Abstract
Purpose
Deep anterior lamellar keratoplasty (DALK) is a challenging procedure that often results in conversion to penetrating keratoplasty (PKP). Preservation of Descemet’s membrane (DM) relies on indirect visualization of surgical planes.
We describe a technique for enhanced visualization of key steps in DALK with intraoperative optical coherence tomography (iOCT).
Methods
Utilizing a microscope-mounted spectral-domain OCT system, high-resolution images of various steps.
Results
Specifically, images were obtained of trephination depth and proximity of the cannula tract to DM. Other key steps such as air cannula placement, assessment of DM position and integrity after attempted big bubble delivery, and assessment of graft-host apposition were readily visualized. Presence of intrastromal emphysema after air injection decreased visualization of deeper structures.
Conclusion
iOCT allows visualization of depth-dependent anatomy and changes from specific surgical interventions during DALK not appreciated with en face operating microscope view and has the potential to facilitate big bubble delivery.
Keywords: intraoperative optical coherence tomography (iOCT), deep anterior lamellar keratoplasty (DALK), keratoplasty
Introduction
Deep anterior lamellar keratoplasty (DALK) has become a preferred corneal transplant technique for many anterior corneal stromal diseases including keratoconus, corneal scarring and corneal stromal dystrophies. The key advantages of DALK over full-thickness penetrating keratoplasty include lower graft rejection rates and longer graft survival.1–2 Anwar et al. developed the big-bubble technique, which is now the favored approach.3 Many iterations of the big-bubble technique have followed. The key step in the big-bubble protocol is the use of air injection into the host pre-DM space to facilitate removal of the host stromal tissue. Also critical is to avoid perforating DM. Arguably, these remain the most challenging practical aspects of implementing big-bubble DALK.
Optical Coherence Tomography (OCT) has revolutionized clinical ophthalmology and continues to offer new potential for diagnosis and management of eye disease. In 1994, Izatt et al described the use of OCT for the anterior segment at near-histology level resolution.4 OCT has now become one of the most important diagnostic tests across the ophthalmic subspecialties. Given the potential advantages of imaging during surgery, the translation of OCT to the operating room is a natural progression of the technology. Intraoperative OCT (iOCT), though still in its infancy, has been explored for both anterior and posterior segment surgical applications and lamellar keratoplasty.5–10 The premise behind iOCT is that it provides a unique feedback mechanism for the surgeon providing real-time feedback and may help facilitate the achievement of surgical objectives.
For DALK, iOCT may provide critical information to the surgeon on relative depth information, tissue planes, and optimal instrument localization. This feedback may lead to changes in operative technique and even surgical outcomes. The purpose of this study is to prospectively examine the role of iOCT in DALK in the PIONEER study.
Materials and Methods
PIONEER (ClinicalTrials.gov: NCT02423161) is an IRB-approved prospective iOCT multi-surgeon, single center study examining the feasibility and utility of iOCT in ophthalmic surgery.9 All tenets of the Declaration of Helsinki were followed. Consent for surgery and for participation in the PIONEER study was obtained for all patients. This study represents the DALK subgroup of the PIONEER study. Two surgeons were involved in the DALK portion of the study. A standardized surgeon feedback form and intraoperative time log was utilized to evaluate the impact of iOCT on surgeon decision-making and case time. A custom microscope-mounted system was utilized to obtain iOCT images during DALK utilizing the Bioptigen EnVisu (Bioptigen, Research Triangle Park, NC) portable system with anterior scan head, as previously described.9 A 12 mm x 12 mm volume scan was obtained at each time point. This system has 3.3 μm axial resolution (2.4 μm in tissue) and 3.4mm scan depth (2.5mm in tissue). The handheld probe was mounted to the microscope to facilitate rapid and reproducible imaging with foot pedal control of X-Y-Z directions. The probe angle can be oriented according to surgeon preference. Scans sweep across the cornea such that the entire cornea can be imaged with one capture. These sequential parallel images can be scrolled through to isolate a particular section of interest that correlates clinically to reference line on an en face image.. iOCT images were obtained at surgical milestones throughout the surgery. Specifically, post-host trephination images refer to those captured after the initial partial thickness trephination was completed using a Hessburg-Barron trephine. Pre-big bubble, cannula tunnel images were captured following dissection with the Fogla 27-gauge dissector prior to final placement of the air injection cannula. Following air injection, iOCT images were again captured. Imaging was again performed following dissection of the stromal tissue. Finally imaging was performed following suturing of the donor tissue. Surgical technique adhered to the “Big-bubble technique,” with minor modifications.3 Namely, a Fogla 27-gauge pointed dissector (Bausch & Lomb, Rochester, NY) was used to create a cannula tunnel and a Fogla 27-gauge air injection cannula (Bausch & Lomb) was used for air injection.
For all post-trephination images captured, we measured depth of initial trephination and residual posterior stromal depth. For all post-cannula tunnel creation we measured the distance of the cannula tract to the posterior most aspect of the cornea. All measurements were made using the standard Bioptigen reading software following the surgical procedure. Measurements were taken with the caliper function of the software. Measurements can be performed immediately after obtaining the scan intraoperatively or may be performed later following surgery.
Results
Eighteen eyes (12 right and 6 left) underwent attempted DALK with concurrent iOCT imaging. Seven patients had keratoconus without prior episodes of hydrops. Four had keratoconus with visually significant corneal scarring from presumed hydrops episodes. Big-bubbles were attempted on cases of suspected hydrops. Seven patients had stromal scars from other causes. All 18 eyes had attempted big-bubble. Eleven eyes had successful big-bubbles, while 7 eyes were converted to PKP.
iOCT imaging was successfully obtained in all 18 eyes, although not every eye had imaging performed at each time point. An average of 3.8±0.5 scans were performed per case. The average total additional time used to obtain images was 284±42 seconds. No intraoperative adverse events occurred related to iOCT. From the successful big-bubble cases, we successfully captured iOCT images of post-host corneal trephination (Figure 2), pre-big bubble cannula placement prior to air injection (Figure 3), post-big bubble injection (Figure 4), post-initial anterior stromal bed dissection, prior to entering the pre-DM space (Figure 5), bare DM (Figure 6) and post-suturing of donor graft (Figure 7).
Figure 2.
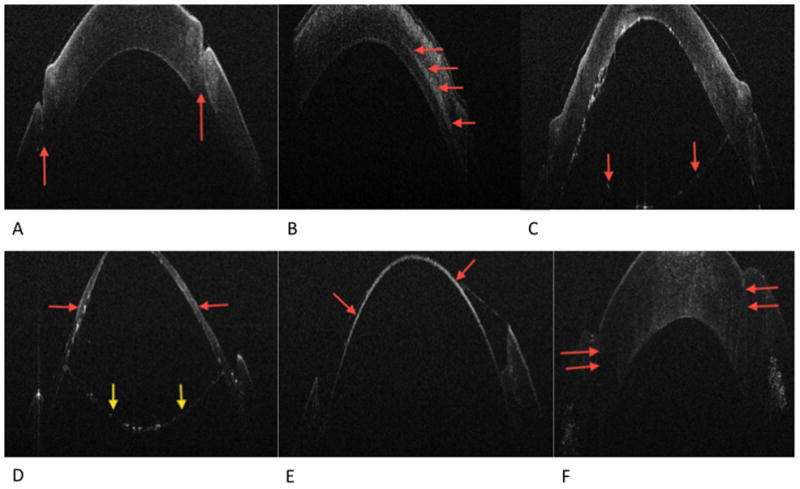
Figure 2A. Post-host partial thickness trephination. The red arrows indicated trephination groove
Figure 2B. Post-cannula tunnel creation. The red arrows indicated cannula tunnel once the Fogla pin was removed
Figure 2C. Post-big-bubble. The red arrows indicated the Descemet’s membrane/endothelial complex separated by the overlying stroma by air
Figure 2D. Post-initial anterior stromal manual dissection. The red arrows indicated residual stroma following the initial anterior lamellar dissection. The yellow arrows indicate the Descemet’s membrane/endothelial complex
Figure 2E. Post final dissection, bared Descemet’s membrane indicated by the red arrows
Figure 2F. Post-graft suturing. The red arrows indicate the graft host stromal apposition. There is an apparent anterior wound gap, which actually represents the donor edge tucked under
Figure 1 shows the post-host trephination tract outlined by a hyper-reflective demarcation line on either side of the tract. Initial trephination groove depth measured 74.8±4.5% depth (n=4) in the completed DALK group versus 64.5±12.6% depth (n=2) in the PKP group (p=0.4). Surgeon feedback forms indicated that in 5 of 9 cases (40%), post-trephination iOCT resulted in a decision to manually extend the trephine cut to a deeper level prior to creating the tunnel.
Figure 1.
Bioptigen iOCT mounted on operating microscope
The cannula tract (Figure 2) is highlighted as a hyper-reflective plane separated from the pre-DM space by a thin layer of residual stromal bed following cannula removal. No correlation between the tract depth and the ability to generate a big bubble was identified.
Figures 4 and 5 show the post-big bubble and post-initial manual anterior stromal dissection images, respectively. The images demonstrate a hyporeflective air bubble in the pre-DM space anterior to a hyperreflective plane bowing posteriorly, representing DM and the endothelium. In these images there is partial loss of interface air such that the big bubble no longer extends across the entire host button. This loss occurred upon removal of the cannula post-air injection to allow imaging. Following stromal dissection (Figure 6), the DM and endothelial cell layer complex is represented by a hyperreflective band. In this same image, a thin hyperreflective line extending from the anterior most trephination site on either side represents balanced salt solution/tear film. This can be seen also in many of the other images. Finally, iOCT images were obtained following graft suturing (Figure 7). The graft-host junction is best identified on the right side of the image as a hyper-reflective line. There is a slight indentation to the left of this line, representing the 10-0 nylon suture indenting the anterior cornea.
Discussion
The application of OCT to surgical techniques may be a useful tool for gauging tissue planes at various steps in DALK surgery utilizing the big-bubble technique. It provides a quantitative assessment of depth of trephination, position of the cannula, location of the big bubble, post-dissection residual stromal bed, and alignment of the graft-host junction. Of the mentioned steps above, the two that appear to be of the greatest utility are the post trephination step and post cannula tunnel step.
Current alternatives to visualizing tissue planes with hand held or microscope mounted slit beams.11 The potential advantages of iOCT over the use of slit beam are several. iOCT presumably offers increased sensitivity with highly opacified corneas with poor visualization. There is typically no need to adjust the angle of the OCT, as you would with a microscope, since an entire corneal “cube” is obtained and can be captured to review individual frames during or after surgery. Also, the use of a handheld microscope can allow breaks in sterility based on the necessary position of the examiners hands and proximity to the sterile field. Small adjustments in the position of the patient’s head are typically all that is needed if the surgeon only wants to capture an iOCT image in the far periphery.
Recently, Sorcia, et al. utilized iOCT to measure trephination depth during DALK in keratoconus patients.12 They found a significant difference in successfully completed big bubble cases favoring a shorter distance between the end of the cannula tunnel and DM. Measuring depth of initial trephination seems to be helpful to determine if the depth is adequate or if there is a need for further manual dissection to achieve ideal depth for subsequent cannula placement. In our series, we observed a 10% greater percentage trephination depth for successfully completed DALK versus PKP. This was not statistically significant, however our study was not powered to detect such a difference. A non-significant trend toward deeper cannula tracts in completed DALK procedures was also observed.
In the future, assessment of the cannula position prior to air injection on attempted big bubble may prove to be a useful application of this modality in the success rate of achieving big bubble. Currently, the ideal depth has not been established, but OCT is the best objective quantitative tool to potentially identify if an ideal depth exists.
Images of the intrastromal tunnel created for cannula placement prior to injection of air were also captured. This allowed for assessment of relative distance of the cannula tip to the pre-DM plane. Real-time, informed alterations to the tract are, therefore, possible as deemed fit before attempted big-bubble. Future studies may be able to more clearly assess the relationship of cannula position prior to big-bubble initiation.
Due to the reflectivity characteristics of current instrumentation, visualization of the instruments within the tract is limited. These issues regarding instrument properties have been previously described utilizing a microscope integrated system.13–15 In this study, the cannula was removed prior to image capture of the tract. This additional step can potentially lead to excessive manipulation of the cannula tract from removal and reinsertion and therefore compromise the seal of the cannula around the tract. Future real-time visualization and microscope integration of OCT systems may help to provide significant improvements in identifying the cannula in the tract.
Following initial injection of air and attempt to achieve a big bubble, images were captured to verify the extent of the air dissection plane through the pre-DM space past the trephination mark Potentially, images at this step could alert the surgeon to sites of inadequate separation of the stromal button from DM. Cases in which a big bubble is not obtained, the iOCT gives information as to how deep the dissection is (i.e. how much residual stroma is left). It is sometimes very difficult to discern how much corneal stroma remains after manual dissection has been initiated. iOCT gives the surgeon the confidence to attempt deeper dissection or possibly attempt another big bubble, when significant stroma is still present. Alternatively, if imaging shows that only minimal posterior stroma remains the surgeon may stop dissection and attach the donor tissue without baring DM completely. Similar to the shadowing effect of the cannula mentioned above, the presence of intrastromal emphysema after air injection was noted to decrease visualization of deeper structures, limiting feedback to the surgeon.
The residual host bed may also be assessed following manual dissection. Bared DM host bed was imaged to verify that complete removal of stromal tissue was achieved. Optimal donor/host apposition was achieved with a completely bared host bed. Finally, following placement and suturing of the host button, we imaged the relative position of the two tissues may be assessed. The junction of the donor stroma to host DM was imaged, to search for potential air separating the two tissues. Optimization of graft-host approximation is yet another possible step that may be aided with this use of iOCT.
The intraoperative imaging system utilized has some important limitations. First, the OCT system was external to the microscope requiring cessation of the surgical procedure. However, this additional time on average amounted to less than 5 minutes per case. Additionally, the external nature of the OCT system does not allow for real-time visualization of the tissue-instrument interaction. A solution to this problem has been previously described in the form of microscope integration of the OCT scanner.13–15 This would eliminate the need pause the surgery, or at least eliminate the need to move the microscope from away from the surgical view.
Microscope integration would also potentially allow for an additional significant advancement in the technique: real-time imaging surgical maneuvers with immediate feedback to the surgeon regarding instrument localization. Both the static nature of OCT scans and instrument properties will need to be addressed to optimize real-time feedback. Techniques to optimize real-time OCT-based visualization of surgical maneuvers have been described utilizing spatial compounding and custom scan sequences to capture near video level feedback.16 Optimized software packages are also needed to improve qualitative and quantitative feedback to the surgeon during the surgical procedure. We are currently actively researching novel instrumentation and feedback mechanisms to help couple this technology to the real-time surgical experience.
Additional study limitations include the small sample size and lack of mandatory scanning at every surgical step. The small patient cohort size had insufficient power to definitively exclude or confirm a difference between groups (e.g. successful big-bubble versus unsuccessful big bubble).
In summary, iOCT seems to offer potential useful intraoperative information, which may be helpful in DALK. Here, we demonstrate successful acquisition of iOCT images at various surgical milestones with high-resolution information of tissue planes and alterations. With improved understanding of the role of iOCT and advancements in integrative OCT technology, iOCT may help facilitate increased success in DALK procedures with reduction in rate of conversion to PKP.
Acknowledgments
Financial support: This work was supported in part by an Ohio Third Frontier Innovation Platform Award from the State of Ohio (JPE, SKS, WJD), NIH R01 EY023381 (WJD), NIH K23 EY022947-01A1 (JPE), Research to Prevent Blindness Challenge and Unrestricted Grants to the Dept. of Ophthalmology, Cleveland Clinic Lerner College of Medicine.
Footnotes
Data previously presented at The Association for Research in Vision and Ophthalmology (ARVO), Seattle, WA, USA, May 7, 2013
Financial Interests: WJD has received research funding from Zeiss and Topcon, who manufacturer OCT devices. JPE and SKS have filed intellectual property and received royalties for the intraoperative mount described in this paper, which has been licensed to Bioptigen. JPE and SKS also serve as consultants for Leica Microsystems, Zeiss Meditec. JPE also serves as a consultant to Bioptigen. JPE and SKS have intellectual properties licensed with Synergetics.
References
- 1.Borderie V, Sandali O, Bullet J, et al. Long-term Results of Deep Anterior Lamellar versus Penetrating Keratoplasty. Ophthalmology. 2012;119:249–255. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 2.Sari ES, Kubaloglu A, Unal M, et al. Deep Anterior Lamellar Keratoplasty versus Penetrating Keratoplasty for Macular Corneal Dystrophy: A Randomized Trial. Am J Ophthalmol. 2013;15:267–274. doi: 10.1016/j.ajo.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Anwar M, Teichman KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 4.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112:1584–9. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 5.Geerling G, Muller M, Winter C, et al. Intraoperative 2-Dimensional Optical Coherence Tomography as a New Tool for Anterior Segment Surgery. Arch Ophthalmol. 2005:253–257. doi: 10.1001/archopht.123.2.253. [DOI] [PubMed] [Google Scholar]
- 6.Knecht PB, Kaufmann C, Menke MN, et al. Use of intraoperative fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am J Ophthalmol. 2010;150:360–365. doi: 10.1016/j.ajo.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Ide T, Wang J, Tao A, Leng T, et al. Ophthalmic Surg Lasers Imaging. Intraoperative use of three-dimensional spectral-domain optical coherence tomography. 2010 Mar;41(2):250–4. doi: 10.3928/15428877-20100303-15. [DOI] [PubMed] [Google Scholar]
- 8.Juthani VV, Goshe JM, Srivastava SK, et al. Association between Transient Interface Fluid on Intraoperative OCT and Textural Interface Opacity After DSAEK Surgery in the PIONEER Study. Cornea. 2014;33(9):887–92. doi: 10.1097/ICO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical Coherence TomogRaphy (PIONEER) Study: 2-year Results. Am J Ophthalmol. 2014;158(5):999–1007. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers JP, Xu D, Kaiser PK, et al. Intrasurgical dynamics of macular hole surgery: An assessment of surgery-induced ultrastructral alteration with intraoperative optical coherence tomography. Retina. 2014;34(2):213–21. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart ZN, Feng MT, Price MO, et al. Handheld slit beam techniques to facilitate DMEK and DALK. Cornea. 2013;32:722–4. doi: 10.1097/ICO.0b013e31827797e7. [DOI] [PubMed] [Google Scholar]
- 12.Scorcia V, Busin M, Lucisano A, et al. Anterior segment optical coherence tomography-guided big-bubble technique. Ophthalmology. 2013;120:471–6. doi: 10.1016/j.ophtha.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52:3115–3317. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers JP, Srivastava SK, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS One. 2014;20:9, e105224. doi: 10.1371/journal.pone.0105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014 Oct;98:1329–32. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers JP, Tao YK, Farsiu S, et al. Visualization of real-time intraoperative maneuvers with spectral domain optical coherence tomography system. Retina. 2013;33:232–6. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]