Abstract
Awareness increases that the tumor biology influences treatment outcome and prognosis in cancer. Tumor hypoxia is thought to decrease sensitivity to radiotherapy and some forms of chemotherapy. Presence of hypoxia may be assessed by investigating expression of endogenous markers of hypoxia (EMH) using immunohistochemistry (IHC). In this systematic review we investigated the effect of EMH expression on local control and survival according to treatment modality in head and neck cancer (head and neck squamous cell carcinoma [HNSCC]). A search was performed in MEDLINE and EMBASE. Studies were eligible for inclusion that described EMH expression in relation to outcome in HNSCC patients. Quality was assessed using the Quality in Prognosis Studies (QUIPS) tool. Hazard ratios for locoregional control and survival were extracted. Forty studies of adequate quality were included. HIF-1a, HIF-2a, CA-IX, GLUT-1, and OPN were identified as the best described EMHs. With exception of HIF-2a, all EMHs were significantly related to adverse outcome in multiple studies, especially in studies where patients underwent single-modality treatment. Positive expression was often correlated with adverse clinical characteristics, including disease stage and differentiation grade. In summary, EMH expression was common in HNSCC patients and negatively influenced their prognosis. Future studies should investigate the effect of hypoxia-modified treatment schedules in patients with high In summary, EMH expression. These may include ARCON, treatment with nimorazole, or novel targeted therapies directed at hypoxic tissue. Also, the feasibility of surgical removal of the hypoxic tumor volume prior to radiotherapy should be investigated.
Keywords: head and neck neoplasms, hypoxia, hypoxia-inducible factor 1, personalized medicine, tumor microenvironment
Introduction
Despite improvement of surgical and radiotherapeutic techniques, as well as the introduction of systemic therapies including cisplatin or cetuximab, 5-year survival rates for patients with head and neck squamous cell carcinoma (HNSCC) remain low 1. Currently, staging and treatment selection is based only on clinical staging using the AJCC TNM-classification. However, awareness increases that not all squamous cell carcinomas are the same, but have different tumor biology 2. These differences could have an even greater impact on treatment outcome than mere clinical staging. An example is infection with the human papillomavirus (HPV) in oropharyngeal squamous cell carcinoma (OPSCC): HPV-associated (HPV+) OPSCC cancers show a much better response to radio- and chemotherapy than non-HPV-associated (HPV−) OPSCC 3,4. In this line there is a clear need for other novel biomarkers to predict sensitivity to a particular treatment modality, or to identify which patients might benefit from adjuvant therapies.
One possible target or prognosticator is tumor hypoxia. Hypoxia is defined as a mismatch between cellular oxygen demand and supply. The causes of hypoxia can roughly be divided into two categories: acute or chronic hypoxia. Acute, or perfusion-limited, hypoxia, occurs when there is insufficient oxygen supply to cells due to compromise of the supplying blood vessels. Acute hypoxia causes electrolyte imbalances and an increase in intracellular hydrogen sulfide. When this occurs in specialized hypoxia-sensing cells, such as glomus or smooth muscle cells, this leads to a systemic response, such as vasodilation 5. In contrast, chronic hypoxia triggers a cellular response in individual cells only after several hours of hypoxia 6. Chronic hypoxia is often caused by diffusion-limitations which occur when the distance from a cell to the nearest blood vessel is too large for adequate cellular oxygenation 7. Because of expansive tumor growth, chronic hypoxia often occurs in solid tumors, including HNSCC 8. HNSCC is often treated with radiotherapy which depends on oxygen for free radical formation to induce DNA strand breaks and cell death. Because of the need for oxygen, tumor hypoxia causes decreased sensitivity to radiotherapy. Separately, hypoxia is thought to induce tumor progression and a more aggressive phenotype 9. Therefore, the hypoxic status of a tumor could possibly contribute in identifying the treatment option that offers the best prognosis to an individual patient. For example, surgical removal of the hypoxic component before radiotherapy might be preferable above radiotherapy alone, when reduced sensitivity to primary radiotherapy is expected. Alternatively, hypoxia-sensitizing radiotherapy schedules such as accelerated radiotherapy with carbogen-breathing and nicotinamide (ARCON) or addition of a hypoxia-sensitizing drug like nimorazole may be considered 10,11.
Several ways of assessing tumor hypoxia have been described 12. This includes the invasive Eppendorf pO2 histography, that uses polarographic needles to measure tissue pO2 in vivo. Tissue biomarkers for hypoxia may also be used to assess tumor hypoxia histologically. The use of exogenous biomarkers, for instance of the nitroimidazole class, is also invasive, as they have to be administered to patients intravenously before excision of the tissue. Finally, various endogenous biomarkers for hypoxia (EMHs) exist, that can be used to assess the hypoxic state using IHC, with no need for additional invasive procedures other than routine diagnostic biopsy. The most important endogenous biomarkers are part of the hypoxia-inducible factor 1(HIF)-1 pathway. HIF-1 is upregulated under hypoxia to improve cellular survival in a hypoxic microenvironment. This basic helix-loop-helix transcription factor consists of an alpha (HIF-1a) and beta (HIF-1b or ARNT) subunit. Both are constitutively expressed, but under normoxic conditions HIF-1a is quickly degraded by hydroxylation and binding to the VHL protein 13,14. In the hypoxic state, hydroxylation of HIF-1a is inhibited, causing stabilization, enabling interaction with HIF-1b and increased transcription of its downstream targets. Another EMH is osteopontin (OPN), which is expressed independently of HIF-1a and is involved in the adhesive cell–matrix interaction and is considered a protein involved in tumor development and progression 15,16. A brief review of the studied EMHs is shown in Box Box 1.
Box 1 Endogenous markers of hypoxia.
| Biomarker | Role |
|---|---|
| HIF-1a | HIF-1alpha is the alpha subunit of the HIF-1 transcription factor, which is part of the cellular defense mechanisms to survive in a hypoxic state. Under normoxic conditions it is quickly degraded by prolyl hydroxylase (PHD) 1–3. Under hypoxic conditions, PHD activity is inhibited, causing overexpression of HIF-1a. As a transcription factor, HIF-1 stabilization causes increased transcription of its downstream targets through hypoxia-responsive elements (HRE) in the DNA |
| HIF-2a | HIF-2alpha is also a transcription factor in the HIF family, but has distinct other downstream targets. HIF-2a stabilization under hypoxia occurs through the same mechanism as HIF-1a |
| CA-IX | As hypoxic cells rely on anaerobic metabolism, intracellular pH will drop because of lactate formation. Carbonic anhydrase (CA) IX is a downstream target of HIF-1 involved in pH regulation 85. |
| GLUT-1 | A downstream target of HIF-1a. In hypoxic conditions, additional glucose is required for the anaerobic metabolism. There are many members in the glucose transporter (GLUT-) family, but GLUT-1 is specifically upregulated by HIF-1a |
| OPN | Osteopontin (OPN) is an integrin-binding protein of the SIBLING family (small integrin-binding ligand N-linked glycoprotein) and was first discovered in bone tissue. It promotes cellular survival through the NF-κβ pathway by reducing cell peroxide levels 86. It is upregulated independent of HIF-1a under hypoxia |
HIF-1, hypoxia-inducible factor 1.
Several (narrative) reviews are currently available on the effect of HIF-1a expression on local control and survival in patients with HNSCC. However, to our knowledge, no systematic reviews have studied EMH expression from a clinical approach, by systematically comparing the effect of all EMHs according to treatment outcome and taking into account differences between subsites. In the present study, we investigate which biomarkers are used to determine tumor hypoxia in HNSCC, as well as the effect of overexpression on clinical outcome.
Methods
Search strategy
A systematic review was performed in PubMed/MEDLINE and EMBASE. The search strategy is shown in Table S1. Briefly, a search was performed for studies that described the domain (“HNSCC”) and the determinant (“hypoxia”/EMHs) or synonyms of these terms in the title or abstract or as MeSH terms. The MEDLINE GENE database was used to identify synonyms of the various EMHs. Abstracts were screened based on predetermined in- and exclusion criteria by two authors independently (Fig.1). Full-text analysis of potentially relevant abstracts was performed and a final selection was made. At all stages, differences were resolved by discussion. The review was limited to EMHs that were studied in more than two articles.
Figure 1.
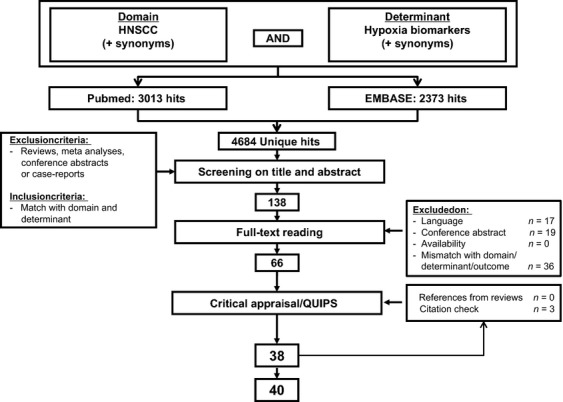
Study selection process. Study selection flowchart. Of the 66 suitable papers, 38 were found of adequate quality. A citation check yielded three additional results, of which two were of adequate quality. In total, 40 studies were included.
Relevant full text papers were appraised for risk of bias using the Quality in Prognosis Studies (QUIPS) tool, that has been developed for systematic appraisal in studies of prognostic factors 17. Using QUIPS, a risk of bias is determined, based on the study design and the reported results. For each of the six domains within QUIPS, the risk of bias was judged low (0 points), moderate (1 point) or high (2 points), based on three to seven predefined criteria per domain. For the current review, the following criteria were used: the source population should consist of a consecutive cohort of patients. Baseline characteristics should include T- and N-staging, as well as the treatment modality. Studies disclosing loss to follow-up and that confirmed whether censored patients were known to be alive at the moment of analysis were valued highest in the “study attrition” appraisal. In the correction for confounding appraisal, studies that investigated potential confounding effects of T- and N-staging, as well as treatment modality were valued highest. Finally, studies that scored a low risk of bias (≤3) were included.
Data extraction and meta-analysis
Extracted data included number of patients, disease stage, tumor subsite, treatment, biomarkers, and corresponding cutoffs and outcome. The studied outcomes were the hazard ratios (HR) for locoregional control (LRC), overall survival (OS), disease-free survival (DFS) and disease-specific survival (DSS). If a HR was not described, but a Kaplan–Meier curve was available, the curve was digitized using the open-source Engauge Digitizer software (http://digitizer.sourceforce.net) and a univariate HR was estimated through the methods of Tierney et al. 18. Meta-analysis was considered only if studies used the same cutoff values for EMH positivity and described patient cohorts were comparable in terms of treatment and disease stage. A review protocol was not previously published. Results are presented in accordance with the PRISMA statement for systematic reviews 19.
Results
Study selection
The search in EMBASE and PubMed yielded 4684 unique publications. Abstract screening yielded 138 potentially interesting papers, of which the full text was requested. Of these papers, 17 were not in English, Dutch or German, 19 were conference abstracts with no full text paper available and 36 were excluded for various reasons of mismatch with the domain, determinant, or outcome. Sixty-six papers remained for critical appraisal. Relevant reviews were read, references were screened and a citation check was performed using Web of Science. This yielded three additional papers. The study selection process is shown in Figure1.
Critical appraisal
Using the QUIPS criteria, the 66 and 3 papers identified through the search and citation checks, respectively, were appraised (Table1 for included studies, Table S2 for excluded studies). In many papers it was not described whether the cohort was consecutive. Also, loss to follow-up and the characteristics of patients lost to follow-up were rarely reported, resulting in a high risk of bias in the “Study Attrition” domain. Many studies used data-dependent cutoffs for their prognostic factor assessment, scoring lower on the “Prognostic factor” domain. Also, the treatment modality was often not included in multivariate analysis. As the effect of hypoxia might be different for each treatment modality (e.g., surgery, radiotherapy, or chemoradiation [CRT]), these papers scored a higher risk of bias in the “Confounding” domain, except when the cohort received uniform treatment. A score was calculated as described before, however, the study attrition score was omitted in this final risk of bias score. Forty papers remained for final analysis.
Table 1.
Critical appraisal of included studies and description of biomarkers
| Study | SP | SA | PF | O | C | AR | B | HIF-1a | HIF-2a | CA-IX | GLUT-1 | OPN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aebersold et al. 20 | L | H | L | L | M | L | 1 | • | ||||
| Avirović et al. 35 | M | L | M | L | M | L | 3 | • | ||||
| Brockton et al. 48 | L | H | M | L | M | L | 2 | • | ||||
| Brockton et al. 33 | M | H | M | L | M | L | 3 | • | • | |||
| Cabanillas et al. 53 | H | H | L | L | M | L | 3 | • | ||||
| Chien et al. 36 | H | H | L | L | L | M | 3 | • | ||||
| Chien et al. 37 | M | L | L | L | M | L | 2 | • | ||||
| Choi et al. 57 | M | H | L | L | M | M | 3 | • | ||||
| Choi et al. 38 | M | L | L | L | M | M | 3 | • | ||||
| Dos Santos et al. 39 | M | L | L | L | L | M | 2 | • | ||||
| Douglas et al. 22 | L | H | L | L | L | L | 0 | • | • | |||
| Dunkel et al. 40 | L | H | L | L | M | M | 2 | • | ||||
| Eckert et al. 41 | H | L | L | L | M | L | 3 | • | • | |||
| Eckert et al. 42 | H | L | L | L | L | L | 2 | • | • | |||
| Eriksen et al. 61 | L | L | L | L | L | L | 0 | • | ||||
| Fillies et al. 49 | H | H | M | L | L | L | 3 | • | ||||
| Grimm et al. 44 | H | L | L | L | M | L | 3 | • | ||||
| Hong et al. 56 | L | L | L | L | L | L | 0 | • | ||||
| Hui et al. 31 | L | H | L | L | L | L | 0 | • | • | |||
| Jonathan et a. 30 | M | L | L | L | L | L | 1 | • | • | |||
| Kim et al. 51 | H | L | M | L | L | L | 3 | • | ||||
| Kitagawa et al. 32 | H | L | M | L | L | L | 3 | • | ||||
| Kwon et al. 23 | H | L | L | L | M | L | 3 | • | • | • | ||
| Le et al. 58 | H | L | M | L | L | L | 3 | • | • | |||
| Liang et al. 45 | L | H | L | L | H | L | 2 | • | • | |||
| Nordsmark et al. 29 | M | L | L | L | M | M | 3 | • | • | • | ||
| Pérez-Sayáns et al. 55 | M | L | L | L | M | M | 3 | • | ||||
| Rademakers et al. 24 | M | L | M | L | L | L | 2 | • | ||||
| Rahimi et al. 60 | L | H | L | L | L | L | 0 | • | • | |||
| Roh et al. 52 | L | L | M | L | M | L | 2 | • | • | • | ||
| Schrijvers et al. 25 | L | L | M | L | L | L | 1 | • | • | • | ||
| Silva et al. 21 | M | H | L | L | M | L | 2 | • | ||||
| Van den Broek (2009) | L | H | M | L | L | L | 1 | • | • | |||
| Wachters et al. 26 | L | H | L | L | L | L | 0 | • | • | • | ||
| Wan et al. 59 | M | H | M | L | L | L | 2 | • | ||||
| Wildeman et al. 27 | M | L | L | L | M | L | 2 | • | • | |||
| Winter et al. 54 | M | L | L | L | M | L | 2 | • | • | • | ||
| Xueguan et al. 28 | L | L | L | L | M | M | 2 | • | ||||
| Zheng et al. 46 | M | H | L | L | M | M | 3 | • | ||||
| Zhu et al. 47 | L | H | M | L | L | L | 1 | • | • | |||
| Totals | 27 | 3 | 21 | 7 | 6 |
Low, 0; Moderate, 1; High, 2 points. SA was not included. Studies with a bias score >3 were excluded. Appraisal of these studies is described in supplementary table S2. SP, study participation; SA, study attrition; PF, prognostic factor; O, Outcome; C, Confounding; AR, statistical analysis and reporting. B, Bias score according to QUIPS; HIF-1, hypoxia-inducible factor 1. The EMHs that were described in each study are shown with a filled dot (example of filled dot).
Endogenous markers of hypoxia
The included studies described the effect of EMH expression across all subsites in the head and neck area. However, many studies analyzed different staining patterns, methods, and cutoff values to define EMH positivity. For instance, some studies used an H-score that combined staining proportion and intensity, while others only scored staining proportion or intensity. Because of this heterogeneity, it was deemed that data pooling and subsequent meta-analysis were not appropriate, as this might introduce bias.
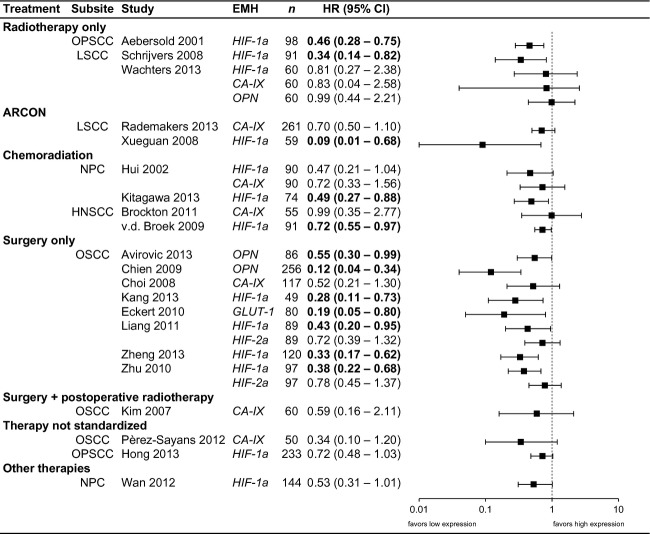
EMH expression was often correlated with adverse clinical parameters, such as T-stage, N-stage, or differentiation grade, as shown in Tables2 through 7. The effect of EMH expression is discussed per treatment strategy, as hypoxia may influence outcome of various treatment strategies differently. As a summary, a forest plot for OS across all treatment strategies is shown in Figure2.
Table 2.
Clinical outcome: radiotherapy/ARCON
| Study | Treatment | Stage | EMH | Pos/n | Cutoff | Correlations | LRC | OS | DFS | DSS |
|---|---|---|---|---|---|---|---|---|---|---|
| Oropharyngeal carcinoma | ||||||||||
| Aebersold et al. 20 | XRT | Any | HIF-1a | 92/98 | 10% N | Tumor grade | 0.46 (0.28–0.75) | 0.50 (0.30–0.83) | ||
| Silva et al. 21 | XRT | HIF-1a | 43/79 | 10% | Low Hb | 0.2 (0.1–0.42) | ||||
| Laryngeal carcinoma | ||||||||||
| Douglas et al. 22 | XRT | I–II | HIF-1a | 124/271 | 10% N | None | 0.96 (0.79–1.16) | LR P = 0.23 | ||
| Kwon et al. 23 | XRT | I–II | HIF-1a | 7/42 | 50% N | ns | 0.13 (0.02–0.82) | |||
| I–II | CA-IX | 17/42 | 30% M | ns | 0.11 (0.01–0.96) | |||||
| Rademakers et al. 24 | ARCON/XRT1 | III–IV | CA-IX | 132/261 | Med2 | None | 0.7 (0.5–1.1) | |||
| Schrijvers et al. 253 | XRT | I–II | HIF-1a | 46/91 | 0.5% N | None | 0.34 (0.14–0.82) | |||
| I–II | CA-IX | 39/91 | 12.5% M | None | 0.34 (0.14–0.85) | ns | ||||
| I–II | GLUT-1 | 53/91 | 35% M | ns | ns | |||||
| Wachters et al. 26 | XRT | I–II | HIF-1a | 47/60 | 0.5% N | None | 0.93 (0.26–3.45) | 0.81 (0.27–2.38) | ||
| I–II | CA-IX | 11/60 | 12.5% M | None | 0.83 (0.04–2.58) | |||||
| I–II | OPN | 20/60 | 0.5% C | ns | 0.99 (0.44–2.21) | |||||
| Wildeman et al. 27 | XRT | Any | HIF-1a | 59 | N/M %4 | ns | 1.08 (0.91–1.29)5 | |||
| Any | HIF-1a | 59 | Int | ns | 0.92 (0.56–1.49)5 | |||||
| Any | CA-IX | 59 | int | ns | 1.21 (0.96–1.52)5 | |||||
| Nasopharyngeal carcinoma | ||||||||||
| Xueguan et al. 28 | ARCON | Any | HIF-1a | 40/59 | 10% N | None | 0.41 (0.06–2.69) | 0.09 (0.01–0.68)6 | 0.26 (0.07–0.97) | |
| Multiple subsites | ||||||||||
| Nordsmark et al. 29 | XRT | Any | HIF-1a | 19/597 | 50% N | ns | 0.22 (0.06–0.81) | |||
| CA-IX | 26/577 | 10% M | ns | 0.35 (0.12–1.01) | ||||||
| OPN | 17/57 | Int D | ns | 0.83 (0.35–2.00) | ||||||
| Jonathan et al. 30 | ARCON | Any | CA-IX | 29/58 | 25% M | ns | 4.23 (1.07–16.76)6 | ns | ||
| GLUT-1 | 29/58 | Int D | ns | ns | LR P = 0.001 | |||||
The outcomes locoregional control (LRC), overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) are shown as hazard ratio (95% confidence interval). Hazard ratios <1 indicate beneficial prognosis for nonhypoxic tumors. Significant values are shown in bold Cutoff: EMHs were scored according to nuclear (N), membranous (M), cytoplasmic (C), or diffuse (D) staining patterns. XRT: radiotherapy. ARCON; accelerated radiotherapy, carbogen gas breathing and nicotinamide. Pos: number of patients with staining above the mentioned cutoff. LR: Logrank test. ns: not specified. Multiple subsites, patients were not analyzed per subsite. EMH, endogenous markers of hypoxia; HIF-1, hypoxia-inducible factor 1.
Patients were randomized between ARCON and XRT.
Computerized image analysis was performed and the median value was used in statistical analyses.
Supraglottic carcinomas only.
Analyses were performed using the proportion of membranous or nuclear staining cells as a continuous variable, no cutoff was used. Number of positive staining patients is therefore not relevant.
Presented numbers are odds-ratios for 2-year locoregional recurrence, not hazard ratios.
Last surviving patient was scored as deceased to enable HR-calculation, because of 100% survival in one arm.
Data on immunohistochemical analysis were available for 59 of 67 patients (HIF-1a) and 57/67 patients (CA-IX).
Figure 2.
Forest plot: Overall survival and EMH Expression. Visual summary of studies that described overall survival. ARCON, accelerated radiotherapy, carbogen gas, and nicotinamide. HRs < 1 indicate beneficial prognosis for nonhypoxic tumors. Therapy not standardized: All treatment modalities were analyzed in a single cohort and results were not presented according to therapy. The studies of Pérez-Sayáns and Hong describe their entire cohort of patients, receiving any treatment. In the study of Wan, patients were randomized between neoadjuvant radiotherapy or chemoradiation, followed by concurrent chemoradiation. EMH, endogenous markers of hypoxia.
Primary radiotherapy or ARCON
Eleven studies were identified that studied the clinical effect of EMHs in patients treated with radiotherapy (XRT) or the hypoxia-sensitizing treatment of accelerated radiotherapy, in combination with carbogen gas breathing and intravenously administered nicotinamide (ARCON) 20–30. Results are summarized in Table2. Most studies identified a worse outcome in patients with high EMH expression. This finding appears to be present across all subsites within the head and neck region, including the oropharynx 20,21, the larynx 23,25, and the nasopharynx 28, as well as in a study that analyzed patients with cancers of several subsites 29. In this last study multiple biomarkers of hypoxia were studied. HIF-1a expression predicted significantly worse LRC. While LRC was lower in patients with high expression of CA-IX and OPN, this did not reach statistical significance.
The study of Rademakers et al. 24 of 261 patients randomized between treatment with XRT or ARCON did not find better OS in low CA-IX expressing laryngeal cancer patients (LSCC). Unfortunately, no separate data were presented for XRT and ARCON. The authors did report differences in OS between different staining patterns: a perinecrotic staining pattern, in which cells stain more strongly as the distance to the nearest blood vessel increases, was associated with worse OS (P < 0.01) and LRC (P = 0.01) when compared to diffuse or no expression of CA-IX. Surprisingly, in the study of Jonathan et al. 30 of 58 HNSCC patients treated only with ARCON, a better outcome was observed in patients with high EMH expression. In this small study, CA-IX expression was mostly low. Using a cutoff at the median value of expression, the authors describe no significant correlation with the outcome. Finally, the 80th percentile (25% membranous expression) was used as a cutoff value that found a significantly better outcome for patients with high CA-IX levels.
Primary CRT
Only four studies were available that studied EMH expression in a cohort of patients treated with CRT only 31–34. A significant effect of EMH expression on survival was found in two 32,34. Kitagawa et al. 32 described a cohort of 74 nasopharyngeal cancer (NPC) patients treated with CRT (with exempt of seven patients that did not receive chemotherapy because of kidney failure), patients with more than 10% HIF-1a expression had significantly worse OS. Van den Broek et al. studied HIF-1a expression in a cohort of 91 HNSCC patients and described worse OS, but not LRC in patients with higher HIF-1a expression 34. Hui et al. studied a cohort of 90 NPC patients and found a trend toward better outcome in low HIF-1a-expressing patients, but did not find a similar trend for CA-IX 31. Brockton et al. studied CA-IX and GLUT-1 expression in a smaller cohort of 58 patients with primary tumors from various subsites in the head and neck and also did not find a correlation with survival 33 Results are summarized in Table3.
Table 3.
Clinical outcome: primary chemoradiation
| Study | Stage | EMH | Pos/n | Cutoff | Correlations | LRC | OS |
|---|---|---|---|---|---|---|---|
| Nasopharyngeal cancer | |||||||
| Hui et al. 31 | III–IV | HIF-1a | 32/90 | 5% N | None | 0.47 (0.21–1.04) | |
| CA-IX | 32/90 | 5% M | None | 0.72 (0.33–1.56) | |||
| Kitagawa et al. 32 | Any | HIF-1a | 27/741 | 10% N | None | 0.49 (0.27–0.88) | |
| Multiple subsites | |||||||
| Brockton et al. 33 | II–IV | CA-IX | 23/462 | Med3 | None | 0.99 (0.35–2.77) | |
| GLUT-1 | 24/472 | Med | None | LR P = 0.79 | |||
| Van den Broek et al. 34 | Any | HIF-1a | 91 | N/M4 | ns | 0.64 (0.36–1.12) | 0.72 (0.55–0.97) |
| CA-IX | 91 | M/C4 | ns | 0.73 (0.43–1.23) | ns | ||
The outcomes locoregional control (LRC) and overall survival (OS) are shown as hazard ratio (95% confidence interval). None of the studies reported disease free or disease-specific survival. Hazard ratios <1 indicate beneficial prognosis for nonhypoxic tumors. Significant values are shown in bold. Cutoff: EMHs were scored according to nuclear (N), membranous (M), cytoplasmic (C), or diffuse (D) staining patterns. Pos: number of patients with staining above the mentioned cutoff. LR: Logrank test. ns: not specified. Multiple subsites: patients were not analyzed per subsite. EMH, endogenous markers of hypoxia; HIF-1, hypoxia-inducible factor 1.
Seven of 74 patients received radiotherapy only due to kidney failure.
Total 55 patients, data were missing because of missing or folded TMA cores.
Computerized image analysis was performed, staining pattern was not taken into account.
Nuclear or membranous expression was analyzed as a continuous variable.
Primary surgery
Thirteen studies studied EMH expression in patients treated with primary surgery only 35–47. All studies concerned oral cavity carcinoma (OSCC) and all studies, except two, described that in surgically treated patients, HIF-1a expression significantly decreased the prognosis. Choi et al. 38 investigated CA-IX expression in a cohort of 118 patients and did not find an association with prognosis. The study of Dos Santos et al. 39 describes a small subgroup of 36 patients treated only with surgery in a larger cohort of 66 OSCC patients and did not find a difference between high and low HIF-1a-expressing patients. Results are summarized in Table4.
Table 4.
Clinical outcome: primary surgery
| Study | Stage | EMH | Pos/n | Cutoff | Correlations | OS | DFS | DSS | |
|---|---|---|---|---|---|---|---|---|---|
| Oral cavity | |||||||||
| Avirović et al. 35 | Any | OPN | 48/86 | 71% C | N-stage, disease stage | 0.55 (0.3–0.99) | |||
| Chien et al. 36 | Any | OPN | 30/94 | 10% C | T-stage, N-stage, tumor thickness, tumor necrosis | LR P < 0.001 | |||
| Chien et al. 371 | Any | OPN | 192/2566 | 10% C | T-stage, N-stage, disease stage | 0.12 (0.04–0.34) | |||
| Choi et al. 38 | Any | CA-IX | 64/117 | 5% M | None | 0.52 (0.21–1.30) | |||
| Dos Santos et al. 39 | Any | HIF-1a | 15/36 | 6/92 | None | LR P = 0.7 | |||
| Dunkel et al. 40 | I | HIF-1a | 16/44 | Int | ns | LR P = 0.022 | LR P = 0.29 | ||
| Kang et al. 433 | I–III | HIF-1a | 43/49 | 10%3 | T-stage, N-stage, tumor grade | 0.28 (0.11–0.73) | 0.34 (0.15–0.79) | ||
| Eckert et al. 41 | Any | HIF-1a | 804 | 3–4 vs. 6–8 C5 | T-stage | 0.21 (0.06–0.72) | |||
| 3–4 vs. 9–12 C5 | 0.19 (0.05–0.8) | ||||||||
| Eckert et al. 42 | Any | GLUT–1 | 806 | 0–2 vs. 3–4 M5 | None | 0.71 (0.2–2.54) | |||
| 0–2 vs. 6–8 M5 | 0.29 (0.12–0.71) | ||||||||
| 0–2 vs. 9–12 M5 | 0.5 (0.15–1.63) | ||||||||
| Grimm et al. 44 | Any | GLUT–1 | 161 | 50% M/C7 | ns | 0.58 (0.37–0.91) | |||
| Liang et al. 45 | Any | HIF-1a | 89 | 25% N/C7 | Tumor grade, N-stage | 0.43 (0.20–0.95) | |||
| HIF-2a | 89 | 25% N/C7 | T-stage | 0.72 (0.39–1.32) | |||||
| Zheng et al. 46 | Any | HIF-1a | 120 | 1% N | N-stage, disease stage | 0.33 (0.17–0.62) | 0.30 (0.16–0.75) | ||
| Zhu et al. 47 | Any | HIF-1a | z97 | 1% N | T-stage, N-stage, tumor grade | 0.38 (0.22–0.68) | 0.44 (0.26–0.75) | ||
| HIF-2a | 974 | 1% N | T-stage, microvessel density | 0.78 (0.45–1.37) | 0.87 (0.51–1.47) | ||||
The outcomes overall survival (OS), disease free survival (DFS) and disease specific survival (DFS) are shown as hazard ratio (95% confidence interval). None of the studies reported locoregional control. Hazard ratios <1 indicate beneficial prognosis for nonhypoxic tumors. Significant values are shown in bold. Cutoff: EMHs were scored according to nuclear (N), membranous (M), cytoplasmic (C), or diffuse (D) staining patterns. Pos: number of patients with staining above the mentioned cutoff. LR: Logrank test. ns: not specified. Multiple subsites: patients were not analyzed per subsite. EMH, endogenous markers of hypoxia; HIF-1, hypoxia-inducible factor 1.
A score range 0–9 was calculated based on staining proportion and intensity. Staining pattern was not disclosed.
The scored staining pattern was not disclosed.
Negative expression (score 0–2): 11, weak (3, 4): 24, moderate (6–8) 38, strong (9–12): 7 patients.
A score range 0–12 was calculated based on staining proportion and intensity.
Negative expression (score 0–2): 32, weak (3, 4): 13, moderate (6–8) 21, strong (9–12): 11 patients.
Both membranous and cytoplasmic (M/C) or nuclear and cytoplasmic (N/C) staining cells were scored positive.
Surgery and postoperative radiotherapy
Eight studies were available that studied EMH expression in a cohort of patients treated with surgery and postoperative radiotherapy 39,48–54. Surprisingly, two studies described better prognosis in high HIF-1a expressing patients 39,49. The study of Fillies et al. 49 described the effect of HIF-1a expression on clinical outcome and found that HIF-1a expression above 5% results in better survival in OSCC patients. The study of Dos Santos et al. describes a small subgroup of 30 patients treated with surgery and postoperative radiotherapy within a study of 66 OSCC patients 39. The study of Winter et al. 54 describe significantly worse outcome for HNSCC patients with high HIF-1a expression. All other studies did not show a difference in outcome when patients were stratified according to EMH expression. Results are summarized in Table5.
Table 5.
Clinical outcome: primary surgery + postoperative radiotherapy
| Study | Stage | EMH | Pos/n | Cutoff | Correlations | Favor | LRC | OS | DFS | DSS |
|---|---|---|---|---|---|---|---|---|---|---|
| Oral cavity | ||||||||||
| Brockton et al. 48 | Any | CA-IX | 17/61 | p751 | ns | L | 0.26 (0.06–1.05) | |||
| Dos Santos et al. 39 | Any | HIF-1a | 16/302 | H | 3.41 (1.13–10.34) | |||||
| Fillies et al. 49 | I–II | HIF-1a | 45/85 | 5% N | None | H | LR P < 0.053 | LR P = 0.02 | ||
| Han et al 50 | II | HIF-1a | 4/33 | 10% N | ns | L | ||||
| Kim et al. 51 | Any | CA-IX | 38/60 | 10% C/M | Tumor grade, subsite, smoking | L | 0.59 (0.16–2.11) | 0.85 (0.33–2.23) | ||
| Roh et al. 52 | II | HIF-1a | 6/43 | 1% N | None | L | LR P = 0.154 | 0.37 (0.13–1.03) | ||
| CA-IX | 26/43 | 10% M | Tumor thickness | L | LR P = 0.857 | LR P = 0.159 | ||||
| GLUT-1 | 31/43 | 50% M | Tumor thickness, n-stage | L | LR P = 0.416 | LR P = 0.060 | ||||
| Laryngeal cancer | ||||||||||
| Cabanillas et al. 53 | Any | HIF-1a | 75/106 | 10% N | T-stage, disease stage | N | LR P = 0.8 | LR P = 0.5 | ||
| Multiple subsites | ||||||||||
| Winter et al. 54 | Any | HIF-1a | 45/151 | medN3 | Disease stage | L | LR P = 0.08 | LR P = 0.02 | LR P = 0.02 | |
| HIF-2a | 21/151 | Med N/C3 | None | L | LR P = 0.43 | LR P = 0.10 | LR P = 0.16 | |||
| CA-IX | 92/151 | Med M3 | ns | – | LR P = 0.3 | LR P = 0.2 | LR P = 0.1 | |||
The outcomes locoregional control (LRC), overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) are shown as hazard ratio (95% confidence interval). Hazard ratios <1 indicate beneficial prognosis for nonhypoxic tumors. Significant values are shown in bold. Cutoff: EMHs were scored according to nuclear (N), membranous (M), cytoplasmic (C), or diffuse (D) staining patterns. Pos: number of patients with staining above the mentioned cutoff. Favor: L, better outcome for low expression; H, better outcome for high expression; N, no difference between arms. LR: Logrank test. ns: not specified. Multiple subsites: patients were not analyzed per subsite. EMH, endogenous markers of hypoxia; HIF-1, hypoxia-inducible factor 1.
Computerized image analysis, scoring pattern and value not disclosed.
Subgroup within a larger study of 66 patients.
Exact value not disclosed.
Other treatment strategies
Seven studies describe data from larger cohorts treated with various treatment modalities, depending on localization and staging 55–61. Only the studies of Rahimi and Le identified a significant correlation between EMH expression and outcome. Rahimi found significant better DFS and improved, but not significantly different LRC for patients expressing no or very low levels (<1%) of HIF-1a 60. Similar results were not obtained for CA-IX. Le et al. 58 studied both CA-IX and OPN expression and found better survival in patients with low CA-IX expression. Pérez-Sayáns et al. (CA-IX), Hong (HIF-1a), and Choi (HIF-1a) did not find a correlation with outcome 55–57. Wan et al. 59 studied 144 NPC patients randomized to receive neoadjuvant radiotherapy and neoadjuvant CRT followed by CRT and found better, although not statistically significant, OS in low HIF-1a expressing patients. The same results were obtained when both treatment arms were analyzed separately. Results are summarized in Table6.
Table 6.
Clinical outcome: other treatment strategies
| Study | Treatment | Stage | EMH | Pos/n | Cutoff | Correlations | LRC | OS | DFS | DSS |
|---|---|---|---|---|---|---|---|---|---|---|
| Oral cavity cancer | ||||||||||
| Pérez-Sayáns et al. 55 | Any | Any | CA-IX | 23/501 | 50% M | Disease stage | 0.34 (0.1–1.2)2 | |||
| Oropharyngeal cancer | ||||||||||
| Hong et al. 56 | Any | Any | HIF-1a | 137/233 | 10% N | T-stage, tumor grade | 0.72 (0.48–1.03) | 0.75 (0.46–1.22) | ||
| Rahimi et al. 60 | XRT/CRT3 | Any | HIF-1a | 26/58 | 1% N | 0.76 (0.55–1.01) | 0.81 (0.67–0.99) | |||
| HIF-1a | NS/58 | 1% C | 1.10 (0.72–1.67) | 1.06 (0.83–1.35) | ||||||
| Any | CA-IX | NS/57 | 1% C | 1.52 (0.71–3.23) | 1.03 (0.81–1.32) | |||||
| CA-IX | NS/57 | 1% M | 0.93 (0.77–1.12) | 1.01 (0.88–1.15) | ||||||
| Wan et al. 59 | nC+R/nC+CRT4 | Any | HIF-1a | 66/1441 | 5/16 C/N5 | 0.53 (0.31–1.01) | ||||
| Multiple subsites | ||||||||||
| Choi et al. 57 | Any | Any | HIF-1a | 25/76 | 1% C | None | LR P = 0.237 | 0.55 (0.33–1.15) | ||
| Eriksen et al. 61 | 6 | Any | CA-IX | 370 | M7 | None | LR P = 0.8 | |||
| Le et al. 58 | Any | Any | CA-IX | 29/948 | Int C9 | LR P = 0.011 | LR P = 0.030 | |||
| Any | OPN | 70/84 | Int D | NS | ||||||
The outcomes locoregional control (LRC), overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) are shown as hazard ratio (95% confidence interval). Hazard ratios <1 indicate beneficial prognosis for nonhypoxic tumors. Significant values are shown in bold. Cutoff: EMHs were scored according to nuclear (N), membranous (M), cytoplasmic (C) or diffuse (D) staining patterns. Pos: number of patients with staining above the mentioned cutoff. LR: Logrank test. ns: not specified. Multiple subsites: patients were not analyzed per subsite. EMH, endogenous markers of hypoxia; HIF-1, hypoxia-inducible factor 1.
Twenty-three patients had intense staining, 18 patients had moderate staining and 9 patients had no staining.
Strong vs. no CA-IX staining.
Chemotherapy was added in the case of T4 or N3 disease.
Patients participated in a RCT between neoadjuvant chemotherapy followed by either radiotherapy or chemoradiation
A score was calculated from 0–16, based on staining proportion and intensity. Both cytoplasmic and nuclear patterns were scored.
Patients were randomized between radiotherapy or radiotherapy and the radiosensitizer nimorazole
Patients were analyzed in groups: <1%, 1–10%, 10–30%, and above 30%. None of these subgroups showed significantly better improvement compared to the other groups
Results for CA-IX and OPN were available for 94 and 84 patients, respectively, because of TMA core availability.
Expression was scored as negative, weak or strong by a single pathologist.
Discussion
In this systematic review, we investigated expression of biomarkers for tumor hypoxia in relation to clinical outcome and treatment strategy. We identified 40 high-quality studies. EMH expression was common and associated to worse survival or LRC in most studies, although statistical significance was not always reached. In addition, EMH expression was often correlated with worse clinicopathological characteristics. Surprisingly, three studies found EMH expression to be associated to better outcome, but these mostly had a small sample size 30,39,49. Moreover, in studies that investigated multiple EMHs, high HIF-1a expression was often associated with worse outcome, while this was not always true for the other EMHs.
Chronic hypoxia is an important and highly prevalent problem in solid tumors 9. We observed that several adverse clinical parameters were often associated with higher EMH expression, such as the presence of cervical lymph node metastasis, higher T-stages, and worse differentiation grade. The latter two correlations support the hypothesis that hypoxia occurs more often in larger and faster growing tumors. Despite the correlations to clinical parameters, the presence of hypoxia was often an independent predictor of adverse outcome. One explanation might be that in the hypoxic microenvironment, several mechanisms are activated that improve cellular survival under these adverse circumstances. As the HIF-1a transcription factor is stabilized, transcription of proteins increases, including those involved in pH regulation (CA-IX), cellular metabolism (GLUT-1), but also genes involved in angiogenesis or oxygen transport. OPN expression occurs through a hypoxia-dependent, HIF-independent pathway and reduces cell death and apoptosis in hypoxic or reoxygenated cells, it may therefore signify a more aggressive tumor phenotype 15,16,62,63.
Radiotherapy relies on the formation of free oxygen radicals to induce DNA strand breaks and cell death 64. Also, radiotherapy causes apoptosis through stabilization of p53. The HIF-1 pathway upregulates proteins involved in epithelial-to-mesenchymal transition (EMT), including the transcription factor Snail 65,66. Radiation-induced DNA damage is reduced by EMT by emergence of cancer stem cells that express high levels of free radical-scavenging proteins 67. Moreover, Snail causes radioresistance by suppressing p53-mediated apoptosis 64,67. Snail also contributes to cisplatin resistance, which is often concurrently administered to patients as a radiosensitizer 68. Thus, hypoxia does not only directly affect (chemo-)radiosensitivity, but also indirectly through EMT.
In this review we identified several studies that show that increased EMH expression leads to worse LRC and survival. However, in patients that were surgically treated only, EMH expression was also associated with worse outcome 35–37,43,45. This supports the hypothesis that hypoxia also contributes to a more aggressive tumor phenotype as described above. Surprisingly, only few studies on HNSCC patients treated with surgery and postoperative (chemo)radiotherapy found an association between EMH expression and survival. A possible explanation is that decreased tumor volume, will lead to better sensitivity to radiotherapy, as was shown by Pameijer and Chen 69,70. Dunst and colleagues even described that the hypoxic tumor volume is a better prognosticator than total tumor volume to predict outcome after radiotherapy 71.
Although EMH expression has been well described in HNSCC in current literature, there are still opportunities for future studies. In many publications, EMH expression was studied using tissue microarrays (TMAs) 72. In a TMA, tumor tissues from multiple patients are placed on a single histological slide 73. This technique allows for high throughput in determining biomarker expression in patients, but also introduces the risk of sampling bias. Unfortunately, EMH expression may vary widely within tumors, as hypoxia may occur more often in cells that are located distantly from microvessels. This will result in intratumor heterogeneity in the expression of EMH. Positive staining for hypoxic markers is often found in areas of necrosis (perinecrotic staining patterns), which is by some considered proof of “actual hypoxia.” Alternatively, diffuse expression of HIF-1a may also be observed, and is thought to derive from an oncogene-driven overexpression or stabilization of HIF-1a. These expression patterns may be visualized better in whole-slide tissue sections, rather than TMAs. Unfortunately, staining patterns in whole slides have only been described in two studies. Perinecrotic CA-IX staining was associated with worse outcome in a large cohort of LSCC patients treated with ARCON 24. In fact, staining pattern was a stronger predictor of outcome than the percentage of positive staining cells. In a smaller study of OPSCC patients, there was no difference between perinecrotic and diffuse HIF-1a staining patterns 20.
In the past years, HPV+ HNSCC has emerged as a separate entity with a difference in tumor biology, but also a better response to therapy. There may be differences in the prevalence of tumor hypoxia in HPV+ cancers and tumor hypoxia might also affect the prognosis of HPV+ cancers differently than HPV− cancers. This should be investigated in future studies. Finally, the effect of therapies that focus on hypoxia, either through improvement of tumor oxygenation, or by targeting hypoxic tumor cells should be the subject of future studies. Promising therapies include ARCON, the combination of radiotherapy with carbogen gas breathing and nicotinamide administration, which is currently tested in phase III trials 10,74. Delivering increased radiotherapy doses to hypoxic areas in a tumor by IMRT “dose-painting” is also considered, but not yet widely applied 75,76. Alternatively, the hypoxia-sensitizer nimorazole may be added to primary radiotherapy 11. The efficacy of nimorazole administration in HNSCC has been shown in trials within the DAHANCA group 77. Finally, surgery may also be a treatment option for hypoxic tumors, to decrease the hypoxic or therapy-resistant fraction. While devascularization of the surgical field may lead to hypoxia in the direct postoperative phase, revascularization occurs as early as several days after surgery 78. This may increase oxygenation, making remaining tumor cells more susceptible to postoperative radiotherapy. Future studies should investigate the feasibility of such a multimodal approach and the effect on survival of patients with hypoxic tumors.
Several limitations of this review and the identified literature should be considered. In the literature, many ways to score biomarker positivity were used. Most studies scored percentages of positive staining cells, while others also took into account staining intensity, or combined the two using a H-score. If HIF-1a expression will be used in treatment selection, a validated, and reproducible scoring strategy should be employed, preferably without software imaging analysis, that may not be available in all centers. Moreover, the different cutoff points, as well as large heterogeneity in terms of tumor subsite and tumor stages did not allow for proper meta-analysis of the extracted data. As the cutoff points used in the individual studies were most often the ideal cutoff values for each individual data set, data pooling may introduce bias, and give an overestimation of the effect. Therefore, we have not performed this, in contrast to an earlier review on HIF-1a only 79. To provide a visual overview of the results, a forest plot is provided.
Another limitation of this review is that we did not include hypoxia gene expression profiles in the search. Several articles describe such profiles in head and neck cancer 80–84. In the present study we have chosen to focus on IHC, as HNSCC is still highly prevalent in resource-limited areas. Compared to techniques like quantitative PCR (qPCR) or the use of microarrays, IHC may be performed at relatively low cost.
Conclusion
In this systematic review, we identified HIF-1a, HIF-2a, CA-IX, GLUT-1, and OPN as the best studied endogenous markers of tumor hypoxia. In general, expression of these biomarkers was associated with worse survival, almost regardless of the therapy provided. These proteins are not only biomarkers, but are also part of cellular survival mechanisms. Therefore, EMH overexpression may result in worse prognosis not only due to hypoxia, but also because of a more aggressive tumor phenotype. The effect of tumor hypoxia in HNSCC patients warrants further investigation. Studies should investigate the best treatment option for hypoxic tumors, for instance hypoxia-modified radiotherapy schedules, targeted therapies against hypoxic cells or excision of the hypoxic tissue to improve radiation sensitivity. Knowledge on the tumor hypoxia status will help clinicians to select tailored treatments for each individual patient and thus enable personalized cancer care.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1.Search strategy.
Table S2. Critical appraisal of the excluded studies.
Table S3. PRISMA checklist.
References
- Pulte D. Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15:994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJM. Brakenhoff RH. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 2010;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- Masterson L, Moualed D, Liu ZW, Howard JEF, Dwivedi RC, Tysome JR, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur. J. Cancer. 2014;50:2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taabazuing CY, Hangasky JA. Knapp MJ. Oxygen sensing strategies in mammals and bacteria. J. Inorg. Biochem. 2014;133:63–72. doi: 10.1016/j.jinorgbio.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LK, Cavadas MA, Scholz CC, Fitzpatrick SF, Bruning U, Cummins EP, et al. A dynamic model of the hypoxia-inducible factor 1 (HIF-1) network. J. Cell Sci. 2013;126:1454–1463. doi: 10.1242/jcs.119974. [DOI] [PubMed] [Google Scholar]
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- Höckel M. Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Mayer A, Vaupel P. Hypoxia, lactate accumulation, and acidosis: siblings or accomplices driving tumor progression and resistance to therapy? In: Van Huffel S, Naulaers G, Caicedo A, Bruley DF, Harrison DK, editors. Advances in experimental medicine and biology. Vol. 789. New York, NY: Springer; 2013. pp. 203–209. Adv Exp Med Biol. [DOI] [PubMed] [Google Scholar]
- Kaanders JHAM, Bussink J. van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol. 2002;3:728–737. doi: 10.1016/s1470-2045(02)00929-4. [DOI] [PubMed] [Google Scholar]
- Timothy AR, Overgaard J. Overgaard M. A phase I clinical study of Nimorazole as a hypoxic radiosensitizer. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:1765–1768. doi: 10.1016/0360-3016(84)90545-5. [DOI] [PubMed] [Google Scholar]
- Hoogsteen IJ, Marres HAM, Bussink J, van der Kogel AJ. Kaanders JHAM. Tumor microenvironment in head and neck squamous cell carcinomas: predictive value and clinical relevance of hypoxic markers. A review. Head Neck. 2007;29:591–604. doi: 10.1002/hed.20543. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouvès A, Volmat V, Roux D. Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Denhardt DT, Cao H, Sutphin PD, Koong AC, Giaccia AJ, et al. Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene. 2005;24:6555–6563. doi: 10.1038/sj.onc.1208800. [DOI] [PubMed] [Google Scholar]
- Le Q-T, Denko NC. Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- Hayden JA, van der Windt DA, Cartwright JL, Côté P. Bombardier C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- Tierney JF, Stewart LA, Ghersi D, Burdett S. Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J. Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- Silva P, Slevin NJ, Sloan P, Valentine H, Cresswell J, Ryder D, et al. Prognostic significance of tumor hypoxia inducible factor-1alpha expression for outcome after radiotherapy in oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:1551–1559. doi: 10.1016/j.ijrobp.2008.07.051. [DOI] [PubMed] [Google Scholar]
- Douglas CM, Bernstein JM, Ormston VE, Hall RC, Merve A, Swindell R, et al. Lack of prognostic effect of carbonic anhydrase-9, hypoxia inducible factor-1α and bcl-2 in 286 patients with early squamous cell carcinoma of the glottic larynx treated with radiotherapy. Clin. Oncol. 2013;25:59–65. doi: 10.1016/j.clon.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Kwon OJ, Park JJ, Ko GH, Seo JH, Jeong B, Kang KM, et al. HIF-1α and CA-IX as predictors of locoregional control for determining the optimal treatment modality for early-stage laryngeal carcinoma. Head Neck. 2014;37:505–510. doi: 10.1002/hed.23620. [DOI] [PubMed] [Google Scholar]
- Rademakers SE, Hoogsteen IJ, Rijken PF, Oosterwijk E, Terhaard CH, Doornaert PA, et al. Pattern of CAIX expression is prognostic for outcome and predicts response to ARCON in patients with laryngeal cancer treated in a phase III randomized trial. Radiother. Oncol. 2013;108:517–522. doi: 10.1016/j.radonc.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Schrijvers ML, van der Laan BF, de Bock GH, Pattje WJ, Mastik MF, Menkema L, et al. Overexpression of intrinsic hypoxia markers HIF1alpha and CA-IX predict for local recurrence in stage T1-T2 glottic laryngeal carcinoma treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:161–169. doi: 10.1016/j.ijrobp.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Wachters JE, Schrijvers ML, Slagter-Menkema L, Mastik M, de Bock GH, Langendijk JA, et al. Prognostic significance of HIF-1a, CA-IX, and OPN in T1-T2 laryngeal carcinoma treated with radiotherapy. Laryngoscope. 2013;123:2154–2160. doi: 10.1002/lary.23831. [DOI] [PubMed] [Google Scholar]
- Wildeman MA, Gibcus JH, Hauptmann M, Begg AC, van Velthuysen MLF, Hoebers FJ, et al. Radiotherapy in laryngeal carcinoma: can a panel of 13 markers predict response? Laryngoscope. 2009;119:316–322. doi: 10.1002/lary.20069. [DOI] [PubMed] [Google Scholar]
- Xueguan L, Xiaoshen W, Yongsheng Z, Chaosu H, Chunying S. Yan F. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor expression are associated with a poor prognosis in patients with nasopharyngeal carcinoma receiving radiotherapy with carbogen and nicotinamide. Clin. Oncol. 2008;20:606–612. doi: 10.1016/j.clon.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Eriksen JG, Gebski V, Alsner J, Horsman MR. Overgaard J. Differential risk assessments from five hypoxia specific assays: the basis for biologically adapted individualized radiotherapy in advanced head and neck cancer patients. Radiother. Oncol. 2007;83:389–397. doi: 10.1016/j.radonc.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Jonathan RA, Wijffels KIEM, Peeters W, de Wilde PCM, Marres HAM, Merkx MAW, et al. The prognostic value of endogenous hypoxia-related markers for head and neck squamous cell carcinomas treated with ARCON. Radiother. Oncol. 2006;79:288–297. doi: 10.1016/j.radonc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Hui EP, Chan ATC, Pezzella F, Turley H, To K, Poon TCW, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin. Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- Kitagawa N, Kondo S, Wakisaka N, Zen Y, Nakanishi Y, Tsuji A, et al. Expression of seven-in-absentia homologue 1 and hypoxia-inducible factor 1 alpha: novel prognostic factors of nasopharyngeal carcinoma. Cancer Lett. 2013;331:52–57. doi: 10.1016/j.canlet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Brockton N, Dort J, Lau H, Hao D, Brar S, Klimowicz A, et al. High stromal carbonic anhydrase ix expression is associated with decreased survival in p16-negative head-and-neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:249–257. doi: 10.1016/j.ijrobp.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Van den Broek GB, Wildeman MAM, Rasch CRN, Armstrong N, Schuuring E, Begg AC, et al. Molecular markers predict outcome in squamous cell carcinoma of the head and neck after concomitant cisplatin-based chemoradiation. Int. J. Cancer. 2009;124:2643–2650. doi: 10.1002/ijc.24254. [DOI] [PubMed] [Google Scholar]
- Avirović M, Matušan-Ilijaš K, Damante G, Fabrro D, Cerović R, Juretić M, et al. Osteopontin expression is an independent factor for poor survival in oral squamous cell carcinoma: a computer-assisted analysis on TMA sections. J. Oral Pathol. Med. 2013;42:620–626. doi: 10.1111/jop.12055. [DOI] [PubMed] [Google Scholar]
- Chien C, Su C, Chuang H, Fang F, Huang H-Y, Chen C-M, et al. Clinical significance of osteopontin expression in T1 and T2 tongue cancers. Head Neck. 2008;30:776–781. doi: 10.1002/hed.20783. [DOI] [PubMed] [Google Scholar]
- Chien C-Y, Su C-Y, Chuang H-C, Fang F-M, Huang H-Y, Chen C-H, et al. Comprehensive study on the prognostic role of osteopontin expression in oral squamous cell carcinoma. Oral Oncol. 2009;45:798–802. doi: 10.1016/j.oraloncology.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Choi S-W, Kim J-Y, Park J-Y, Cha I-H, Kim J. Lee S. Expression of carbonic anhydrase IX is associated with postoperative recurrence and poor prognosis in surgically treated oral squamous cell carcinoma. Hum. Pathol. 2008;39:1317–1322. doi: 10.1016/j.humpath.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Dos Santos M, Mercante AMDC, Louro ID, Gonçalves AJ, de Carvalho MB, da Silva EHT, et al. HIF1-alpha expression predicts survival of patients with squamous cell carcinoma of the oral cavity. PLoS One. 2012;7:e45228. doi: 10.1371/journal.pone.0045228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel J, Vaittinen S, Grénman R, Kinnunen I. Irjala H. Prognostic markers in stage I oral cavity squamous cell carcinoma. Laryngoscope. 2013;123:2435–2441. doi: 10.1002/lary.23888. [DOI] [PubMed] [Google Scholar]
- Eckert AW, Schutze A, Lautner MHW, Taubert H, Schubert J. Bilkenroth U. HIF-1alpha is a prognostic marker in oral squamous cell carcinomas. Int. J. Biol. Markers. 2010;25:87–92. doi: 10.1177/172460081002500205. [DOI] [PubMed] [Google Scholar]
- Eckert AW, Lautner MHW, Schütze A, Taubert H, Schubert J. Bilkenroth U. Coexpression of hypoxia-inducible factor-1α and glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology. 2011;58:1136–1147. doi: 10.1111/j.1365-2559.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- Kang F-W, Gao Y, Que L, Sun J. Wang Z-L. Hypoxia-inducible factor-1α overexpression indicates poor clinical outcomes in tongue squamous cell carcinoma. Exp. Ther. Med. 2013;5:112–118. doi: 10.3892/etm.2012.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M, Munz A, Teriete P, Nadtotschi T. Reinert S. GLUT-1(+)/TKTL1(+) coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014;117:743–753. doi: 10.1016/j.oooo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Liang X, Zheng M, Jiang J, Zhu G, Yang J. Tang Y. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol. 2011;47:92–97. doi: 10.1016/j.oraloncology.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ni Y, Huang X, Wang Z. Han W. Overexpression of HIF-1α indicates a poor prognosis in tongue carcinoma and may be associated with tumour metastasis. Oncol. Lett. 2013;5:1285–1289. doi: 10.3892/ol.2013.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Tang Y, Li L, Zheng M, Jiang J, Li X, et al. Hypoxia inducible factor 1α and hypoxia inducible factor 2α play distinct and functionally overlapping roles in oral squamous cell carcinoma. Clin. Cancer Res. 2010;16:4732–4741. doi: 10.1158/1078-0432.CCR-10-1408. [DOI] [PubMed] [Google Scholar]
- Brockton NT, Klimowicz AC, Bose P, Petrillo SK, Konno M, Rudmik L, et al. High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral Oncol. 2012;48:615–622. doi: 10.1016/j.oraloncology.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Fillies T, Werkmeister R, van Diest PJ, Brandt B, Joos U. Buerger H. HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer. 2005;5:84. doi: 10.1186/1471-2407-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MW, Lee HJ, Cho K-J, Kim JS, Roh J-L, Choi S-H, et al. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck. 2012;34:1395–1402. doi: 10.1002/hed.21945. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Shin HJ, Jung K-Y, Baek S-K, Shin BK, Choi J, et al. Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn. J. Clin. Oncol. 2007;37:812–819. doi: 10.1093/jjco/hym121. [DOI] [PubMed] [Google Scholar]
- Roh J-L, Cho K-J, Kwon GY, Ryu CH, Chang HW, Choi S-H, et al. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol. 2009;45:63–68. doi: 10.1016/j.oraloncology.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Cabanillas R, Rodrigo JP, Secades P, Astudillo A, Nieto CS. Chiara MD. The relation between hypoxia-inducible factor (HIF)-1alpha expression with p53 expression and outcome in surgically treated supraglottic laryngeal cancer. J. Surg. Oncol. 2009;99:373–378. doi: 10.1002/jso.21243. [DOI] [PubMed] [Google Scholar]
- Winter SC, Shah KA, Han C, Campo L, Turley H, Leek R, et al. The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer. 2006;107:757–766. doi: 10.1002/cncr.21983. [DOI] [PubMed] [Google Scholar]
- Pérez-Sayáns M, Suárez-Peñaranda JM, Pilar G-D, Supuran CT, Pastorekova S, Barros-Angueira F, et al. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J. Oral Pathol. Med. 2012;41:667–674. doi: 10.1111/j.1600-0714.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- Hong A, Zhang M, Veillard A-S, Jahanbani J, Lee CS, Jones D, et al. The prognostic significance of hypoxia inducing factor 1-α in oropharyngeal cancer in relation to human papillomavirus status. Oral Oncol. 2013;49:354–359. doi: 10.1016/j.oraloncology.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Choi HG, Kim J-S, Kim KH, Kim KH, Sung M-W, Choe J-Y, et al. Expression of hypoxic signaling markers in head and neck squamous cell carcinoma and its clinical significance. Eur. Arch. Otorhinolaryngol. 2014;272:219–228. doi: 10.1007/s00405-014-2954-1. [DOI] [PubMed] [Google Scholar]
- Le Q-T, Kong C, Lavori PW, O’byrne K, Erler JT, Huang X, et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:167–175. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Wan X-B, Fan X-J, Huang P-Y, Dong D, Zhang Y, Chen M-Y, et al. Aurora-A activation, correlated with hypoxia-inducible factor-1α, promotes radiochemoresistance and predicts poor outcome for nasopharyngeal carcinoma. Cancer Sci. 2012;103:1586–1594. doi: 10.1111/j.1349-7006.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi AS, Wilson DD, Saylor DK, Stelow EB, Thomas CY, Reibel JF, et al. p16, Cyclin D1, and HIF-1α Predict Outcomes of Patients with Oropharyngeal Squamous Cell Carcinoma Treated with Definitive Intensity-Modulated Radiation Therapy. Int. J. Otolaryngol. 2012;2012:685951. doi: 10.1155/2012/685951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JG. Overgaard J. Lack of prognostic and predictive value of CA IX in radiotherapy of squamous cell carcinoma of the head and neck with known modifiable hypoxia: an evaluation of the DAHANCA 5 study. Radiother. Oncol. 2007;83:383–388. doi: 10.1016/j.radonc.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P, et al. Osteopontin as a therapeutic target for cancer. Expert Opin. Ther. Targets. 2014;18:883–895. doi: 10.1517/14728222.2014.925447. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Lopez CA, Rollo EE, Hwang SM, An XR. Walther SE. Osteopontin-induced modifications of cellular functions. Ann. N. Y. Acad. Sci. 1995;760:127–142. doi: 10.1111/j.1749-6632.1995.tb44625.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Jonathan E, Bernhard EJ. McKenna WG. How does radiation kill cells? Curr. Opin. Chem. Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- Tsai Y-P. Wu K-J. Hypoxia-regulated target genes implicated in tumor metastasis. J. Biomed. Sci. 2012;19:102. doi: 10.1186/1423-0127-19-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor -1α in hepatocellular carcinoma. BMC Cancer. BMC Cancer. 2013;13:108. doi: 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantajit D, Lin D. Li JJ. The network of epithelial–mesenchymal transition: potential new targets for tumor resistance. J. Cancer Res. Clin. Oncol. 2014 doi: 10.1007/s00432-014-1840-y. DOI: 10.1007/s00432-014-1840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DSS, Lan HY, Huang CH, Tai SK, Chang SY, Tsai TL, et al. Regulation of excision repair cross-complementation group 1 by snail contributes to cisplatin resistance in head and neck cancer. Clin. Cancer Res. 2010;16:4561–4571. doi: 10.1158/1078-0432.CCR-10-0593. [DOI] [PubMed] [Google Scholar]
- Pameijer FA, Mancuso AA, Mendenhall WM, Parsons JT. Kubilis PS. Can pretreatment computed tomography predict local control in T3 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy? Int. J. Radiat. Oncol. Biol. Phys. 1997;37:1011–1021. doi: 10.1016/s0360-3016(96)00626-8. [DOI] [PubMed] [Google Scholar]
- Chen S-W, Yang S-N, Liang J-A, Tsai M-H, Shiau A-C. Lin F-J. Value of computed tomography-based tumor volume as a predictor of outcomes in hypopharyngeal cancer after treatment with definitive radiotherapy. Laryngoscope. 2006;116:2012–2017. doi: 10.1097/01.mlg.0000237804.38761.81. [DOI] [PubMed] [Google Scholar]
- Dünst J, Stadler P, Becker A, Lautenschläger C, Pelz T, Hänsgen G, et al. Tumor volume and tumor hypoxia in head and neck cancers. Strahlenther. Onkol. 2003;179:521–526. doi: 10.1007/s00066-003-1066-4. [DOI] [PubMed] [Google Scholar]
- Kallioniemi OP, Wagner U, Kononen J. Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum. Mol. Genet. 2001;10:657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- Noorlag R, van der Groep P, Leusink FKJ, van Hooff SR, Frank MH, Willems SM, et al. Nodal metastasis and survival in oral cancer: association with protein expression of SLPI, not with LCN2, TACSTD2, or THBS2. Head Neck. 2014 doi: 10.1002/hed.23716. DOI: 10.1002/hed.23716. [DOI] [PubMed] [Google Scholar]
- Janssens GO, Rademakers SE, Terhaard CH, Doornaert P, Bijl HP, van den Ende P, et al. Improved recurrence-free survival with ARCON for anemic patients with laryngeal cancer. Clin. Cancer Res. 2014;20:1345–1354. doi: 10.1158/1078-0432.CCR-13-1730. [DOI] [PubMed] [Google Scholar]
- Chang JH, Wada M, Anderson NJ, Lim Joon D, Lee ST, Gong SJ, et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using (18)F-FMISO PET: a biological modeling study. Acta Oncol. 2013;52:1723–1729. doi: 10.3109/0284186X.2012.759273. [DOI] [PubMed] [Google Scholar]
- Grosu AL, Souvatzoglou M, Röper B, Dobritz M, Wiedenmann N, Jacob V, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:541–551. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- Bentzen J, Toustrup K, Eriksen JG, Primdahl H, Andersen LJ. Overgaard J. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 2015 doi: 10.3109/0284186X.2014.992547. DOI: 10.3109/0284186X.2014.992547. [DOI] [PubMed] [Google Scholar]
- Kumar I, Staton CA, Cross SS, Reed MWR. Brown NJ. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br. J. Surg. 2009;96:1484–91. doi: 10.1002/bjs.6778. [DOI] [PubMed] [Google Scholar]
- Gong L, Zhang W, Zhou J, Lu J, Xiong H, Shi X, et al. Prognostic value of HIFs expression in head and neck cancer: a systematic review. PLoS One. 2013;8:e75094. doi: 10.1371/journal.pone.0075094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67:3441–3449. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
- Buffa FM, Harris AL, West CM. Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br. J. Cancer. 2010;102:428–435. doi: 10.1038/sj.bjc.6605450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace A, Mani N, Span PN, Irlam JJ, Taylor J, Nj G, et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin. Cancer Res. 2013;19:4879–4888. doi: 10.1158/1078-0432.CCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toustrup K, Sørensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- Toustrup K, Sørensen BS, Lassen P, Wiuf C, Alsner J. Overgaard J. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother. Oncol. 2012;102:122–129. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J. Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014;4:400. doi: 10.3389/fphys.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M. Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001;60:1645–1657. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Search strategy.
Table S2. Critical appraisal of the excluded studies.
Table S3. PRISMA checklist.