concentrations declined log-linearly without any significant distribution phase observed. The terminal half-life (t1/2) was measured to be 30–39 min under all conditions tested. On C1D1, the geometric mean (GM) of Cmax for IL-13-PE was 17.5 ng/mL when treated at 1 μg/kg, and 41.2 ng/mL at 2 μg/kg. After multiple dosing in the first cycle of therapy, no significant accumulation was observed, as the GMRs of AUC0–∞ and Cmax (C1D3 vs. C1D1) were 1.23 and 1.20 in the 1 μg/kg dose group and 1.14 and 1.00 in the 2 μg/kg dose group, respectively. In contrast, in the two patients who completed a second cycle of therapy at the 1 μg/kg dose, the GMRs of AUC0–∞ and Cmax (C2D1 vs. C1D1) were decreased by 73% and 72%, respectively, in Pt.5, but were not decreased in Pt.1 (Fig.2). The PK of IL-13-PE increased relatively proportionally when the dose was doubled, as the GMRs of AUC0–∞ and Cmax (2 μg/kg vs. 1 μg/kg dose groups) were 2.54 and 2.31, respectively.
Figure 2.
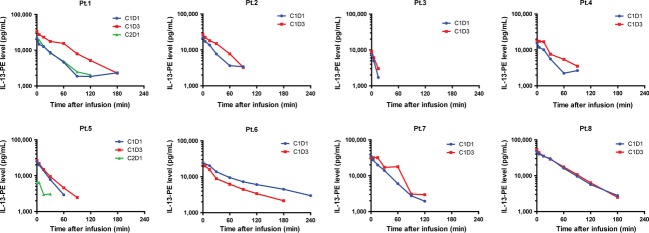
Serum pharmacokinetics of interleukin-13-Pseudomonas exotoxin (IL-13-PE) for each patient enrolled. Only serum samples with IL-13-PE concentration above the lower limit of quantification (LLQ) are included. Pt.1 to Pt.6 received 1 μg/kg IL-13-PE, whereas Pt.7 and Pt.8 received 2 μg/kg IL-13-PE.
Table 3.
Geometric mean (GM) pharmacokinetic parameters of IL-13-PE treatment
| Dose | Cycle, day | No. of patients | Cmax (ng/mL) | AUClast (min·ng/mL) | AUC0–∞ (min·ng/mL) | % AUCextrap | t1/2 (min) | Vz (mL) | CL (mL/min) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | CV | GMR | GM | CV | GMR | GM | CV | GMR | GM | CV | GM | CV | GM | CV | GM | CV | |||
| 1 μg/kg | C1D1 | 6 | 17.5 | 33.8 | NA | 1134 | 57 | NA | 1262 | 59 | NA | 9 | 36 | 30 | 78 | 3169 | 26 | 72 | 86 |
| C1D3 | 6 | 21 | 36.6 | 1.2 | 1410 | 51 | 1.24 | 1553 | 49 | 1.23 | 9 | 37 | 34 | 55 | 2917 | 21 | 59 | 82 | |
| C2D1 | 2 | 11.7 | 79.9 | 0.67 | 665 | 92 | 0.59 | 836 | 79 | 0.66 | 15 | 87 | 36 | 15 | 7320 | 78 | 142 | 87 | |
| 2 μg/kg | C1D1 | 2 | 41.2 | 19 | NA | 2963 | 41 | NA | 3089 | 41 | NA | 4 | 9 | 38 | 26 | 1153 | 31 | 21 | 55 |
| C1D3 | 2 | 41.4 | 36.1 | 1 | 3374 | 34 | 1.14 | 3531 | 33 | 1.14 | 4 | 33 | 39 | 12 | 1068 | 37 | 19 | 47 | |
| 1 μg/kg | All days | 6 | 17.9 | 39.4 | NA | 1154 | 56 | NA | 1300 | 55 | NA | 9.8 | 61 | 33 | 60 | 3447 | 73 | 73 | 87 |
| 2 μg/kg | All days | 2 | 41.3 | 23.8 | 2.31 | 3162 | 31 | 2.74 | 3303 | 31 | 2.54 | 4.2 | 21 | 38 | 16 | 1110 | 28 | 20 | 43.2 |
IL-13-PE, interleukin-13-Pseudomonas exotoxin; Cmax, maximum serum concentration; t1/2, terminal half-life; AUClast, area under the curve from time 0 to last quantifiable time point; AUC0–∞, area under the curve extrapolated from time 0 to infinity; % AUCextrap, percentage AUC extrapolation; Vz, volume of distribution; CL, clearance; CV, coefficient of variation; GMR, geometric mean ratio; NA, not applicable.
Immunogenicity of IL-13-PE
To determine if neutralizing antibodies against PE were present before treatment or generated against IL-13-PE during treatment, serum samples before each treatment cycle plus serum samples from C1D15 (if available) were collected and tested in a nonisotopic cytotoxicity assay. Interestingly, baseline anti-PE antibodies were detected in 2 (25%) of the eight patients at a low titer (50) prior to initiation of C1 treatment (Fig.3, Table S2). By C1D15, neutralizing antibodies were detected in four (67%) of six patients with available serum samples. These included the two patients who had baseline antibodies and two new patients who developed anti-IL-13-PE antibodies after treatment. At the end of C1 (day 29, immediately prior to C2D1), anti-IL-13-PE antibodies were detected in all three tested patients (100%), one of which developed neutralizing antibodies for the first time. Once the antibodies were generated, they were produced rapidly in large quantities. Within 14–28 days of initial detection, the antibody titer was as high as 105.
Figure 3.
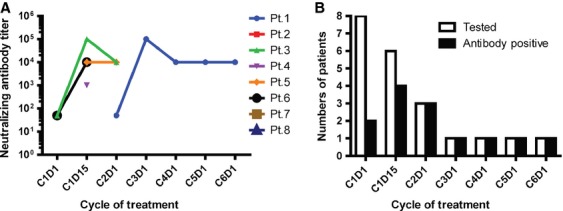
Immunogenicity of interleukin-13-Pseudomonas exotoxin (IL-13-PE). The presence of neutralizing antibodies against IL-13-PE was monitored over time. (A) Neutralizing antibody titer detected in each patient during treatment. (B) Numbers of patients tested (Open bar) and positive (black bar) for neutralizing antibodies against PE or IL-13-PE at each treatment cycle.
Treatment response
Although this was a Phase I trial, we were able to evaluate response to treatment in five of the six patients who received the 1 μg/kg dose (Table S2). Pt.2 was taken off study due to disease progression before the end of C1, thus the response was not measured. Of the five patients assessed, one (20%, Pt.1) had stable disease for 5.5 months and underwent six cycles of treatment, but disease progressed at the end of C6; two patients (40%, Pt.3 and Pt.5) had stable disease for 2 months, but disease progressed at the end of C2; the other two patients (40%, Pt.4 and Pt.6) had progressive disease after C1.
Discussion
IL-13-PE represents a novel therapeutic strategy that specifically targets cells overexpressing IL-13Rα2. Here, we provide the results of the first Phase I clinical trial designed to examine the safety profile and effects of systemic IV administration of IL-13-PE in patients with metastatic ACC overexpressing IL-13Rα2. Based on our study, IV infusion of 1 μg/kg was determined as the MTD for IL-13-PE. At this dose, the most common adverse effects included low grade anemia, proteinuria, fatigue, and increase in ALT, AST, and creatinine.
The first two patients treated with 2 μg/kg of IL-13-PE developed Grade 3 and 4 toxicities most consistent with thrombotic microangiopathy but only required supportive care. Although a kidney biopsy sample was not obtained to determine thrombotic microangiopathy, this toxicity has been previously observed in Phase I trials of other immunotoxins 26. The self-limited toxicities occurred after completion of the first week of IL-13-PE infusion. The mechanism behind immunotoxin-induced thrombotic microangiopathy is not well understood 26. Several mechanisms have been proposed to explain immunotoxin-mediated thrombotic microangiopathy, including toxin-mediated endothelial damage (off-target and targeted effect) and a general proinflammatory response. We analyzed the cytokine profile in all patients before and after IL-13-PE infusion but observed no appreciable change in proinflammatory cytokine profiles before and after IL-13-PE infusion to support the latter hypothesis. As IL-13Rα2 protein expression was negative in normal human tissues as examined by IHC in human tissue array (Fig. S2), and IL-13Rα2 gene expression was also undetected in 14 cases of human activated lymphocytes (with IL-2) and 21 cases of blood lymphocytes (data not shown), it is unlikely that the toxicities exhibited in Pt.7 and Pt.8 were due to off-target effect of IL-13-PE.
As IL-13-PE is a recombinant cytotoxin, which contains domain II and III of PE 16, immunogenicity to this cytotoxin may be expected in patients. Neutralizing antibodies present from prior exposure to PE or produced de novo during treatment represents a challenge for therapy using this chimeric cytotoxin. In this study, we observed that antibodies against PE was present at baseline in 2 (25%) of the eight patients. During IL-13-PE treatment, the generation of high titers of antibodies was fairly rapid (within 14–28 days of treatment initiation) and prevailed in all patients who remained on treatment at MTD. Generation of neutralizing antibody may alter PK and subsequently diminish the efficacy of IL-13-PE. For example, the PK data for C1 and C2 were available for both Pt.1 and Pt.5. In Pt.1 the Cmax and CL were comparable on C1D1 and C2D1. In contrast, the Cmax achieved in Pt.5 was much lower on C2D1 than on C1D1 (Fig.2). This would be consistent with the finding that on C2D1, the anti-IL-13-PE antibody titer was much higher in Pt.5 (104) than in Pt.1 (50) (Fig.3A). High levels of existing anti-IL-13-PE antibodies putatively neutralized most of the administered IL-13-PE during treatment, thus the concentration of free IL-13-PE measured in serum was significantly lower in Pt.5.
PK of IL-13-PE showed overall rapid elimination from serum and apparent linear disposition among drug dose levels of 1 and 2 μg/kg, based on evaluation of Cmax and AUC (drug exposure) on each treatment day and cycle. However, due to limited patient numbers, interpatient variability in weight, age, and other factors that may also influence the PK of IL-13-PE, further investigation is needed in future clinical trials involving IL-13-PE. We also do not know if mitotane therapy could have affected the PK data but this is unlikely given that the patients had normal organ function.
Of the five patients treated at MTD and assessed for response, Pt.1 had stable disease for the longest time (5.5 months) before disease progression. Interestingly, Pt.1 had the highest IL-13Rα2 expression level in the tumors and the latest development of neutralizing antibodies against IL-13-PE (Figs.1 and 3A). However, the limited number of patients makes it difficult to determine whether patient response would correlate with the expression level of IL-13Rα2.
In conclusion, this is the first Phase I trial designed to test the safety and effects of systemic IV administration of IL-13-PE in patients with metastatic ACC. Our study demonstrated that IV infusion of IL-13-PE at dose 1 μg/kg is safe and tolerated well by patients when administered every other day for three times during week 1 of a 4-week cycle. However, the generation of neutralizing antibody might hinder the effectiveness of the treatment. In future clinical trials, it would be worthwhile to consider strategies for immunodepletion before IL-13-PE treatment to reduce the development of neutralizing antibodies to this immunotoxin. Alternatively, other IL-13 fusion proteins with no or low immunogenicity should be developed.
Acknowledgments
This research was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (grant # 1 ZIA BC011286 05).
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Expression levels of 22 cytokines that were detected in at least one patient using human 30-plex cytokine array. Human Cytokine Magnetic 30-Plex Panel (Life technologies, Frederick, MD) was used to quantitatively determine the level of 30 different cytokines in patient serum. Assay plates were run on Luminex100 platform and data analysis was performed using Bio-Plex manager 6.0 (Bio-Rad, Hercules, CA). Pt.1 to Pt.6 received 1 µg/kg IL-13-PE, whereas Pt.7 and Pt.8 received 2 µg/kg IL-13-PE. Only data within the detection limit of the assay are shown. For RANTES, missing data points were all above the highest detection limit of the assay. For the remaining cytokines, missing data points were all below the lowest detection limit of the assay. No significant changes in cytokine levels were observed that could be associated with DLT. (A) Proinflammatory cytokines. (B) Anti-inflammatory cytokines. Compared to other patients, the cytokine expression profile of Pt.2 was very different. Pt.2 had heavier disease burden than other patients, and the adverse event exhibited in this patient (Grade 3 bone pain, Grade 3 hypokalemia and Grade 2 AST increase) was considered unrelated or unlikely attributed to treatment. Thus, the unusual cytokine levels observed in Pt.2 were unlikely to be associated with toxicity.
Figure S2. IL-13Rα2 IHC staining in normal tissue array. FDA998 normal organ tissue array of human, 47 cases/99 cores (US Biomax, Inc., Rockville, MD) was used to stain for IL-13Rα2. (A) Array layout: Ceg, Cerebral gray matter; Cew, Cerebral white and/or gray matter; Ceb, Cerebellum tissue; Adr, Adrenal gland; Ova, Ovary; Pan, Pancreas; Par, Parathyroid gland; Hyp, Hypophysis; Tes, Testis; Thr, Thyroid; Bre, Breast; Spl, Spleen; Ton, Tonsil; Thy, Thymus gland; Bon, Bone; Lun, Lung; Hea, Heart; Eso, Esophagus; Sto, Stomach; Sma, Small intestine; Col, Colon; Liv, Liver; Sal, Salivary gland; Kid, Kidney; Pro, Prostate; Ute, Uterine cervix; Str, Striated muscle; Ski, Skin; Ner, Nerve; Mes, Mesothelium; Eye, Eye; Lar, Larynx. Blue background: malignant tumor; light purple background: cancer adjacent normal tissue; others: normal tissue. (B) IL-13Rα2 staining. All normal tissues were IL-13Rα2-negative as compared to positive control melanoma sample.
Table S1. Study design.
Table S2. Treatment dosage, IL-13-PE neutralizing antibody production and response to treatment.
References
- Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Libe R, Kroiss M. Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat. Rev. Endocrinol. 2011;7:323–335. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- Kebebew E, Reiff E, Duh QY, Clark OH. McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J. Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer. 2013;49:2579–2586. doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Fassnacht M. Allolio B. Clinical management of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23:273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Husain SR, Obiri NI, Gill P, Zheng T, Pastan I, Debinski W, et al. Receptor for interleukin 13 on AIDS-associated Kaposi’s sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin. Cancer Res. 1997;3:151–156. [PubMed] [Google Scholar]
- Kawakami M, Kawakami K, Kasperbauer JL, Hinkley LL, Tsukuda M, Strome SE, et al. Interleukin-13 receptor alpha2 chain in human head and neck cancer serves as a unique diagnostic marker. Clin. Cancer Res. 2003;9:6381–6388. [PubMed] [Google Scholar]
- Kioi M, Kawakami M, Shimamura T, Husain SR. Puri RK. Interleukin-13 receptor alpha2 chain: a potential biomarker and molecular target for ovarian cancer therapy. Cancer. 2006;107:1407–1418. doi: 10.1002/cncr.22134. [DOI] [PubMed] [Google Scholar]
- Kioi M, Seetharam S. Puri RK. Targeting IL-13Ralpha2-positive cancer with a novel recombinant immunotoxin composed of a single-chain antibody and mutated Pseudomonas exotoxin. Mol. Cancer Ther. 2008;7:1579–1587. doi: 10.1158/1535-7163.MCT-07-2131. [DOI] [PubMed] [Google Scholar]
- Joshi BH, Plautz GE. Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- Debinski W. Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol. Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- Jain M, Zhang L, He M, Patterson EE, Nilubol N, Fojo AT, et al. Interleukin-13 receptor alpha2 is a novel therapeutic target for human adrenocortical carcinoma. Cancer. 2012;118:5698–5708. doi: 10.1002/cncr.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura T, Fujisawa T, Husain SR, Joshi B. Puri RK. Interleukin 13 mediates signal transduction through interleukin 13 receptor alpha2 in pancreatic ductal adenocarcinoma: role of IL-13 Pseudomonas exotoxin in pancreatic cancer therapy. Clin. Cancer Res. 2010;16:577–586. doi: 10.1158/1078-0432.CCR-09-2015. [DOI] [PubMed] [Google Scholar]
- Hou L, Du J, Wang J, Liu Y, Sun W, Zheng Y, et al. Expression of IL-13Ralpha2 in liver cancer cells and its effect on targeted therapy of liver cancer. J. Cancer Res. Clin. Oncol. 2010;136:839–846. doi: 10.1007/s00432-009-0724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debinski W, Obiri NI, Pastan I. Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J. Biol. Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- Joshi BH, Puri RK. Optimization of expression and purification of two biologically active chimeric fusion proteins that consist of human interleukin-13 and Pseudomonas exotoxin in Escherichia coli. Protein Expr. Purif. 2005;39:189–198. doi: 10.1016/j.pep.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Puri RK, Leland P, Obiri NI, Husain SR, Keitman RJ, Haas GP, et al. Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR) Blood. 1996;87:4333–4339. [PubMed] [Google Scholar]
- Joshi BH, Kawakami K, Leland P. Puri RK. Heterogeneity in interleukin-13 receptor expression and subunit structure in squamous cell carcinoma of head and neck: differential sensitivity to chimeric fusion proteins comprised of interleukin-13 and a mutated form of Pseudomonas exotoxin. Clin. Cancer Res. 2002;8:1948–1956. [PubMed] [Google Scholar]
- Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- Kioi M, Husain SR, Croteau D, Kunwar S. Puri RK. Convection-enhanced delivery of interleukin-13 receptor-directed cytotoxin for malignant glioma therapy. Technol. Cancer Res. Treat. 2006;5:239–250. doi: 10.1177/153303460600500307. [DOI] [PubMed] [Google Scholar]
- Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61:1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. ; discussion 1037–1038. [DOI] [PubMed] [Google Scholar]
- Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar S, Chang SM, Prados MD, Berger MS, Sampson JH. Croteau D, et al. Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies. Neurosurg. Focus. 2006;20:E15. [PubMed] [Google Scholar]
- Ou W, Marino MP, Suzuki A, Joshi B, Husain SR, Maisner A, et al. Specific targeting of human interleukin (IL)-13 receptor alpha2-positive cells with lentiviral vectors displaying IL-13. Hum. Gene Ther. Methods. 2012;23:137–147. doi: 10.1089/hgtb.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake-Haskins JA, Lechleider RJ. Kreitman RJ. Thrombotic microangiopathy with targeted cancer agents. Clin. Cancer Res. 2011;17:5858–5866. doi: 10.1158/1078-0432.CCR-11-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression levels of 22 cytokines that were detected in at least one patient using human 30-plex cytokine array. Human Cytokine Magnetic 30-Plex Panel (Life technologies, Frederick, MD) was used to quantitatively determine the level of 30 different cytokines in patient serum. Assay plates were run on Luminex100 platform and data analysis was performed using Bio-Plex manager 6.0 (Bio-Rad, Hercules, CA). Pt.1 to Pt.6 received 1 µg/kg IL-13-PE, whereas Pt.7 and Pt.8 received 2 µg/kg IL-13-PE. Only data within the detection limit of the assay are shown. For RANTES, missing data points were all above the highest detection limit of the assay. For the remaining cytokines, missing data points were all below the lowest detection limit of the assay. No significant changes in cytokine levels were observed that could be associated with DLT. (A) Proinflammatory cytokines. (B) Anti-inflammatory cytokines. Compared to other patients, the cytokine expression profile of Pt.2 was very different. Pt.2 had heavier disease burden than other patients, and the adverse event exhibited in this patient (Grade 3 bone pain, Grade 3 hypokalemia and Grade 2 AST increase) was considered unrelated or unlikely attributed to treatment. Thus, the unusual cytokine levels observed in Pt.2 were unlikely to be associated with toxicity.
Figure S2. IL-13Rα2 IHC staining in normal tissue array. FDA998 normal organ tissue array of human, 47 cases/99 cores (US Biomax, Inc., Rockville, MD) was used to stain for IL-13Rα2. (A) Array layout: Ceg, Cerebral gray matter; Cew, Cerebral white and/or gray matter; Ceb, Cerebellum tissue; Adr, Adrenal gland; Ova, Ovary; Pan, Pancreas; Par, Parathyroid gland; Hyp, Hypophysis; Tes, Testis; Thr, Thyroid; Bre, Breast; Spl, Spleen; Ton, Tonsil; Thy, Thymus gland; Bon, Bone; Lun, Lung; Hea, Heart; Eso, Esophagus; Sto, Stomach; Sma, Small intestine; Col, Colon; Liv, Liver; Sal, Salivary gland; Kid, Kidney; Pro, Prostate; Ute, Uterine cervix; Str, Striated muscle; Ski, Skin; Ner, Nerve; Mes, Mesothelium; Eye, Eye; Lar, Larynx. Blue background: malignant tumor; light purple background: cancer adjacent normal tissue; others: normal tissue. (B) IL-13Rα2 staining. All normal tissues were IL-13Rα2-negative as compared to positive control melanoma sample.
Table S1. Study design.
Table S2. Treatment dosage, IL-13-PE neutralizing antibody production and response to treatment.


