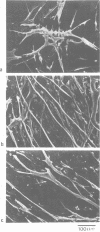
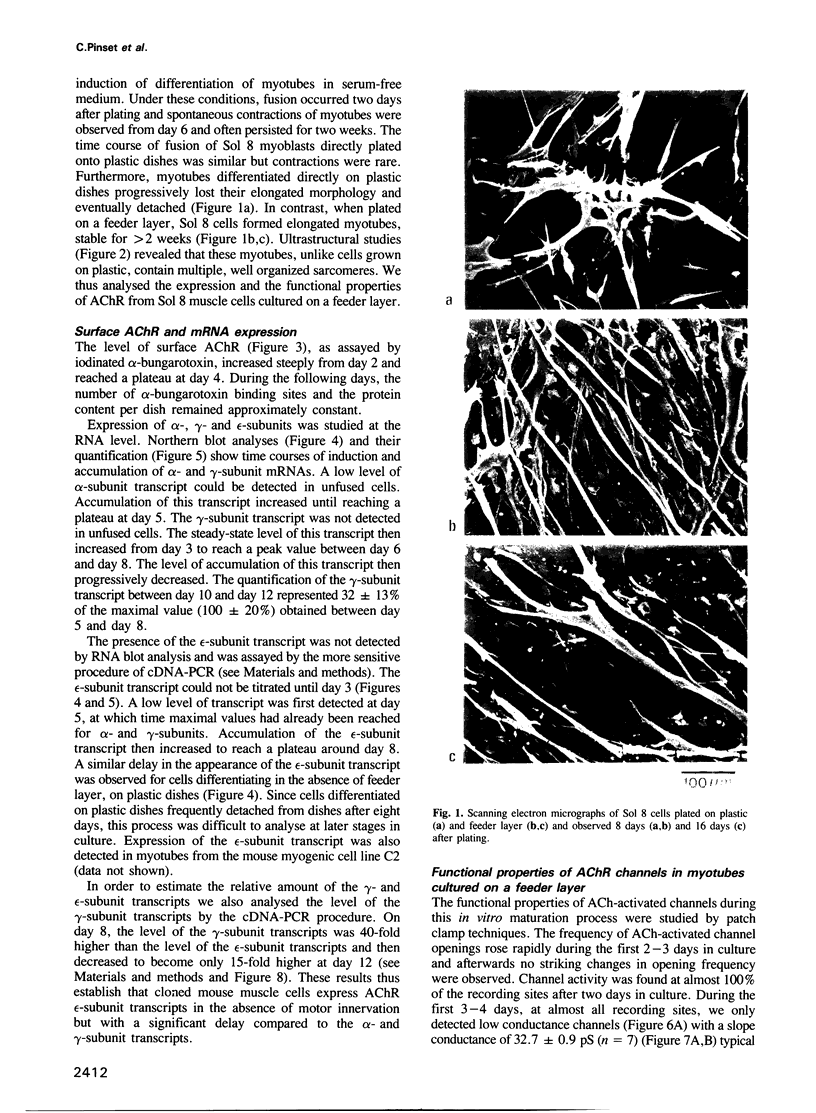
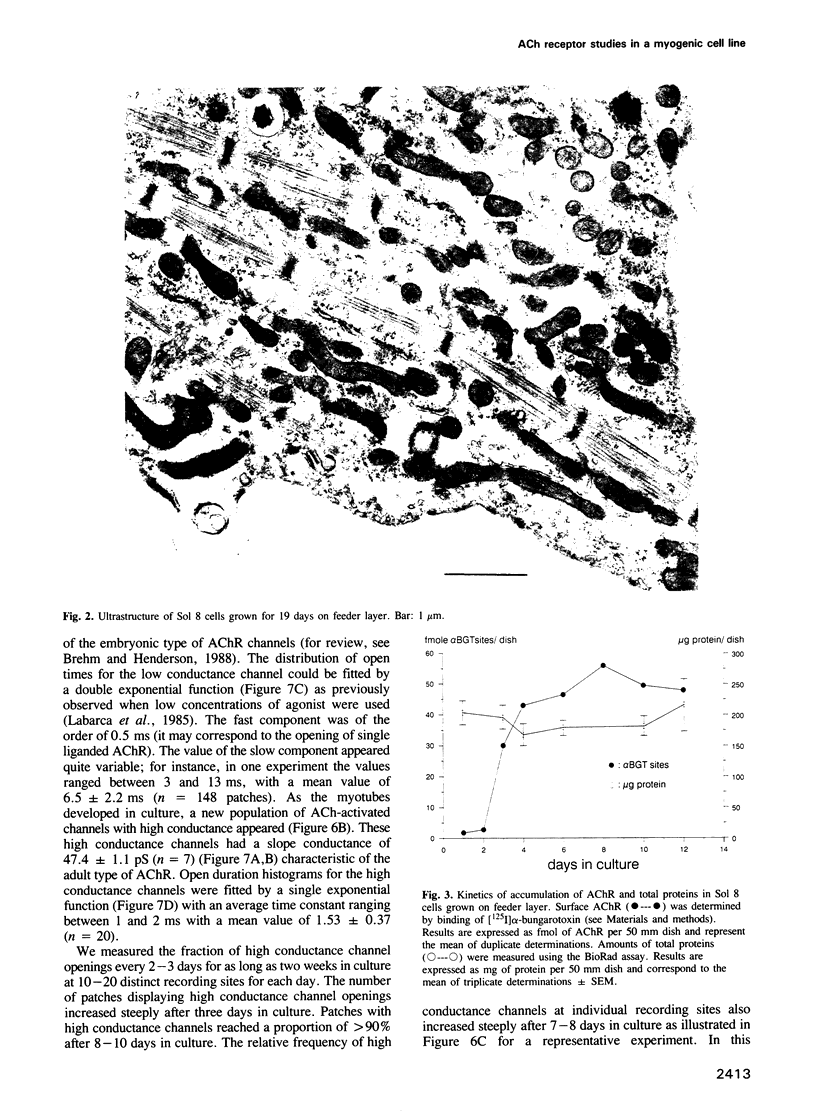
Abstract
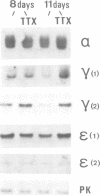
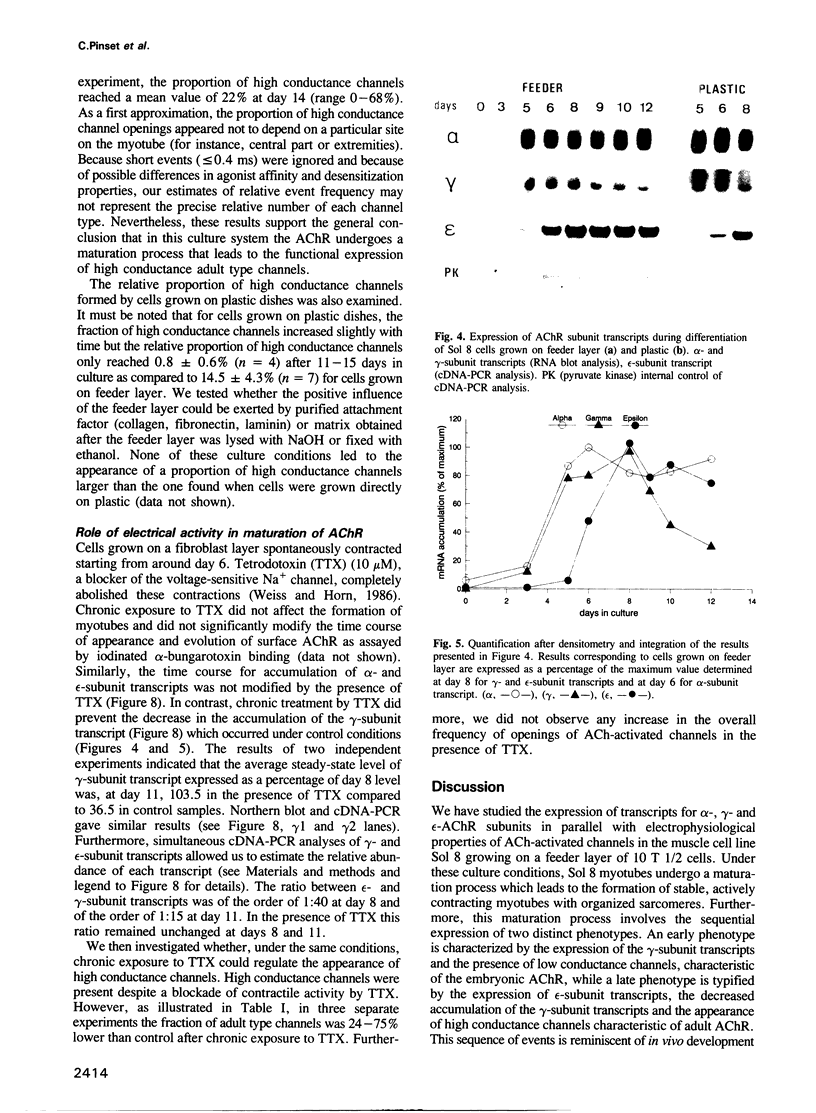
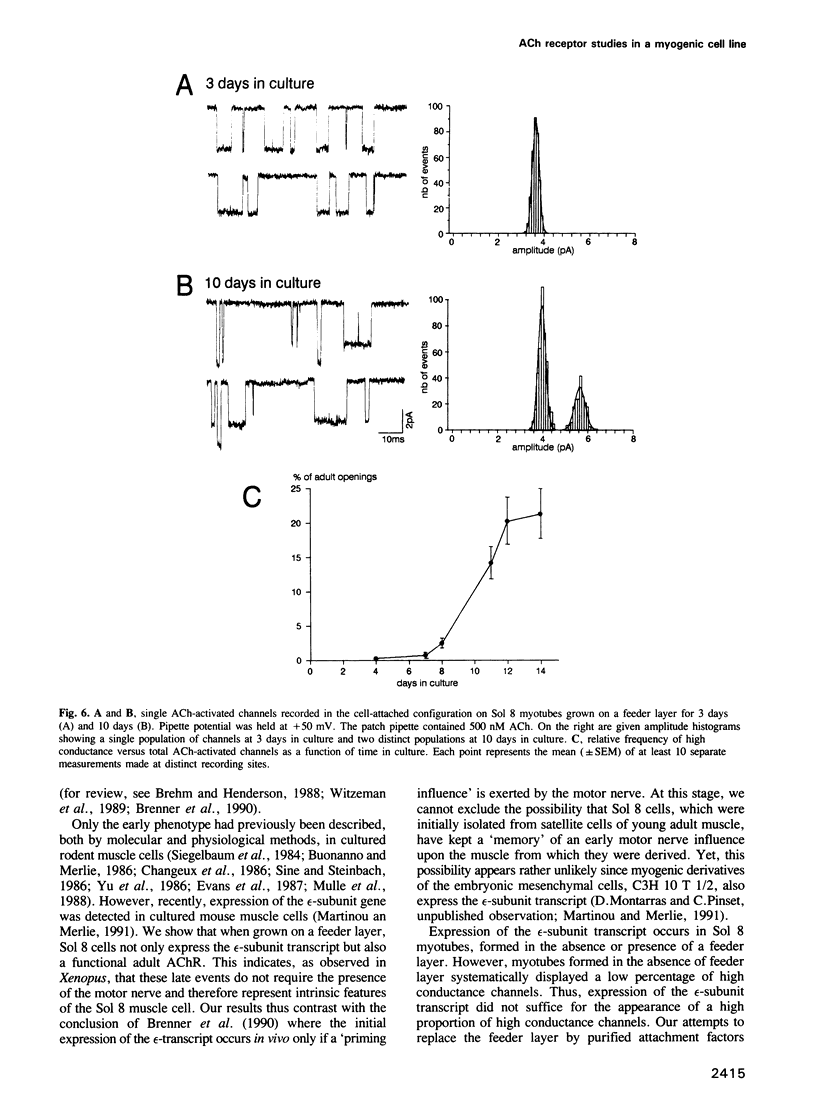
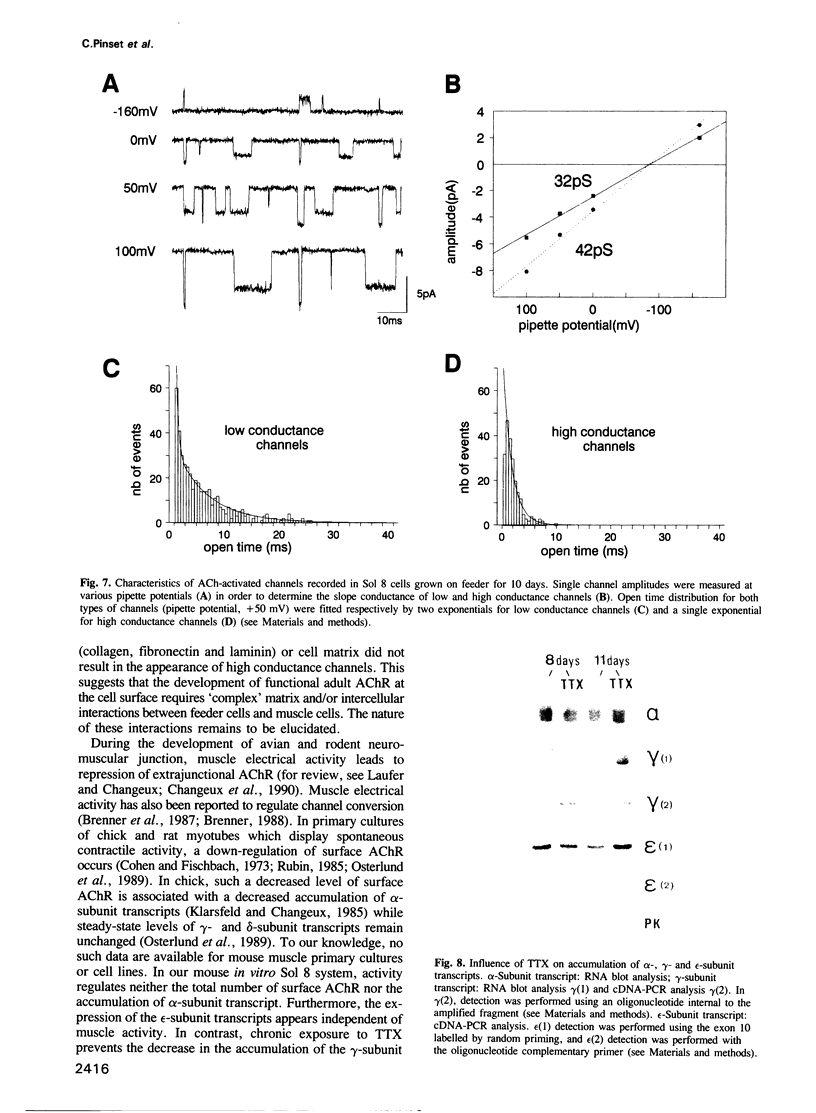
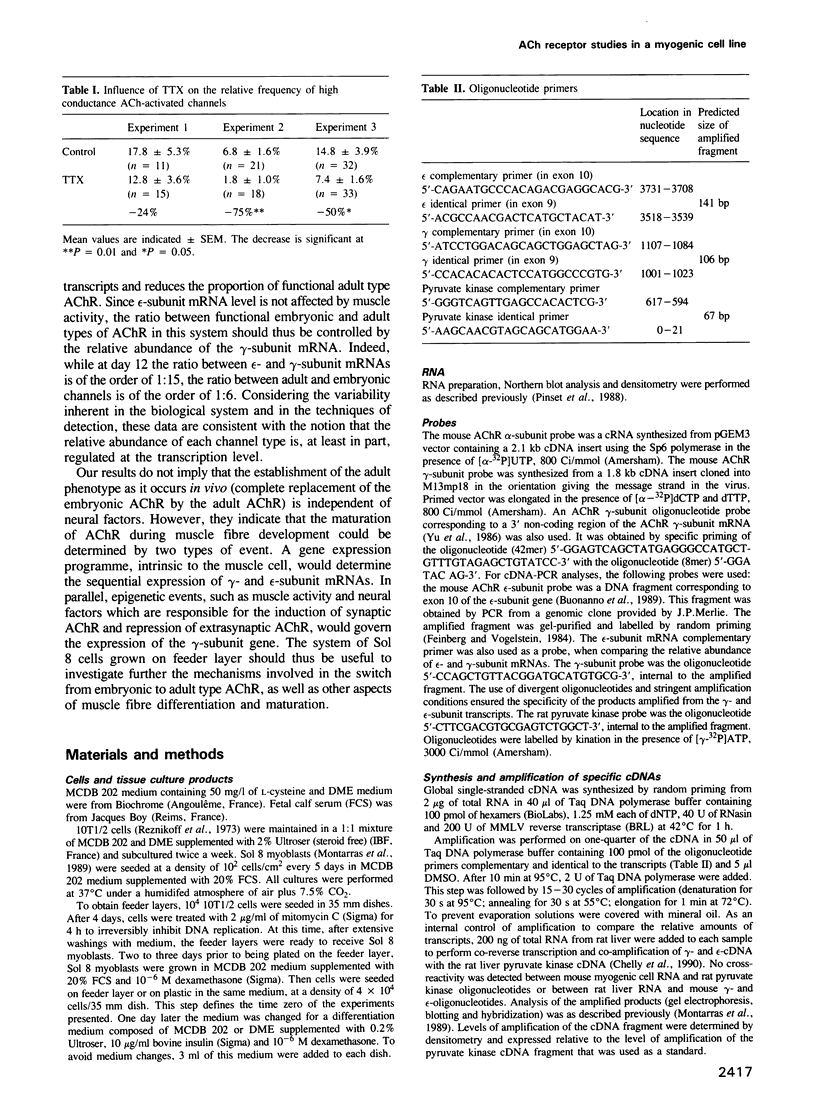
We have defined culture conditions, using a feeder layer of cells from the embryonic mesenchymal cell line, 10T1/2 and a serum-free medium, which allow cells from the mouse myogenic cell line Sol 8 to form contracting myotubes for two weeks. Under these culture conditions, Sol 8 myotubes undergo a maturation process characterized by a sequential expression of two phenotypes. An early phenotype is typified by the expression of the nicotinic acetylcholine receptor (AChR) gamma-subunit transcripts and the presence of low conductance ACh-activated channels, typical of embryonic AChR. A late phenotype is characterized by the expression of AChR epsilon-subunit transcripts, the decreased accumulation of gamma-subunit transcripts and the appearance of high conductance ACh-activated channels, typical of adult AChR. These results indicate that the expression of functional adult type AChR does not require the presence of the motor nerve and therefore represents an intrinsic feature of the Sol 8 muscle cells. Chronic exposure of the cells to the voltage-sensitive Na+ channel blocking agent tetrodotoxin does not affect the appearance of the AChR epsilon-subunit transcripts but prevents the reduction of the steady-state level of the AChR gamma-subunit transcripts and yields a reduced proportion of the adult type channels. Thus, activity seems to facilitate the switch from the embryonic to the adult phenotype of the AChR protein. The Sol 8 cell system might be useful to analyse further the genetic and epigenetic regulation of muscle fibre maturation in mammals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm P., Henderson L. Regulation of acetylcholine receptor channel function during development of skeletal muscle. Dev Biol. 1988 Sep;129(1):1–11. doi: 10.1016/0012-1606(88)90156-x. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kidokoro Y., Moody-Corbett F. Acetylcholine receptor channel properties during development of Xenopus muscle cells in culture. J Physiol. 1984 Dec;357:203–217. doi: 10.1113/jphysiol.1984.sp015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R. Dependence of acetylcholine receptor channel conversion on muscle activity at denervated neonatal rat endplates. Neurosci Lett. 1988 May 26;88(2):161–166. doi: 10.1016/0304-3940(88)90119-x. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Lømo T., Williamson R. Control of end-plate channel properties by neurotrophic effects and by muscle activity in rat. J Physiol. 1987 Jul;388:367–381. doi: 10.1113/jphysiol.1987.sp016619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Witzemann V., Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990 Apr 5;344(6266):544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Merlie J. P. Transcriptional regulation of nicotinic acetylcholine receptor genes during muscle development. J Biol Chem. 1986 Sep 5;261(25):11452–11455. [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Merlie J. P. Isolation and characterization of the beta and epsilon subunit genes of mouse muscle acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7611–7616. [PubMed] [Google Scholar]
- Changeux J. P., Pinset C., Ribera A. B. Effects of chlorpromazine and phencyclidine on mouse C2 acetylcholine receptor kinetics. J Physiol. 1986 Sep;378:497–513. doi: 10.1113/jphysiol.1986.sp016232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Montarras D., Pinset C., Berwald-Netter Y., Kaplan J. C., Kahn A. Quantitative estimation of minor mRNAs by cDNA-polymerase chain reaction. Application to dystrophin mRNA in cultured myogenic and brain cells. Eur J Biochem. 1990 Feb 14;187(3):691–698. doi: 10.1111/j.1432-1033.1990.tb15355.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Regulation of muscle acetylcholine sensitivity by muscle activity in cell culture. Science. 1973 Jul 6;181(4094):76–78. doi: 10.1126/science.181.4094.76. [DOI] [PubMed] [Google Scholar]
- Evans S., Goldman D., Heinemann S., Patrick J. Muscle acetylcholine receptor biosynthesis. Regulation by transcript availability. J Biol Chem. 1987 Apr 5;262(10):4911–4916. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Montal M. S., Lindstrom J. M., Montal M. The occurrence of long openings in the purified cholinergic receptor channel increases with acetylcholine concentration. J Neurosci. 1985 Dec;5(12):3409–3413. doi: 10.1523/JNEUROSCI.05-12-03409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Activity-dependent regulation of gene expression in muscle and neuronal cells. Mol Neurobiol. 1989 Spring-Summer;3(1-2):1–53. doi: 10.1007/BF02935587. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Montarras D., Pinset C., Chelly J., Kahn A., Gros F. Expression of MyoD1 coincides with terminal differentiation in determined but inducible muscle cells. EMBO J. 1989 Aug;8(8):2203–2207. doi: 10.1002/j.1460-2075.1989.tb08343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C., Benoit P., Pinset C., Roa M., Changeux J. P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M., Fontaine B., Devillers-Thiery A., Geoffroy B., Changeux J. P. Acetylcholine receptor expression in primary cultures of embryonic chick myotubes--I. Discoordinate regulation of alpha-, gamma- and delta-subunit gene expression by calcitonin gene-related peptide and by muscle electrical activity. Neuroscience. 1989;32(2):279–287. doi: 10.1016/0306-4522(89)90078-x. [DOI] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. Expression of nicotinic acetylcholine receptors in aneural Xenopus embryos. Dev Biol. 1989 Sep;135(1):12–19. doi: 10.1016/0012-1606(89)90153-x. [DOI] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. In vivo development of nicotinic acetylcholine receptor channels in Xenopus myotomal muscle. J Neurosci. 1989 Mar;9(3):1018–1028. doi: 10.1523/JNEUROSCI.09-03-01018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinset C., Montarras D., Chenevert J., Minty A., Barton P., Laurent C., Gros F. Control of myogenesis in the mouse myogenic C2 cell line by medium composition and by insulin: characterization of permissive and inducible C2 myoblasts. Differentiation. 1988 Jun;38(1):28–34. doi: 10.1111/j.1432-0436.1988.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Rubin L. L. Increases in muscle Ca2+ mediate changes in acetylcholinesterase and acetylcholine receptors caused by muscle contraction. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7121–7125. doi: 10.1073/pnas.82.20.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M., Role L. W. Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Trautmann A., Koenig J. Single acetylcholine-activated channel currents in developing muscle cells. Dev Biol. 1984 Aug;104(2):366–379. doi: 10.1016/0012-1606(84)90092-7. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Noda M., Mishina M., Shimizu S., Furutani Y., Kayano T., Ikeda T., Kubo T., Takahashi H., Takahashi T. Cloning, sequencing and expression of cDNA for a novel subunit of acetylcholine receptor from calf muscle. 1985 Jun 27-Jul 3Nature. 315(6022):761–764. doi: 10.1038/315761a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Criado M., Stein E., Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989 Jan 2;242(2):419–424. doi: 10.1016/0014-5793(89)80514-9. [DOI] [PubMed] [Google Scholar]
- Yu L., LaPolla R. J., Davidson N. Mouse muscle nicotinic acetylcholine receptor gamma subunit: cDNA sequence and gene expression. Nucleic Acids Res. 1986 Apr 25;14(8):3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]