Abstract
Meditation has been associated with relatively reduced activity in the default mode network, a brain network implicated in self-related thinking and mind wandering. However, previous imaging studies have typically compared meditation to rest despite other studies reporting differences in brain activation patterns between meditators and controls at rest. Moreover, rest is associated with a range of brain activation patterns across individuals that has only recently begun to be better characterized. Therefore, this study compared meditation to another active cognitive task, both to replicate findings that meditation is associated with relatively reduced default mode network activity, and to extend these findings by testing whether default mode activity was reduced during meditation beyond the typical reductions observed during effortful tasks. In addition, prior studies have used small groups, whereas the current study tested these hypotheses in a larger group. Results indicate that meditation is associated with reduced activations in the default mode network relative to an active task in meditators compared to controls. Regions of the default mode showing a group by task interaction include the posterior cingulate/precuneus and anterior cingulate cortex. These findings replicate and extend prior work indicating that suppression of default mode processing may represent a central neural process in long-term meditation, and suggest that meditation leads to relatively reduced default mode processing beyond that observed during another active cognitive task.
Keywords: meditation, default mode network, mind wandering, self-related thinking
Introduction
Meditation involves maintaining attention on immediate experience and away from distractions such as self-referential thinking and mind wandering (Bishop et al., 2004). Consistent with this, meditation has been associated with relatively reduced activity in a network of brain regions implicated in self-referential processing known as the default mode network (DMN) in experienced meditators compared to non-meditators (Brewer, Worhunsky, et al., 2011). Likewise, mind wandering has been associated with activity in the DMN (Mason et al., 2007), and reduced DMN activity during meditation has been associated with improved sustained attention outside of the scanner (Pagnoni, 2012). These findings suggest a role for reduced DMN processing during meditation.
Reduced DMN activity during meditation appears to be consistent across different meditation practices. A recent meta-analysis found that DMN activity was consistently reduced during meditation compared to control conditions across neuroimaging studies of meditation involving either focused attention or the repetition of phrases (Tomasino, Fregona, Skrap, & Fabbro, 2012). The same study by our research group found that DMN activity was reduced in meditators compared to controls across three standard mindfulness meditations: focused concentration, loving kindness, and choiceless awareness (Brewer, Worhunsky, et al., 2011). Determining that there are neural mechanisms common across meditation practices may inform the generalizability and potential clinical applications of these techniques.
The DMN has been found to be most highly active when individuals are left to think to themselves undisturbed or during tasks involving self-related processing, and less active during tasks requiring cognitive effort (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001). This network is comprised of a midline core including the anterior medial prefrontal cortex and posterior cingulate cortex/precuneus; a dorsal medial prefrontal cortex subsystem with the temporal pole, lateral temporal cortex and temporoparietal junction; and a medial temporal lobe subsystem with the ventral medial prefrontal cortex, posterior inferior parietal lobule, retrosplenial cortex, parahippocampal complex and hippocampal formation (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010). Several of these brain regions have been shown to have relatively reduced activity during meditation compared to control conditions across neuroimaging studies, including the angular gyrus, middle temporal gyrus, and precuneus (Tomasino et al., 2012), suggesting increased cognitive effort and decreased self-related thinking associated with meditation. In our prior study, meditators relative to controls showed lower activity during meditation as compared to rest in the posterior cingulate cortex/precuneus (Brewer, Worhunsky, et al., 2011). Therefore, this study aimed to replicate this finding in a larger sample, given that most neuroimaging studies of meditation, in particular those involving experienced meditators, have used small groups (mean = 11.7, range = 4–31; Tomasino et al., 2012).
Previous studies have also reported that meditators compared to controls show differences in DMN activity not only during meditation, but also in functional connectivity at rest (Brewer, Worhunsky, et al., 2011; Jang et al., 2011). These findings introduce a potential confound to studies of meditators comparing meditation to rest, as meditation may transform the resting state into a more meditative state. The choice of control condition is a critical problem in cognitive neuroimaging studies and is fundamental for interpreting changes in brain activation patterns (Gusnard & Raichle, 2001; Marx et al., 2004). The resting brain state is expected to be highly variable across individuals, and therefore may be a poorer choice for comparison. To mitigate this confound, some studies have found utility in comparing meditation to active control tasks, such as mental arithmetic (e.g., Holzel et al., 2007). Therefore, this study aimed to compare meditation to another active cognitive task, in order to test the hypothesis that meditation leads to reduced activity in the DMN beyond another active cognitive task.
Methods
Participants
All participants provided written informed consent in accordance with the Human Investigations Committee of the Yale School of Medicine. Twenty experienced meditators and twenty-six non-meditators (controls) took part in the study. Of these participants, six meditators and three controls had participated in our previous study (Brewer, Worhunsky, et al., 2011). All results reported here show similar effects if the analyses are restricted to the new participants only. Meditators were recruited by advertisements and word of mouth, and were all from the Insight meditation (Theravada) tradition. They reported a mean of 9676 ± 1586 practice hours over 14 ± 2 years, including daily practice and retreats. Controls reported no prior meditation experience. Groups were matched on sex, race, age, and years of education (Table 1).
Table 1.
Participant demographics.
| Meditators (n = 20) | Controls (n = 26) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | χ2 | p | |
|
|
||||||
| Sex | .03 | 0.85 | ||||
| Male | 11 | 55 | 15 | 55 | ||
| Female | 9 | 45 | 11 | 45 | ||
| Race | n/a | n/a | ||||
| White (Non-Hispanic) | 20 | 100 | 26 | 100 | ||
|
|
||||||
| Mean | SD | Mean | SD | t | p | |
|
|
||||||
| Age | 45.6 | 11.1 | 42.2 | 13.3 | −.92 | .36 |
| Years of Education | 17.6 | 4.8 | 17.2 | 3.0 | −.36 | .72 |
fMRI protocol
Just before scanning, participants were instructed in three standard mindfulness meditation practices (as previously: Brewer, Worhunsky, et al., 2011; Gunaratana, 2002): (a) Concentration: “Please pay attention to the physical sensation of the breath wherever you feel it most strongly in the body. Follow the natural and spontaneous movement of the breath, not trying to change it in any way. Just pay attention to it. If you find that your attention has wandered to something else, gently but firmly bring it back to the physical sensation of the breath.” (b) Loving kindness: “Please think of a time when you genuinely wished someone well (pause). Using this feeling as a focus, silently wish all beings well, by repeating a few short phrases of your choosing over and over. For example: May all beings be happy, may all beings be healthy, may all beings be safe from harm.” (c) Choiceless awareness: “Please pay attention to whatever comes into your awareness, whether it is a thought, emotion, or body sensation. Just follow it until something else comes into your awareness, not trying to hold onto it or change it in any way. When something else comes into your awareness, just pay attention to it until the next thing comes along.” Participants practiced each meditation condition outside of the scanner prior to fMRI, and confirmed that they understood and could follow the instructions.
Each run began with 30 s eyes open rest period, during which participants were instructed to look at the fixation cross and not think of anything in particular. This was followed by an 8 s display of the instructions for the active cognitive task and then the 90 s active cognitive task. For the active task, participants were asked to make judgments about adjectives in response to a cue indicating that they should judge the word related to “self” (“Does the word describe you?”) or “case” (“Is the word in uppercase letters?”) and to indicate “yes” or “no” using a button box (Kelley et al., 2002). Adjectives were presented using E-Prime 1.2 (http://www.pstnet.com/eprime.cfm) for 2.5 s with a 1–3 s interstimulus fixation interval for 30 trials per run for a total of 180 trials. A total of 60 unique adjectives were drawn from the Anderson Word List (Anderson, 1968) and counterbalanced for valence. Participants practiced the active task to proficiency outside of the scanner prior to scanning. The active task was followed by a 30 s eyes closed rest period. The eyes closed condition was followed by a 30 s recorded meditation instruction (as above) and a 180 s meditation period. At the end of the meditation period, subjects heard an audio prompt to open their eyes and rest until the sound of the scanner stopped, for an additional 20 s eyes open rest period. Each meditation condition was performed twice, for a total of six runs. Meditation conditions were randomized, but the second instance of each meditation was blocked (i.e., AABBCC). After each run, participants were asked to rate how well they were able to follow the instructions and how much their mind wandered during meditation on a scale from 0–10.
fMRI imaging parameters
Scanning was conducted using a Siemens 1.5 Tesla Sonata MRI (Siemens AG, Erlangen, Germany) with an eight-channel receive-only head coil. High resolution T1-weighted 3D anatomical images were acquired using a magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR = 2530 ms, TE = 3.34 ms, field of view = 220 mm, matrix size = 192 × 192, slice thickness = 1.2 mm, flip angle = 8°, 160 slices). Low resolution T1-weighted anatomical images were acquired (TR = 500 ms, TE = 11 ms, field of view = 220 mm, slice thickness = 4 mm, gap = 1 mm, 25 AC-PC aligned axial-oblique slices). Functional image acquisition began at the same slice location as the T1 scan. Functional images were acquired using a T2*-weighted gradient-recalled single shot echo-planar sequence (TR = 2000 ms, TE = 35 ms, flip angle = 90°, bandwidth = 1446 Hz/pixel, matrix size = 64 × 64, field of view = 220 mm, voxel size = 3.5 mm, interleaved, 210 volumes, 2 volumes were acquired at the beginning of the run and discarded).
fMRI data preprocessing
Images were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Functional images were realigned for motion correction and the resultant parameters were used as regressors of no interest in the fMRI model. In addition, Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect) was used to identify global mean intensity and motion outliers in the fMRI time series (outlier thresholds: global signal > 3 standard deviations, motion > 1 mm). Any detected outliers were included as regressors of no interest in the model. A generative model of tissue classification, bias correction, and segmentation (Ashburner & Friston, 2005) was used to estimate spatial normalization parameters to Montreal Neurological Institute (MNI) space. The estimates were then applied to all structural and functional images, and all images were smoothed using a 6 mm full width at half-maximum Gaussian kernel.
Although motion outliers were modeled as regressors of no interest using ART, non-equivalent motion correction may result in bias when modeling group differences. Therefore, mean outliers detected by ART across 6 runs were compared between groups using an independent t-test. No significant difference in mean outliers was found between meditators and controls (meditators = 45, SEM = 6.3; controls = 38, SEM = 5.8; t(44) = .79, P = .43). Outliers were detected in all controls and all but one meditator. Motion outliers in the first and last runs (runs 1 and 6) were compared between groups using a repeated measures analysis of variance. A significant effect of time was found (F = 4.34, P = .04), but no significant group by time interaction was found (F = .01, P = .91), such that mean motion outliers increased from run 1 to run 6 comparably in meditators (run 1 = 5.1, run 6 = 7.8) and controls (run 1 = 5.9, run 6 = 8.3).
fMRI data analysis
Blood oxygen level-dependent (BOLD) signal was modeled using separate regressors for the conditions: active task instructions, active task, meditation instructions, meditation task. Rest periods were combined to form the implicit baseline. The meditation task included the three distinct meditation practices collapsed as a block for the analysis. The active task included “self,” “case,” and fixation trials collapsed as a block for the analysis. The conditions were modeled using a boxcar function convolved with a canonical hemodynamic response function and regressors were fit using SPM8’s implementation of the general linear model. To accommodate the long mediation conditions, the high-pass filter cutoff was 360 s. A first-level model was specified to estimate the parameter for each condition for each subject. A second-level model was specified to estimate the parameter for the main effects of task (meditation, active task) and group (meditation, control), and the interaction effect. A 2-by-2 interaction effect was tested using a repeated measures analysis of variance for group (meditators, controls) by task (meditation, active task), and was exclusively masked with the group effect (meditation versus control) in order to show the voxels in which the interaction was not driven by the main effect of group. All findings are significant at p<.05 Family Wise Error (FWE) cluster-corrected, using a p<.01 cluster-forming threshold and extent threshold of 250 voxels, unless a more conservative threshold is indicated.
Statistics
Statistical analysis was conducted using SPSS 19 (www-01.ibm.com/software/analytic). For participant demographics, paired t-tests were used to determine differences between groups in age and χ2 tests were used to determine differences between groups in sex. Repeated measures analysis of variance was used to determine differences between groups in self-reported mind wandering. For the active task, independent t-tests were used to compare reaction time between groups and χ2 tests were used to compare error rate between groups, with an error defined as an incorrect response to ‘case’ or no response to ‘self.’ All statistical tests were two-tailed and are reported as means ± standard deviation.
Results
Behavioral results
In line with the assumption that meditators and controls performed the active task similarly, no significant difference was found for reaction time between meditators (1.25 ± 0.38 s) and controls (1.26 ± 0.42 s; t=1.46, P=.15). Meditators made significantly fewer errors on the ‘case’ condition (1.7%) than controls (3.5%; χ2 = 13.2, P < .001), whereas no significant difference was found in errors on the ‘self’ condition between groups (meditators = 1.7%; controls = 1.3%; χ2 = 1.1, P = .31).
As expected, meditators reported less mind wandering during meditation relative to controls (F(1,44) = 7.57, P = .009). This finding was apparent for concentration (controls: 4.5 ± 2.1, meditators: 3.5 ± 1.4), loving kindness (controls: 3.8 ± 1.8, meditators: 2.8 ± 1.4), and choiceless awareness meditation (controls: 4.4 ± 2.3, meditators: 2.7 ± 1.6). Both meditators and controls reported being able to follow the instructions to a high degree for concentration (controls: 8.6 ± 1.4, meditators: 8.5 ± 1.4), loving kindness (controls: 8.6 ± 1.4, meditators: 8.8 ± 1.2), and choiceless awareness meditation (controls: 9.0 ± 1.4, meditators: 8.9 ± 0.9). No effect of time was found on mind wandering (meditators: run 1: 3.0 ± 1.6, run 6: 2.9 ± 2.0; controls: run 1: 4.1 ± 2.0, run 6: 4.3 ± 2.5; F(1, 44) = .003, P = .96), and no group by time interaction was found for mind wandering (F(44) = .19, P = .67). Similarly, no effect of time was found on the ability to follow instructions (meditators: run 1: 8.7 ± 1.3, run 6: 8.9 ± 1.4; controls: run 1: 8.6 ± 1.2, run 6: 8.7 ± 1.6; F(1, 44) = 1.14, P = .29), and no group by time interaction was found for the ability to follow instructions (F(1, 44) = .57, P = .45).
fMRI results
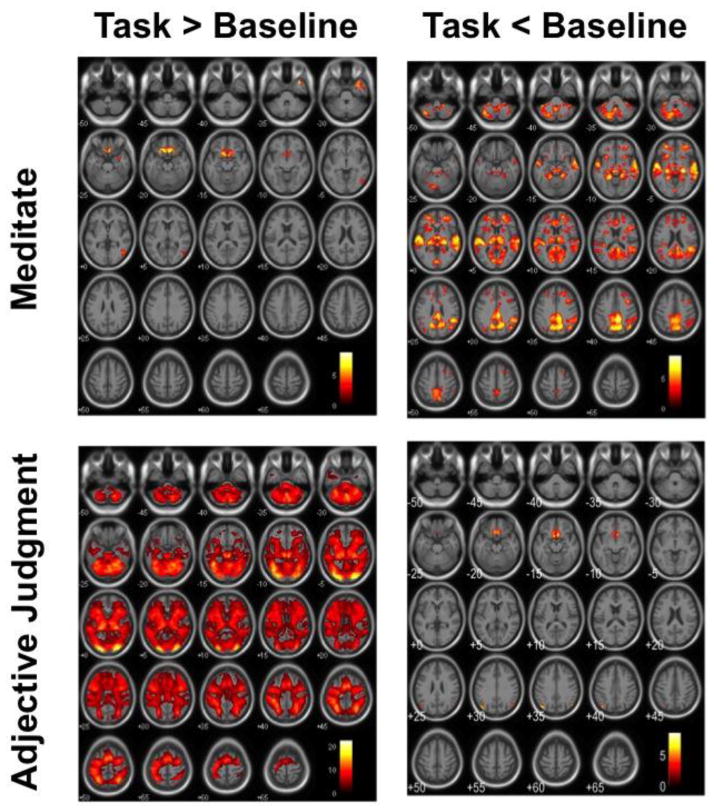
For meditators and controls combined, meditation compared to the implicit baseline was associated with activity increases in the bilateral rectal gyrus and orbitofrontal cortex (Figure 1 top left, Table 2). The same brain regions showed an activity decrease during the active task compared to the implicit baseline in meditators and controls combined (Figure 1, bottom right, Table 2).
Figure 1.
Effects of task in the combined meditator and control groups. Meditation compared to implicit baseline is associated with activity increases bilaterally in the orbitofrontal cortex (top left). The same areas show an activity decrease during the active task compared to implicit baseline (bottom right). Images are displayed in neurological convention with critical thresholds at p<0.001 uncorrected for multiple tests to show the subthreshold extent of the effects.
Table 2.
Brain region peaks showing increased activity with meditation as compared to implicit baseline in both meditators and controls.
| Side | Label | Peak P(FWE-corr) |
Peak Z |
x | y | z |
|---|---|---|---|---|---|---|
| L | Rectal gyrus | 0.00024 | 7.52 | −10 | 14 | −18 |
| R | Rectal gyrus | 0.00024 | 7.08 | −14 | 28 | −22 |
| L | Orbitofrontal cortex | 0.011 | 6.80 | −12 | 26 | −22 |
| R | Rectal gyrus | 0.00020 | 6.29 | 6 | 26 | −20 |
| R | Orbitofrontal cortex | 0.0055 | 6.28 | 16 | 16 | −18 |
| L | Orbitofrontal cortex | 0.022 | 6.09 | −22 | 20 | −20 |
All peaks significant at p<.05 FWE-corrected
A between group difference was found for meditation compared to the implicit baseline. Meditators compared to controls showed reduced activity in the anterior cingulate cortex and the dorsal and ventral precuneus/posterior cingulate cortex during meditation compared to the implicit baseline (Figure 2, supplementary Figure S1).
Figure 2.

A between group contrast of meditation versus implicit baseline revealed effects in the anterior cingulate cortex (ACC) and the dorsal (dPCu) and ventral precuneus (vPCu)/posterior cingulate cortex (M – meditators and C – controls). All three clusters are significant at p<0.05 FWE-corrected, p<.01 cluster-forming threshold, extent threshold 250 voxels. Images are displayed in neurological convention with critical thresholds p<0.01 uncorrected for multiple tests to show the subthreshold extent of the effects.
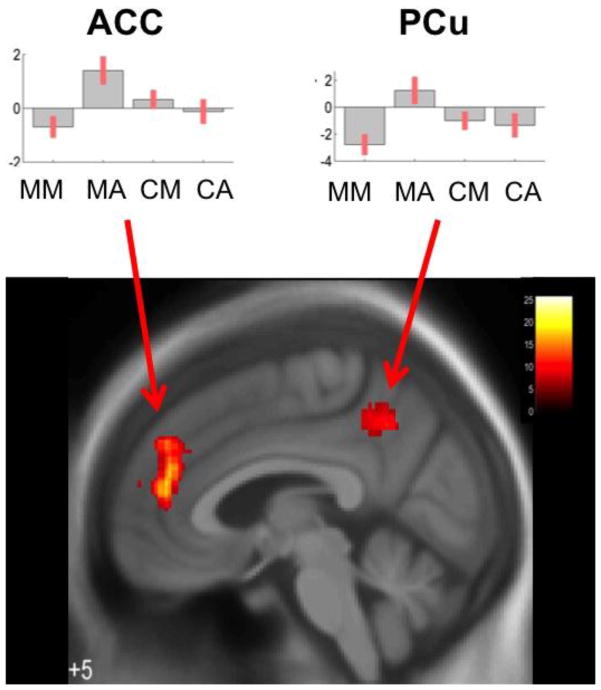
A significant group (meditators, controls) by task (meditation, active task) interaction, exclusively masked by the effects of group, was identified in the middle temporal gyrus, fusiform and hippocampal gyri, anterior cingulate cortex, and precuneus (Figure 3). Plots of the parameter estimates for the anterior cingulate cortex and precuneus demonstrate that activity in these brain regions decreases during meditation and increases during the active control task in meditators, whereas controls did not show this dissociation (Figure 3, insets).
Figure 3.
A group by task interaction exclusively masked with the main effect of group revealed effects in the anterior cingulate cortex (ACC) and the dorsal precuneus (PCu) across groups and task conditions. MM – meditators meditating, MA – meditators performing the active task, CM – controls meditating, CA – controls performing the active task. Both clusters are significant p<.05 FWE-corrected. Images are displayed in neurological convention with critical thresholds of p<0.01 uncorrected for multiple tests to show the subthreshold extent of the effects.
Discussion
In this study, meditation was found to be associated with relatively lower activity in regions of the DMN in meditators compared to controls during meditation compared to another active cognitive task, as indicated by a significant group by task interaction. Brain regions showing relatively reduced activity during meditation in meditators included the anterior cingulate cortex, fusiform gyrus, middle temporal gyrus, and precuneus. Meditators also showed relatively lower activity in DMN regions than controls during meditation as compared to rest.
As described above, the DMN is typically active during task-free resting states (Raichle et al., 2001), where this activity is thought to represent neural processing related to self-related thinking or mind wandering (Buckner et al., 2008). The DMN is further characterized by decreased activity during effortful goal-directed tasks (Fox et al., 2005; Greicius, Krasnow, Reiss, & Menon, 2003). A recent meta-analysis reported that neuroimaging studies of meditation consistently report reduced DMN activity during meditation compared to control conditions in both meditators and non-meditator controls (Tomasino et al., 2012). Although the meta-analysis did not find a difference in DMN activity associated with long-term experience, our prior study found reduced activity in regions of the DMN during meditation relative to rest in experienced meditators compared to non-meditators (Brewer, Worhunsky, et al., 2011). This study replicated that previous study in a larger sample (meditators: 20 versus 12; controls: 26 versus 12).
However, functional connectivity in regions of the DMN, a measure of the temporal correlation of BOLD signal between these regions, has also been found to differ between meditators and controls, not only during meditation but also at rest (Brewer, Worhunsky, et al., 2011; Pagnoni, 2012; Taylor et al., 2013). This suggests that meditation training may alter the behavioral state individuals enter in to when given the standard resting state instructions. Meditators and controls appear to differ in their resting state DMN processing. Therefore, we compared meditation to another active cognitive task. Other studies have found similar utility in comparing meditation with an active task (e.g., Holzel et al., 2007; Tomasino et al., 2012). The current findings add to this work by providing evidence that meditation is associated with relatively reduced DMN activity during meditation as compared to a judgment of adjectives task in meditators versus controls. This finding suggests that meditation by experienced meditators leads to relatively reduced activity in the DMN beyond that expected by general task-based deactivation.
Consistent with other prior findings (Kelley et al., 2002), controls showed a pattern of reduced precuneus/posterior cingulate cortex activity during both the judgment of adjectives task and meditation task (see parameter estimate plots, Figures 2 and 3 insets). It is possible that for controls, reduced activity in this hub of the DMN during meditation and the active task reflects reduced self-related processing and mind wandering during these tasks than during the implicit baseline, which was comprised of resting periods. In support of this, task engagement has been shown to reduce activity in the precuneus/posterior cingulate cortex as compared to rest (Fox et al., 2005). Other studies have reported a high incidence of mind wandering in healthy individuals (Killingsworth & Gilbert, 2010; Whitfield-Gabrieli et al., 2011), and a high incidence of precuneus/posterior cingulate cortex activity associated with mind wandering (Pagnoni, 2012). In contrast, meditators showed increased activity in the precuneus during the judgment of adjectives task (Figure 2), possibly reflecting increased self-related processing relative to the implicit baseline. This interpretation would be consistent with our prior finding that meditators showed altered DMN functional connectivity at rest as compared to non-meditators (Brewer, Worhunsky, et al., 2011). Related to this, we have used real-time fMRI neurofeedback, in which individuals are provided dynamic visual feedback about their ongoing brain activity in real-time, to demonstrate that changes in activity in the posterior cingulate cortex correspond to experienced meditator’s subjective reports of focused attention and mind wandering (Garrison, Santoyo, et al., 2013; Garrison, Scheinost, et al., 2013). The current findings suggest further that long-term meditation experience may lead to changes in DMN activity beyond typical task engagement-related reductions because meditators showed reduced DMN activity during meditation not only compared to rest, but also compared to another active cognitive task. For meditators, this is consistent with the hypothesis that meditation may reduce self-related thinking and mind wandering more than another active task.
This study has several limitations. The use of a mixed design and the comparison of task blocks of different lengths may have reduced design efficiency. Comparing blocks of different lengths can lead to a poorer estimate of the shape of the hemodynamic response to a given stimulus block (Wager, Vazquez, Hernandez, & Noll, 2005). Block length was determined in consideration of both the task requirements and scan time limitations. To improve statistical power, the event-related active task (judgment of adjectives) was analyzed as a block. This may have combined events that increase (e.g., ‘self’) and decrease (e.g., ‘case’) DMN processing, thereby reducing power to detect DMN changes during this active task relative to meditation. Likewise, the meditation conditions (concentration, loving kindness, choiceless awareness) were collapsed to improve power. This design could be optimized to directly compare components of the active task and different meditation practices in a future study. A related limitation is that the meditation and active tasks were not counterbalanced; the active task always preceded the meditation task. Although the fixed order was used to avoid specific effects of state-based meditation on brain activity patterns during the active task, this approach did not account for potential trait-based effects. Finally, interpretation of our results is limited beyond meditation in the research setting. Traditional or cultural meditation practices typically involve contextual components such as intentions for practice, background conceptual beliefs, and the support of a community, among others. In the current study, meditation was performed in an fMRI scanner and thus decontextualized. Despite these drawbacks, since meditators were long-term practitioners with significant commitments to practice, we cannot rule out that larger components of the practice or memory of other contexts were active even during the decontextualized meditation tasks. Due to these empirical differences, further studies are necessary to interpret our findings within the broader field of meditation research. Overall, despite the design limitations, this study found reliable group differences in DMN activity across the different experimental conditions.
These findings provide evidence that reduced DMN processing may represent a central neural process in long-term meditation. This may have clinical implications. Previous work suggests that increased DMN activity may interfere with cognitive performance, and decreased DMN activity is associated with improved performance (for review, see Anticevic et al., 2012). Likewise, increased DMN activity has been associated with depression (Sheline et al., 2009), anxiety (Zhao et al., 2007), and addiction (Garavan et al., 2000), among other disorders. Mind wandering and self-related processing contribute to ruminative thinking which may be a feature of these disorders and has also been associated with decreased well-being (e.g., Killingsworth & Gilbert, 2010). In contrast, meditation, which appears to be associated with reduced activity in the DMN, has been shown to improve attention and working memory performance (Pagnoni, 2012) and promote positive health outcomes (Keng, Smoski, & Robins, 2011). As mindfulness training has shown utility for addiction (Brewer, Mallik, et al., 2011), as well as for pain, anxiety and depression (Goyal et al., 2014), these studies together suggest that a neural mechanism by which meditation results in clinical benefits may be through reducing DMN activity.
Supplementary Material
Effects of group for meditators compared to for meditation (top row) and the active task (bottom row). Images are displayed in neurological convention with critical thresholds at p<0.01 uncorrected for multiple tests to show the subthreshold extent of the effects.
Table 3.
Brain regions identified by a group (meditators/controls) by task (meditation/active task) interaction.
| Side | Label | Cluster p(FWE-corr) |
Cluster k |
Peak Z |
x | y | z |
|---|---|---|---|---|---|---|---|
| R | Middle temporal gyrus | 0.007 | 481 | 4.60 | 66 | −18 | −8 |
| 3.90 | 58 | −4 | −10 | ||||
| 3.25 | 50 | −20 | −10 | ||||
| L | Middle temporal gyrus | 3.228 E-05 | 999 | 4.36 | −48 | −30 | −16 |
| 4.22 | −60 | −28 | −10 | ||||
| 3.86 | −48 | −18 | −22 | ||||
| L | Fusiform and hippocampal gyri | 0.005 | 505 | 4.29 | −20 | −50 | −10 |
| 3.62 | −26 | −60 | −10 | ||||
| 3.38 | −38 | −52 | −20 | ||||
| R | Anterior cingulate cortex | 0.01 | 458 | 4.09 | 12 | 44 | 12 |
| 3.94 | 10 | 40 | 26 | ||||
| 3.51 | 6 | 44 | 36 | ||||
| R | Middle temporal gyrus | 0.0002 | 783 | 4.09 | 46 | −56 | 20 |
| 3.59 | 56 | −36 | 18 | ||||
| 3.56 | 42 | −40 | 20 | ||||
| L | Precuneus | 0.0369 | 350 | 3.63 | −2 | −48 | 46 |
| 3.48 | −14 | −44 | 52 | ||||
| 3.13 | −18 | −36 | 40 |
Cluster forming threshold p<.005; extent threshold 250. All clusters are significant at p<0.05 FWE-corrected.
Acknowledgments
Funding
This work was supported by awards from the National Institutes of Health, National Institute on Drug Abuse (K12-DA00167 to JAB and KAG); the U.S. Veterans Affairs New England Mental Illness Research, Education, and Clinical Center; the American Heart Association (14CRP18200010 to KAG); and private donations from Jeffrey C. Walker, Austin Hearst, and 1440 Foundation.
We thank our participants for their time and effort, Joseph Goldstein and Ginny Morgan for input on meditation instructions, Hedy Kober for input on study design, Thomas Thornhill IV for study coordination, and Hedy Sarofin and staff of the Yale Magnetic Resonance Research Center for help with scanning.
References
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9(3):272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Devins G. Mindfulness: A proposed operational definition. Clinical Psychology-Science and Practice. 2004;11(3):230–241. doi: 10.1093/Clipsy/Bph077. [DOI] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, Rounsaville BJ. Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence. 2011;119(1–2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences. 2011;108(50):20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. In: Kingstone A, Miller MB, editors. The year in cognitive neuroscience 2008. Malden, MA: Blackwell Publishing; 2008. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garrison KA, Santoyo JF, Davis JH, Thornhill TAt, Kerr CE, Brewer JA. Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators’ self-report. Front Hum Neurosci. 2013;7:440. doi: 10.3389/fnhum.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Worhunsky PD, Elwafi HM, Thornhill TA, Thompson E, Brewer JA. Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage. 2013;81:110–118. doi: 10.1016/j.neuroimage.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratana H. Mindfulness in Plain English. Somerville, MA: Wisdom Publications; 2002. [Google Scholar]
- Gusnard DA, Raichle ME. Searching For A Baseline: Functionl Imaging and the Resting Human Brain. Nature Reviews Neuroscience. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, Vaitl D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang DH, Byun MS, Kwon SJ, Choi CH, Kwon JS. Increased default mode network connectivity associated with meditation. Neuroscience Letters. 2011;487(3):358–362. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review. 2011;31(6):1041–1056. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A Wandering Mind Is an Unhappy Mind. Science. 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Marx E, Deutschlander A, Stephan T, Dieterich M, Wiesmann M, Brandt T. Eyes open and eyes closed as rest conditions: impact on brain activation patterns. Neuroimage. 2004;21(4):1818–1824. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Graffon ST, Macrae CN. Wandering Minds: The Default Network and Stimulus-independent Thought. Science. 2007;315(5810):393. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoni G. Dynamical Properties of BOLD Activity from the Ventral Posteromedial Cortex Associated with Meditation and Attentional Skills. Journal of Neuroscience. 2012;32(15):5242–5249. doi: 10.1523/JNEUROSCI.4135-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Gordon LS. A Default Mode of Brain Function. Proceedings of the National Academy of Sciences. 2001;98(2):676. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, Beauregard M. Impact of meditation training on the default mode network during a restful state. Soc Cogn Affect Neurosci. 2013;8(1):4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino B, Fregona S, Skrap M, Fabbro F. Meditation-related activations are modulated by the practices needed to obtain it and by the expertise: an ALE meta-analysis study. Front Hum Neurosci. 2012;6:346. doi: 10.3389/fnhum.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Vazquez A, Hernandez L, Noll DC. Accounting for nonlinear BOLD effects in fMRI: parameter estimates and a model for prediction in rapid event-related studies. Neuroimage. 2005;25(1):206–218. doi: 10.1016/j.neuroimage.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castan A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55(1):225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, Tang XW. Altered default mode network activity in patient with anxiety disorders: an fMRI study. European Journal of Radiology. 2007;63(3):373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of group for meditators compared to for meditation (top row) and the active task (bottom row). Images are displayed in neurological convention with critical thresholds at p<0.01 uncorrected for multiple tests to show the subthreshold extent of the effects.