Abstract
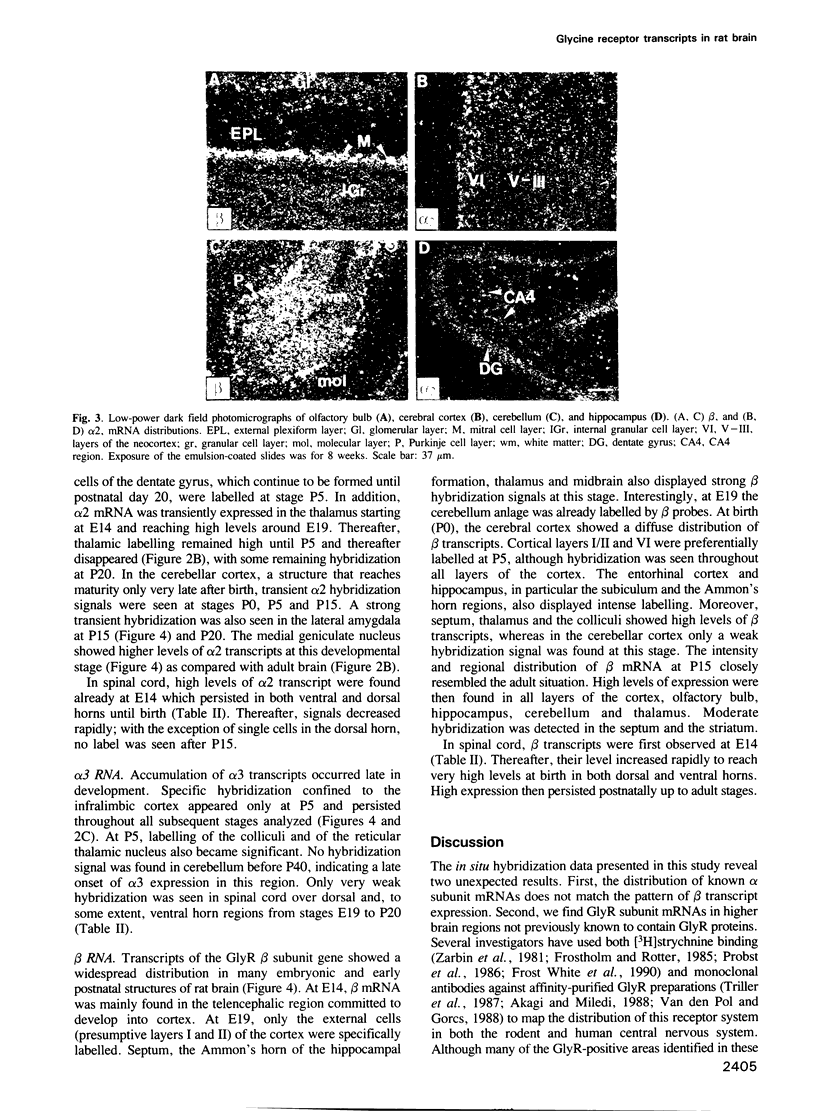
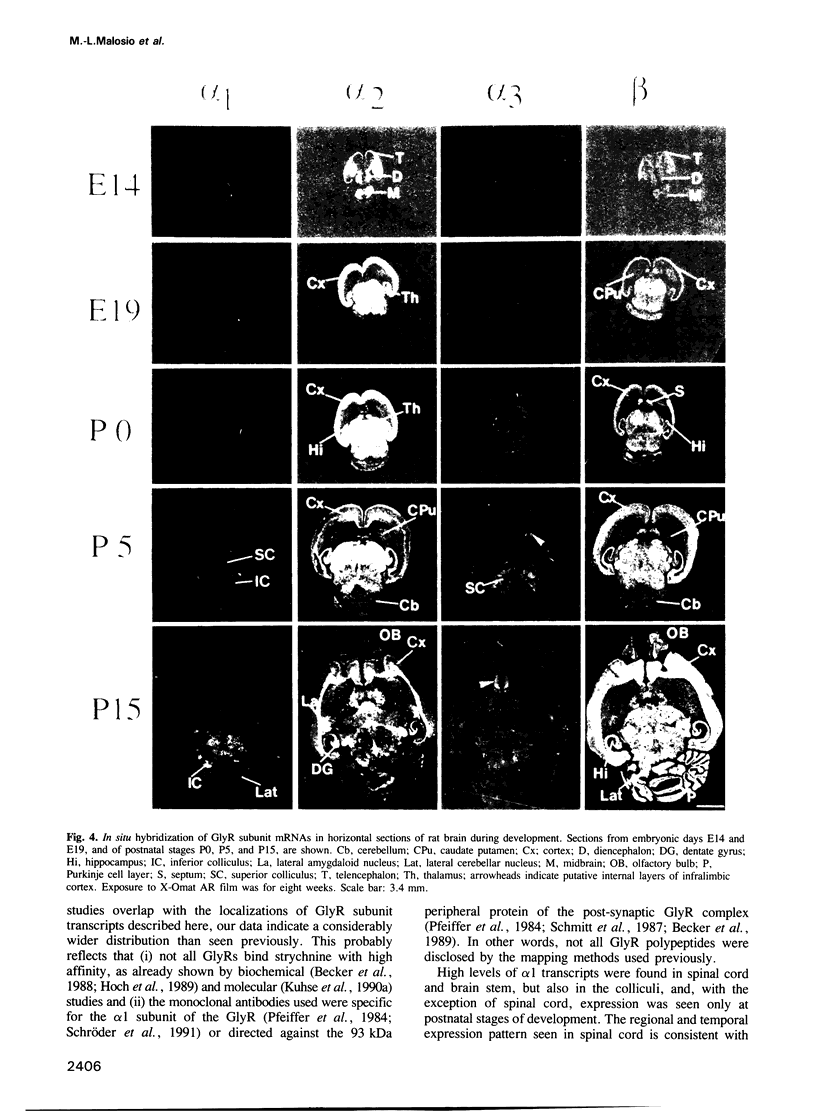
The inhibitory glycine receptor (GlyR) is a ligand-gated ion channel which mediates post-synaptic inhibition in spinal cord and other regions of the vertebrate central nervous system. Previous biochemical and molecular cloning studies have indicated heterogeneity of GlyRs during development. Here, the distribution of GlyR subunit transcripts in rat brain and spinal cord was investigated by in situ hybridization using sequence-specific oligonucleotide probes. In adult animals, GlyR alpha 1 subunit mRNA was abundant in spinal cord, but was also seen in a few brain areas, e.g. superior and inferior colliculi, whereas alpha 2 transcripts were found in several brain regions including layer VI of the cerebral cortex and hippocampus. GlyR alpha 3 subunit mRNA was expressed at low levels in cerebellum, olfactory bulb and hippocampus, while high amounts of beta subunit transcripts were widely distributed throughout spinal cord and brain. During development, alpha 2 mRNA accumulated already prenatally and decreased after birth, whereas alpha 1 and alpha 3 subunit transcripts appeared only in postnatal brain structures. Hybridization signals of beta subunit mRNA were seen already at early embryonic stages and continuously increased to high levels in adult rats. These data reveal unexpected differences in the regional and developmental expression of GlyR subunit mRNAs and point to novel functions of GlyR proteins in the mammalian central nervous system.
Full text
PDF

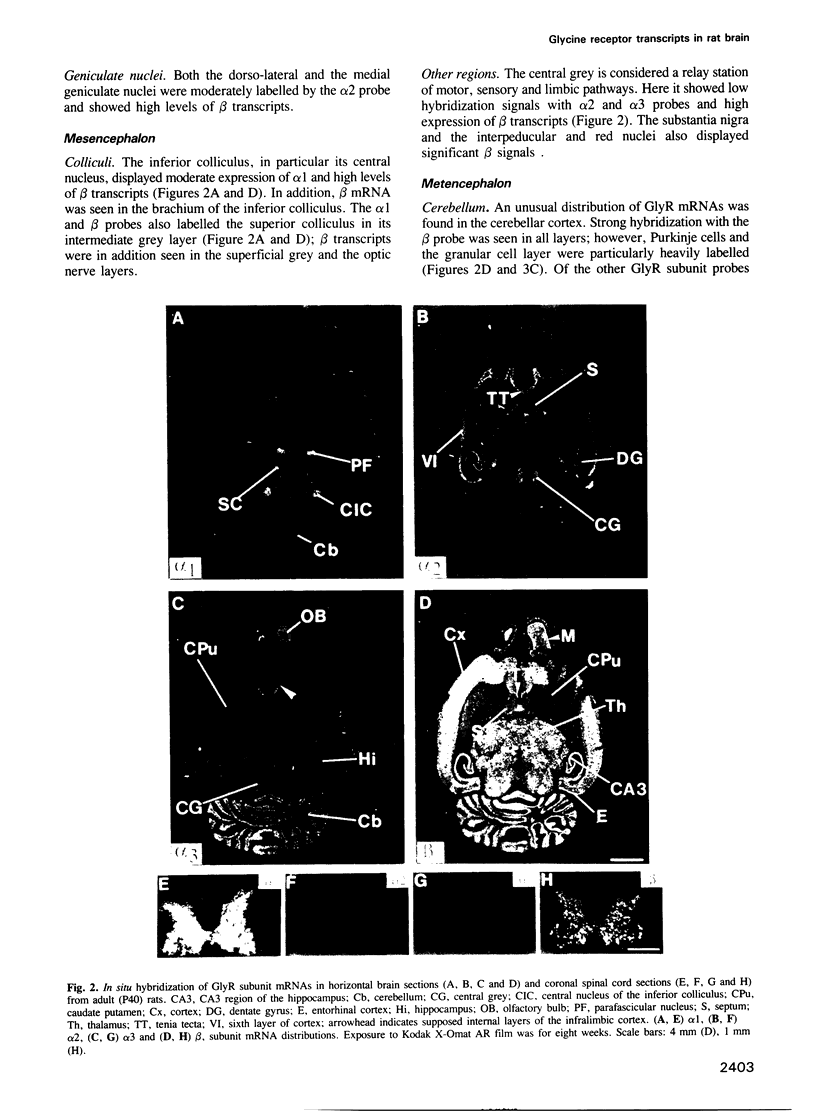



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi H., Miledi R. Heterogeneity of glycine receptors and their messenger RNAs in rat brain and spinal cord. Science. 1988 Oct 14;242(4876):270–273. doi: 10.1126/science.2845580. [DOI] [PubMed] [Google Scholar]
- Altschuler R. A., Betz H., Parakkal M. H., Reeks K. A., Wenthold R. J. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986 Mar 26;369(1-2):316–320. doi: 10.1016/0006-8993(86)90542-1. [DOI] [PubMed] [Google Scholar]
- Angevine J. B., Jr, Sidman R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961 Nov 25;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Araki T., Yamano M., Murakami T., Wanaka A., Betz H., Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988 May;25(2):613–624. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
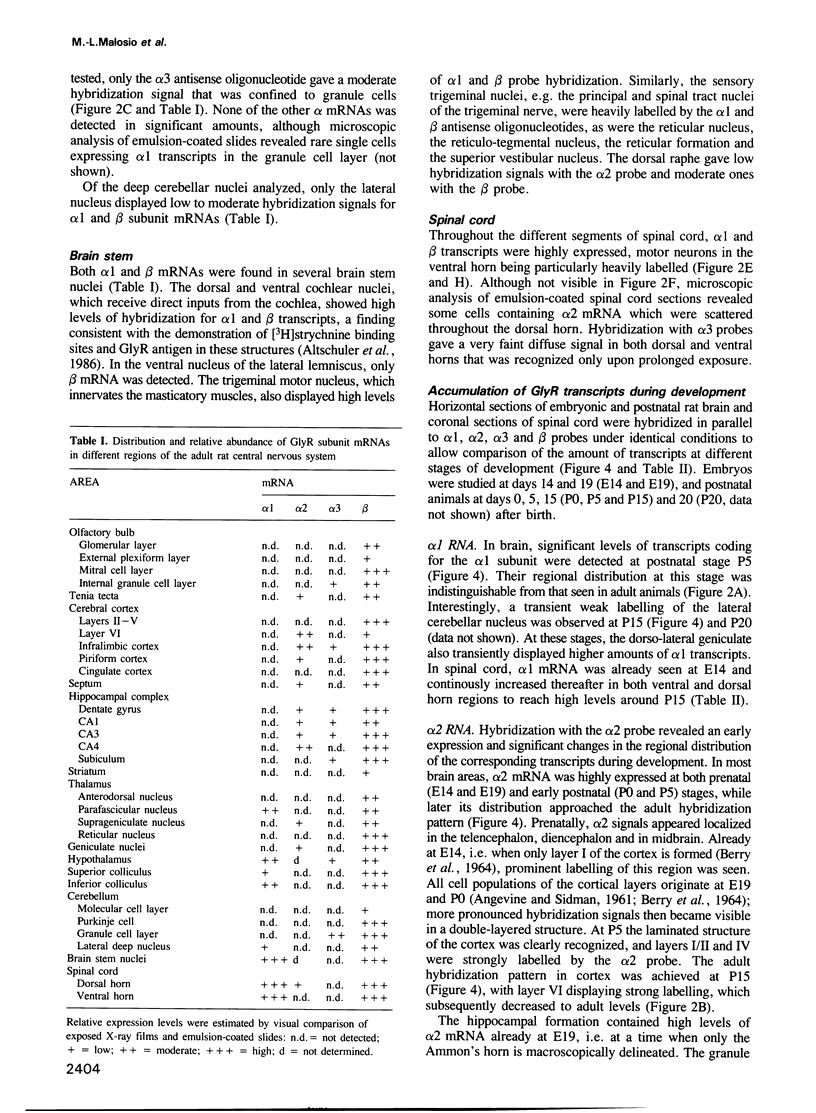
- BERRY M., ROGERS A. W., EAYRS J. T. PATTERN OF CELL MIGRATION DURING CORTICAL HISTOGENESIS. Nature. 1964 Aug 8;203:591–593. doi: 10.1038/203591b0. [DOI] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988 Dec 1;7(12):3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Sensitive immunoassay shows selective association of peripheral and integral membrane proteins of the inhibitory glycine receptor complex. J Neurochem. 1989 Jul;53(1):124–131. doi: 10.1111/j.1471-4159.1989.tb07303.x. [DOI] [PubMed] [Google Scholar]
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Betz H. Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron. 1990 Oct;5(4):383–392. doi: 10.1016/0896-6273(90)90077-s. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow D. R., Bowery N. G., Woodruff G. N. Light microscopic autoradiographic localisation of [3H]glycine and [3H]strychnine binding sites in rat brain. Eur J Pharmacol. 1986 Jul 31;126(3):303–307. doi: 10.1016/0014-2999(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. Coexistence of neuronal messengers and molecular selection. Prog Brain Res. 1986;68:373–403. doi: 10.1016/s0079-6123(08)60252-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Myers S. J., Nicholas R. A. Molecular biology of mammalian amino acid receptors. FASEB J. 1990 Jun;4(9):2636–2645. doi: 10.1096/fasebj.4.9.2161372. [DOI] [PubMed] [Google Scholar]
- Frostholm A., Rotter A. Glycine receptor distribution in mouse CNS: autoradiographic localization of [3H]strychnine binding sites. Brain Res Bull. 1985 Nov;15(5):473–486. doi: 10.1016/0361-9230(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Simler R., Betz H. Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry. 1985 Feb 12;24(4):990–994. doi: 10.1021/bi00325a027. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch W., Betz H., Becker C. M. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989 Sep;3(3):339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Kuryatov A., Maulet Y., Malosio M. L., Schmieden V., Betz H. Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991 May 20;283(1):73–77. doi: 10.1016/0014-5793(91)80557-j. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990 Dec;5(6):867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem. 1990 Dec 25;265(36):22317–22320. [PubMed] [Google Scholar]
- Langosch D., Becker C. M., Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur J Biochem. 1990 Nov 26;194(1):1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio M. L., Grenningloh G., Kuhse J., Schmieden V., Schmitt B., Prior P., Betz H. Alternative splicing generates two variants of the alpha 1 subunit of the inhibitory glycine receptor. J Biol Chem. 1991 Feb 5;266(4):2048–2053. [PubMed] [Google Scholar]
- Morris B. J., Hicks A. A., Wisden W., Darlison M. G., Hunt S. P., Barnard E. A. Distinct regional expression of nicotinic acetylcholine receptor genes in chick brain. Brain Res Mol Brain Res. 1990 May;7(4):305–315. doi: 10.1016/0169-328x(90)90081-n. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Responses to GABA, glycine and beta-alanine induced in Xenopus oocytes by messenger RNA from chick and rat brain. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):201–216. doi: 10.1098/rspb.1988.0019. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Pfeiffer F., Simler R., Grenningloh G., Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A., Cortés R., Palacios J. M. The distribution of glycine receptors in the human brain. A light microscopic autoradiographic study using [3H]strychnine. Neuroscience. 1986;17(1):11–35. doi: 10.1016/0306-4522(86)90222-8. [DOI] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt B., Knaus P., Becker C. M., Betz H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987 Feb 10;26(3):805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- Schröder S., Hoch W., Becker C. M., Grenningloh G., Betz H. Mapping of antigenic epitopes on the alpha 1 subunit of the inhibitory glycine receptor. Biochemistry. 1991 Jan 8;30(1):42–47. doi: 10.1021/bi00215a007. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Korn H. gamma-Aminobutyric acid-containing terminals can be apposed to glycine receptors at central synapses. J Cell Biol. 1987 Apr;104(4):947–956. doi: 10.1083/jcb.104.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- White W. F., O'Gorman S., Roe A. W. Three-dimensional autoradiographic localization of quench-corrected glycine receptor specific activity in the mouse brain using 3H-strychnine as the ligand. J Neurosci. 1990 Mar;10(3):795–813. doi: 10.1523/JNEUROSCI.10-03-00795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Morris B. J., Darlison M. G., Hunt S. P., Barnard E. A. Distinct GABAA receptor alpha subunit mRNAs show differential patterns of expression in bovine brain. Neuron. 1988 Dec;1(10):937–947. doi: 10.1016/0896-6273(88)90151-1. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin M. A., Wamsley J. K., Kuhar M. J. Glycine receptor: light microscopic autoradiographic localization with [3H]strychnine. J Neurosci. 1981 May;1(5):532–547. doi: 10.1523/JNEUROSCI.01-05-00532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988 Feb;8(2):472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]