Abstract
Background
Desmoplastic small round cell tumor (DSRCT) is a very uncommon soft tissue tumor of children and young adults. It has an aggressive course with generally poor survival. In general, the assessment of tumor burden and response has relied upon CT and/or MRI. However these tumors are often metabolically active and can be evaluated using FDG PET/CT imaging.
Objective
The purpose of this study was to determine the metabolic activity of DSRCTs using FDG PET/CT imaging and potential utility of FDG PET/CT imaging in this disease.
Methods
Eight patients (7 male,1 female; ages 2–20 years, median 11 years) with confirmed DSRCT underwent 82 PET/CT scans. PET/CT was used for initial staging (7 patients, 8 scans), monitoring response to therapy (8 patients, 37 scans) and for surveillance of DSRCT recurrence (6 patients, 37 scans).
Results
Each scan performed at diagnosis showed abnormally elevated uptake in the primary tumor. 5 patients had abdominal pelvic involvement, and 2 of those also had thoracic disease. 6 patients whose scans showed no abnormal sites of uptake at the end of therapy have had progression free survivals from 2 – 10 years. One patient whose scan continued to show uptake during treatment died of disease 1.3 years from diagnosis. A 2nd patient with persistent uptake remains in treatment 3 years after initial diagnosis. One surveillance scan identified recurrent disease.
Conclusions
FDG PET CT identified elevated metabolic activity in each patient studied. Despite our small patient sample size, FDG PET/CT scans appear useful for the management of patients with DSCRT. Patients whose studies become negative during or following treatment may have prolonged remissions.
Keywords: tomography, diagnostic imaging, desmoplastic small round cell tumor, PET/CT, FDG, pediatrics
INTRODUCTION
Desmoplastic small round cell tumor (DSRCT) is a member of the small round cell family of soft tissue sarcomas initially described in 1989 by Gerald and Rosai [1]. DSRCT typically affects adolescents and young adults, with a 4 times higher incidence in males. The tumors are characterized by the presence of small, round, blue cells with polyphenotypic differentiation, surrounded by desmoplastic stroma. DSRCT is characterized by a reciprocal translocation between chromosomes 11 and 22 [t(11:22)(p13;q12)], which creates a fusion transcript of the EWS and WT1 genes. The tumors express a variety of histologic markers, with the most common being desmin, keratins (AE1, AE3, and CAM5.2), vimentin, and epithelial membrane antigen [2]. These tumors most frequently arise in the abdomen and pelvic region but also can also occur in other sites, including the scalp, sinus cavities, hands, and paratesticular region [3–6]. DSRCT is an aggressive disease and has a propensity to spread within the peritoneum and omentum and to metastasize to sites including lymph nodes, lungs, and liver [3]. For this reason, the prognosis for DSRCT remains poor, with a mean survival less than 3 years [7].
Diagnosis and staging of DSRCT has relied heavily upon anatomic imaging [8]. Computed tomography (CT) and magnetic resonance imaging (MRI) are useful for identifying sites of disease, but they are limited in their ability to show the viability and metabolic activity of tumors.. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) combined with CT imaging has been well defined as a useful imaging modality for identifying primary tumors of unknown origin and metastases and for monitoring tumor response to therapy as well as recurrence in several other pediatric malignancies, including sarcomas [9–11]. Although FDG PET/CT has been used to assess DSRCT [8, 12–17], a definitive role for functional imaging in this disease has not yet been well established. In previously reported cases of DSRCT, FDG PET/CT has been shown to be a valuable tool for evaluation of disease. In two studies, FDG PET/CT revealed DSRCT metastases that were not detectable by CT alone [15]. In a publication by Rosoff et al.[14], a pelvic mass that was visible by CT imaging after treatment was no longer FDG-avid, providing valuable additional information about the metabolic state of the tumor not measurable by anatomic imaging. Further complicating matters, because of the rarity of the disease, there is no formal staging system for DSRCT. Hayes-Jordan et al. [18] proposed a staging system for DSRCT using the Peritoneal Cancer Index, but this method has not yet been validated.
We hypothesized that FDG PET/CT imaging could lead to more accurate assessment of DSRCT and could become a useful diagnostic tool for disease staging and evaluation. To test this hypothesis, we undertook a retrospective review of the records of patients with this rare disease who underwent FDG PET/CT scans at our institution.
METHODS
To assess the metabolic activity of DSRCT lesions and to determine the potential utility of FDG PET/CT in the management of patients with DSRCT, we conducted a retrospective study of patients with DSRCT who underwent FDG PET/CT scans from April 2002 until July 2014. Patient records were reviewed for demographic data as well as treatment, imaging, pathology, and outcome data. We compared the findings from FDG PET/CT scans with anatomic imaging results to determine the ability of FDG PET/CT to identify DSRCT lesions, verify tumor response to therapy, and assess patient outcome based on the changes in metabolic activity of lesions during therapy.
This study was approved by the St. Jude Children’s Research Hospital institutional review board, and data management was in compliance with the Health Insurance Portability and Accountability Act of 1996.
Patients
We identified 8 patients treated at St. Jude during the study interval with biopsy-proven DSRCT who underwent FDG PET/CT (Table 1). Each patient’s disease was diagnosed based upon clinical, histologic, immunohistochemical, and genetic criteria. Seven of the 8 tumors were positive for the EWS/WT1 gene rearrangement characteristic of DSRCT. The cohort consisted of 7 males and 1 female ranging from 2 to 20 years of age (median 11 years) at the time of diagnosis. There were 82 scans in total. Six patients (7 scans) underwent PET/CT scans at the time of initial diagnosis, and 7 patients (37 scans) were studied to assess response to treatment. Six patients (37 scans) were studied to monitor for residual or recurrent disease after treatment. Patients underwent 2–17 scans each (mean 10, median 8). Patient #1 has been described in a previous study [19]. All studies were performed for clinical management as requested by the treating physician
| Patient | Gender | Age at 1st PET scan |
Reason for PET scan |
Sites of disease | SUVmax | Disease course | ||
|---|---|---|---|---|---|---|---|---|
| Staging # of scans |
Response evaluation # of scans |
Surveillance # of scans |
||||||
| 1 | M | 4y | 0 | 1 | 7 | Abdomen | not applicable | 6.4 at diagnosis Followup study 4 months post surgery showed no uptake to indicate No clinical or radiographic evidence of disease at 10 years after diagnosis |
| 2 | M | 11y | 1 | 3 | 13 | Abdomen-pelvis, liver | 10.0 at staging declined to 3.3 prior to surgery | no clinical or radiographic evidence of disease at 7.5 years after diagnosis |
| 3 | M | 11y | 1 | 4 | 0 | Abdomen, thorax | 10.7 at diagnosis declined to 5.5 during treatment | persistent disease following chemotherapy and radiotherapy died of disease 1.3 years after diagnosis |
| 4 | M | 20y | 1 | 3 | 8 | Supraclavicular mass | 11.7 at diagnosis declined to 4.6 before surgery | no clinical or radiographic evidence of disease at 6 years after diagnosis |
| 5 | M | 20y | 1 | 6 | 2 | Abdomen | 19.1 at diagnosis declined to 8.6 during prior to resection | no clinical or radiographic evidence of disease at 4 years after diagnosis |
| 6 | M | 2y | 1 | 1 | 0 | Right orbit | 6.4 at diagnosis Followup study 4 months post surgery showed no uptake to indicate recurrence | no clinical or radiographic evidence of disease at 3 years after diagnosis |
| 7 | M | 16y | 1 | 11 | 3 | Abdomen-pelvis | Studies became negative during treatment; Surveillance studies showed uptake adjacent to liver; 2 biopsies showed inflammatory changes; subsequently developed pulmonary involvement Progressive disease 3 years after diagnosis |
|
| 8 | F | 20y | 2 | 8 | 4 | Abdomen-pelvis thorax | 15.6 at diagnosis (perisplenic lesion), 3.0 prior to surgery | extensive disease at presentation no clinical or radiographic evidence of disease at 2 years after diagnosis |
| Total scans | 8 | 37 | 37 |
Imaging Studies
Five scans were referred studies from outside institutions with varying imaging techniques. For FDG PET/CT studies conducted at St. Jude, 5.5 MBq/kg FDG (maximum 444 MBq) was injected intravenously after either overnight fasting or a minimum of 4 hours of fasting for afternoon scans. Blood glucose was found to be normal before FDG injection. Patients were kept in a quiet, dark room after injection and were instructed to relax with their arms at their sides in a recumbent position. Approximately 1 hour later, attenuation correction and lesion localization by transmission CT images and PET emission images were acquired using a GE Discovery LS PET/CT system (GE Medical Systems, Waukesha, WI). CT acquisition parameters were: tube rotation 0.8 second, slice thickness 0.5 cm, table speed 1.5 cm/rotation, pitch 1.5:1, 120 kV, 90 mA, with dose modulation. Whole body PET images were obtained from the top of skull to the feet for 5 minutes per bed position in two-dimensional mode. All but one scan included extremities. Scans obtained after July 2011 were acquired on a GE Discovery 690 PET/CT system. Those emission images were acquired in three-dimensional mode.
Image Analysis
Images were reconstructed using standard vendor-supplied software. Two experienced diagnostic imaging physicians (BLS, MBM) without knowledge of clinical information reviewed the FDG PET/CT images. Regions of interest were drawn around areas with uptake greater than that of the surrounding tissue that could not be attributed to accumulation in a normal structure. There was no minimum size for regions of interest, and the size of tumor activity determined the regions of interest. SUVmax was measured by examining the tumor activity in the region of interest over multiple slices. FDG uptake by brown adipose fat was identified by CT scan by the typical location and low attenuation of the tissue. MRI and CT studies were reviewed by an experienced pediatric radiologist (MBM) blinded to the results of the studies and pertinent clinical information.
RESULTS
A summary of patient information and imaging results is presented in Table 1.
Studies at diagnosis (7 patients, 8 scans): Of the 7 patients with DSRCT who underwent PET/CT studies, each had abnormal uptake in FDG in tumor foci (patient #8 underwent repeat scanning as the outside referred study did not include the extremities). At initial staging, 6 had widespread disease and 2 had localized disease. Of those with extensive disease, 3 had abdominal-pelvic involvement only (patients # 1, 2, 5, 7), 2 had thoracic (mediastinal-hilar in both, pulmonary nodules in one) and abdominal-pelvic disease. Patient #2 also had liver metastases. Of the 2 patients with localized disease (#4,6), one had neck disease only, and one orbital involvement only. Patient #2, presented in Figure 1, was first scanned at the initiation of chemotherapy and showed marked uptake in four abdominal masses, one pelvic mass, and one hepatic metastasis. These tumors were all intensely FDG-avid, with SUVmax for each mass ranging from 6.3 to 10.0.
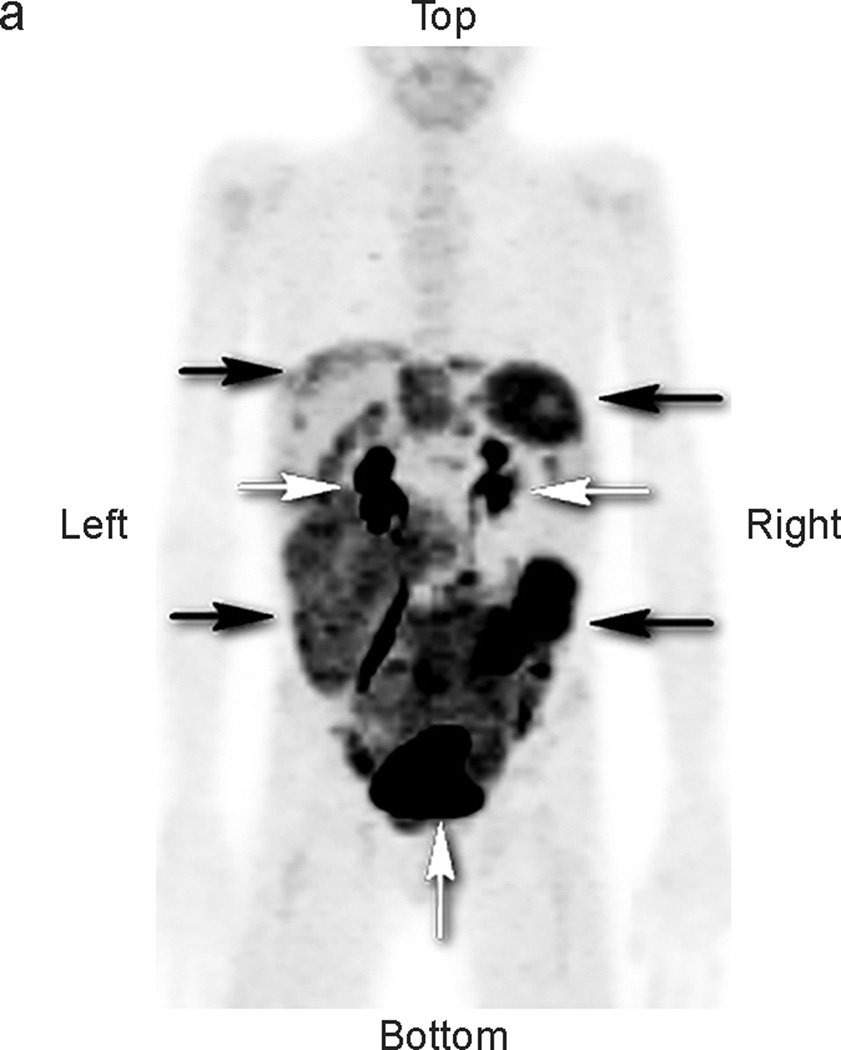
Fig 1.
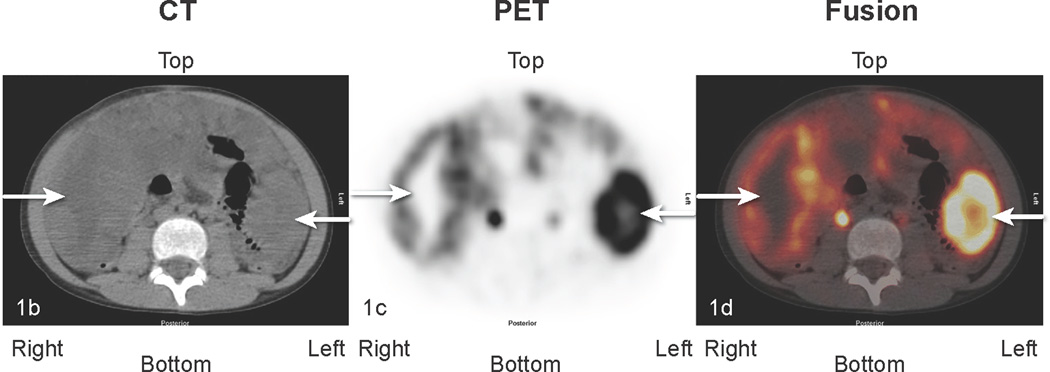
Patient #2: 11-year-old boy with DSRCT showing widespread abdominal and pelvic FDG uptake that decreased in serial scans. a) Anterior maximal intensity projection image (MIP). There is widespread abnormal uptake throughout the abdomen and pelvis (black arrows). Renal excretion and bladder accumulation are noted (white horizontal arrows – kidneys; white vertical arrow at bottom of image – bladder. b,c,d: transverse CT, PET, and fusion images of the mid abdomen. There is extensive tumor present. The white arrows pointing to the right indicate an area of low attenuation on the CT image, corresponding to areas without uptake of FDG on the PET image. The white arrows pointing to the left in each image identify an area of intense tumor uptake.
Studies for response to treatment (8 patients, 37 scans): 8 of the patients with DSRCT underwent PET CT scans (range 1 to 11) to evaluate response to treatment, including surgery. 6 patients had at least one abnormal study during treatment, while in patient #6, the only study performed after staging showed no abnormal uptake that could be attributed to residual or recurrent tumor following chemotherapy and surgery. Patient #2 underwent 3 studies during therapy. These showed progressive decline in uptake (SUVmax 10 at diagnosis, 6.5 at 1 month, 3.3 at 3 months, and no detectable tumor uptake at 5 months, following resection). Pathologic examination of the resected tumor showed evidence of treatment effect, which correlates with the tumor’s loss of FDG avidity, as seen in other studies [20, 21]. After resection, anatomic imaging was unable to distinguish residual disease from postoperative change. PET/CT imaging showed no abnormal uptake while the patient continued chemotherapy and radiation treatment. The patient is clinically free of disease 7.5 years following presentation.
Figure 2 shows persistent disease in patient #3 at 11 months following presentation, during chemotherapy and radiation therapy. The patient did not enter clinical remission and died of disease 1.3 years after diagnosis. Surveillance for disease recurrence (6 patients, 37 scans): 6 patients had followup PET CT scans (range 2–14) to assess for recurrent disease up to 5 years after presentation. The studies of patient #7 showed both false positive and true positive findings (Figure 3). Three studies showed abnormal uptake adjacent to the liver (SUVmax 6.2) that was biopsied twice and showed inflammation and necrosis. One of these scans showed abnormal uptake in the left upper thorax (SUVmax 7.1), surgically removed and confirmed to represent a lung metastasis. Otherwise, 34 scans in the remaining 6 patients showed no evidence for recurrent tumor and these patients (#1,2,4,5,6,8) remain in clinical remission 2–10 years from diagnosis.
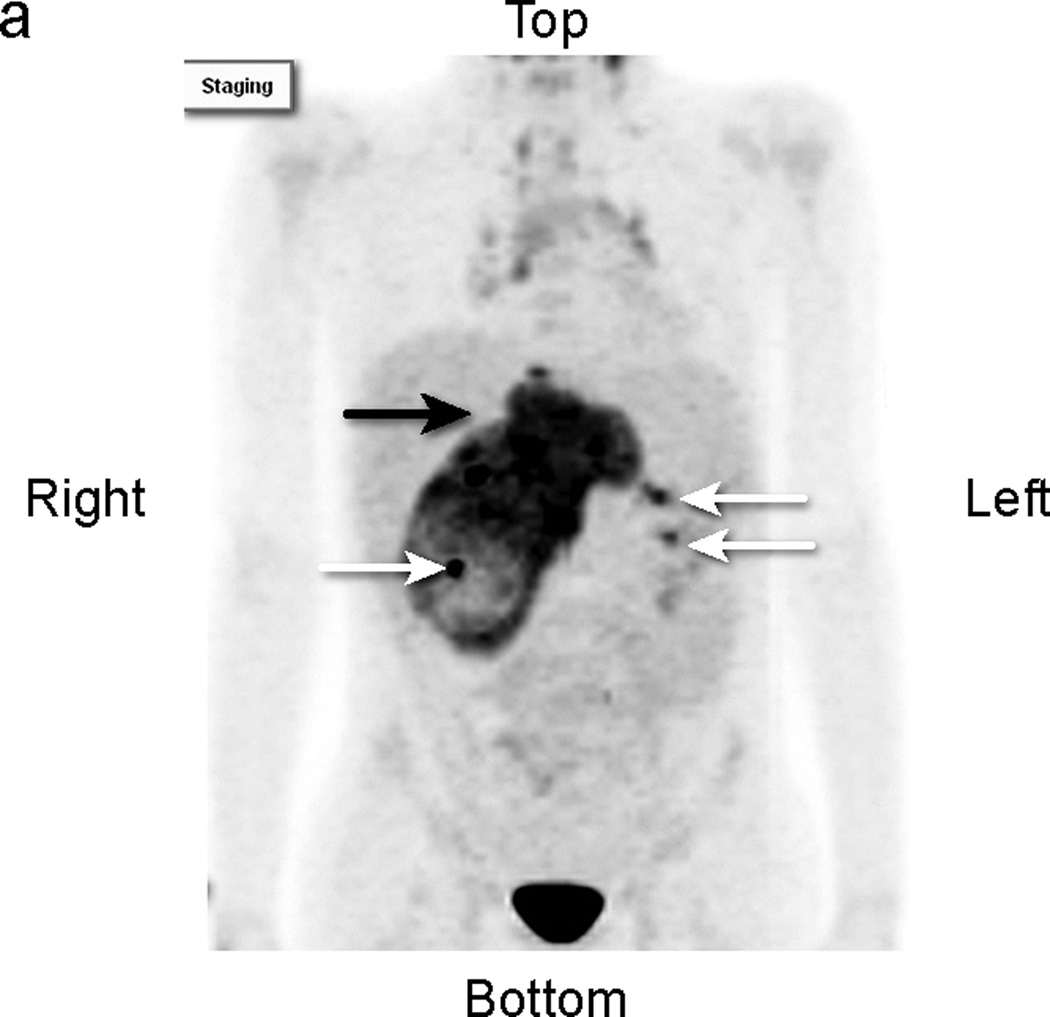
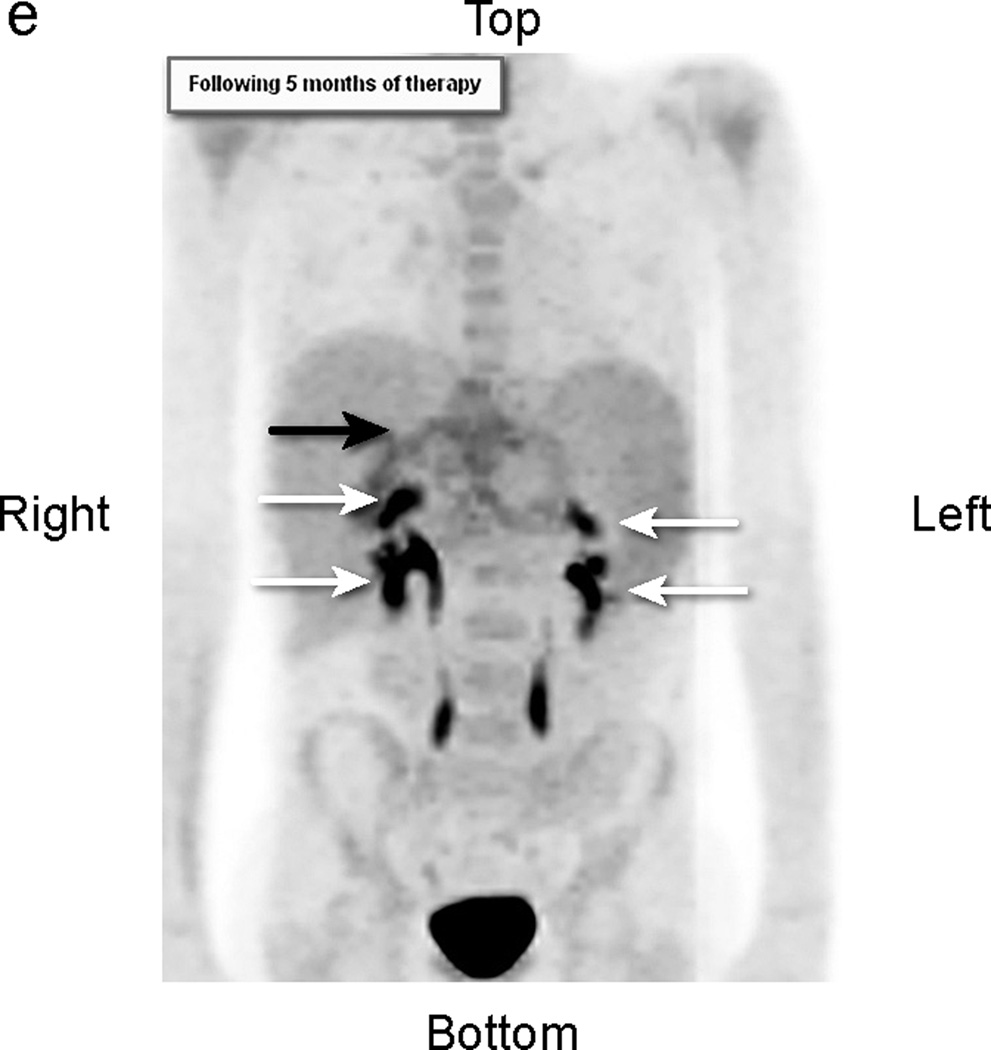
Fig 2.
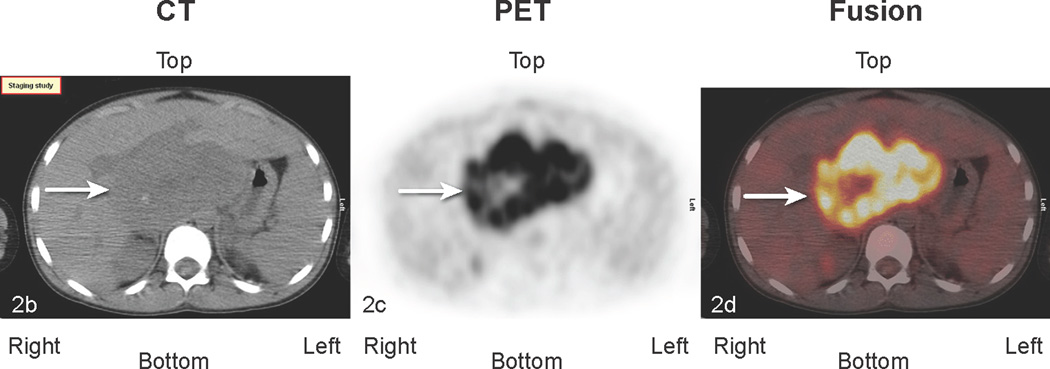
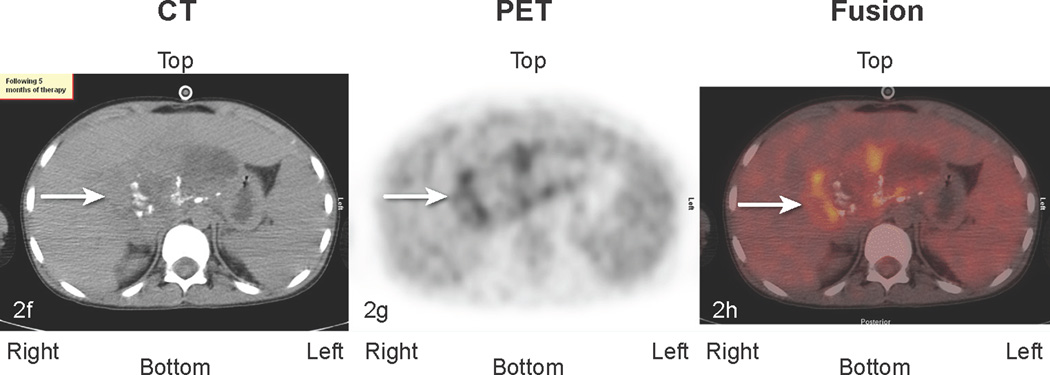
Patient # 3: 11-year-old boy with DSRCT and FDG-avid mass in his abdomen and thoracic lymph nodes. Anterior MIP image from FDG PET/CT scans at a) initial staging shows a very large accumulation of intense activity (black arrow) in the right side of the abdomen and scattered foci of uptake in the chest. White arrows point to renal activity. b,c,d) transverse CT, PET, and fusion images of the mid abdomen show a large area of markedly elevated uptake (white arrows). e) Anterior MIP image shows considerable reduction in mid abdominal activity (black arrow). White arrows show renal accumulation. f,g,h). Transverse CT, PET, and fusion images of the mid abdomen show residual uptake (white arrows) within the central tumor mass, partly calficied. The extent and intensity of uptake have declined considerably.
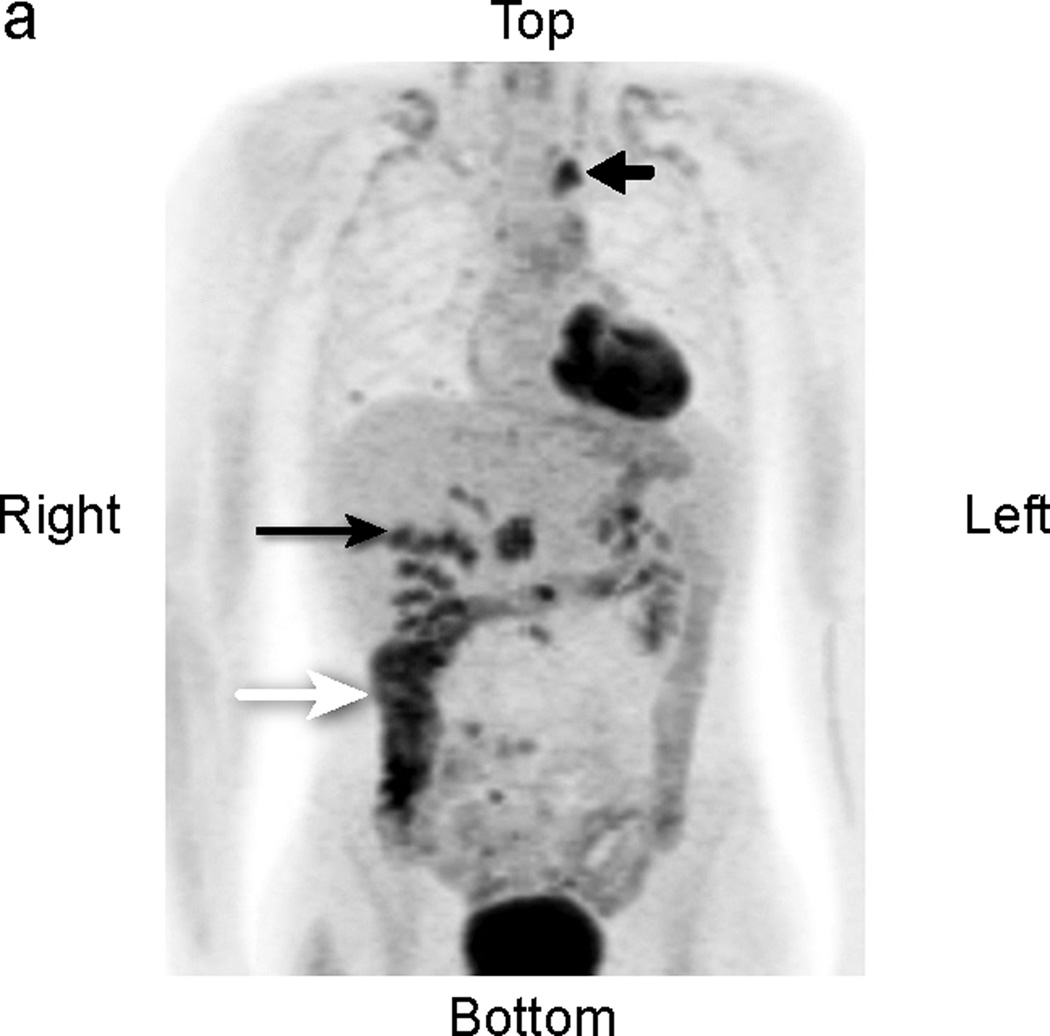
Fig 3.
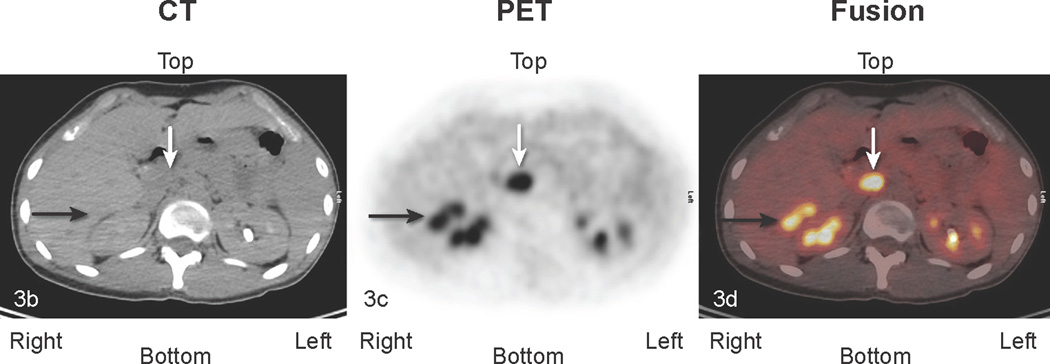
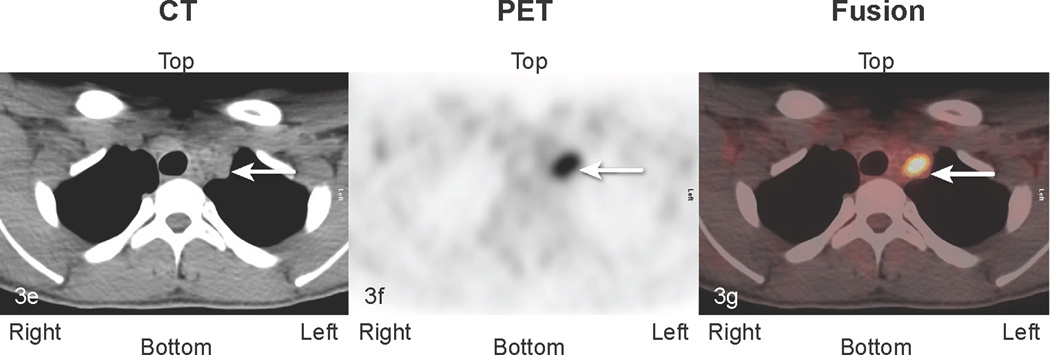
Patient # 7:. 16 year old boy with abdominal DSCRT. True positive and false positive findings 1.5 years following excision of abdominal disease. a) Anterior MIP image: The lower black arrow identifies a site of uptake suspicious for tumor medial to the liver (transverse sections b, c, d below). The top black arrow points to abnormal uptake in the chest (transverse sections e, f, g below). The white arrow depicts physiologic ascending colon uptake. b,c,d:) transverse CT, PET, and fusion images. The black arrow identifies abnormal perihepatic accumulation of tracer. This was biopsied twice and showed inflammatory changes (false positive finding). The white arrow in the midsection of each image points to an area of abnormal uptake. Although this area was not biopsied, it enlarged in subsequent studies consistent with other areas of disease progression (true positive clinically). e, f, g. White arrows point to an area of uptake subsequently excised and shown to represent metastatic DSRCT (true positive).
DISCUSSION
Our retrospective review showed that FDG PET/CT was able to demonstrate metabolic activity in 7 of 8 patients with clinically suspected DSRCT. For those 7 patients, most tumors were intensely FDG-avid when initially imaged, and this avidity was dramatically reduced or completely resolved in the 5 patients who would eventually reach a clinical remission after surgery, although briefly for patient #7. In all of these patients, no new masses identifiable by FDG PET/CT arose after the initial staging study. Only one DSRCT mass, in patient #6, appeared weakly FDG-avid qualitatively at initial presentation due to close proximity to cerebral cortex, but SUVmax of 6.4 confirmed tumor avidity.. Thus, baseline assessment of tumor uptake illustrates metabolic heterogeneity between DSRCT tumors that cannot be seen using other imaging modalities.
Of the three patients who had disease progression, FDG avidity decreased in patient #3 in serial scans, although not as dramatically as in patients who would eventually go into remission. Although evidence of necrosis was present on anatomic and metabolic imaging, FDG avidity did persist in these tumors, and new abdominal, pelvic, and lymph node foci emerged in the final study of this patient.. Patient #7achieved a short term metabolic response followed by disease recurrence and disease stabilization after additional cancer directed therapy.
The uptake of FDG in other soft-tissue sarcomas has been shown to correlate with tumor response to chemotherapy and has even been shown to be a better predictor of histopathologic response than change in tumor size [20, 22–24]. In our study, tumors in four patients that showed reduced FDG uptake in serial PET/CT studies had histologic evidence of treatment effect when analyzed after resection. In patients #8 and #9, histopathologic analysis revealed that the tumors resected after chemotherapy and after they had decreased FDG uptake were less cellular than the original biopsy samples. Analysis of patient #8 also noted that the resected tumor tissue contained more macrophages and had a lower nuclear grade, lower proliferation rate, and increased calcification. These indications of treatment effect are consistent with a loss of FDG avidity after tumors were intensely FDG-avid (SUVmax 9.7) at initial staging. These findings suggest that decreased tumor uptake of FDG correlates with therapeutic response as determined by histopathologic analysis.
In the four patients (#2, #4, #5, and #8) who had FDG-avid tumors initially and reached a clinical remission after surgery, a dramatic decrease in FDG uptake was observed in sequential studies. For example, the SUVmax of the supraclavicular mass in patient #4 decreased from 10.7 to 4.6 after 2 months of chemotherapy. To date, there has been no evidence of residual disease or recurrence after surgical resection 7 years ago. All other patients in this group had decreases in tumor FDG avidity prior to surgical resection, with similar clinical outcomes.. This could indicate that a prolonged remission is more likely to be achieved if the DSRCT tumors become less FDG-avid in response to therapy prior to resection. Patient #7 had recurrent disease after completion of treatment. FDG PET/CT was used to assist selecting a biopsy site and for restaging. Treatment is ongoing.
Because DSRCT is a very rare disease, the cohort for this retrospective review was quite small Our series may be somewhat skewed compared with that of Arora et al [25] in regards to location of disease and patient age. In that study, 62 of 65 patients (95%) had primary abdominal-pelvic involvement and only 3 (5%) had extra-abdominal primary tumor sites, two in bone and one in muscle. In our series, of the six patients studied prior to therapy, four (67%) had abdominal pelvic disease and two (33%) extra abdominal primary tumors (orbital, supraclavicular). Only one patient in our series had hepatic involvement (13%) while 43% of the Avora series had hepatic metastases. The mean age of patients described in the Avora series was 19.5 years, and in our series 11.3 years. Both studies showed male predominance (80% and 87% respectively). To more confidently conclude that FDG PET/CT findings can indicate therapeutic response and clinical outcome, more comprehensive studies are needed to determine whether similar trends can be seen in other cases of DSRCT. These findings are, however, consistent with those of other studies involving FDG PET/CT evaluation of sarcomas that indicate that tumor FDG uptake can be an indicator of prognosis [26, 27]. In a recent report, Arora et al. [25] described their experience using FDG PET/CT in the staging and followup of 11 patients with DSRCT. In an organ-based analysis using biopsy or serial CT or MRI scans, disease was correctly staged using PET/CT in 225 of 231 locations (21 areas in each patient). Sensitivity, specificity, and positive and negative predictive values were >96%. Our study largely supports the use of PET/CT for staging and adds documentation of the clinical utility of the technique at followup in the management of patients.
I Although a lesion by lesion analysis of FDG PET CT was not performed, FDG PET/CT scans in our study allowed identification of sites of increased metabolic activity that were not noted during examination of anatomic imaging. These findings included a hepatic focus in one patient’s initial study, a bony focus in another patient, and lymph node uptake in the initial study of a third patient. These findings were not confirmed by biopsy, and many were not seen on later scans, so it is difficult to determine their relevancy. However, other reported PET/CT studies of patients with DSRCT have been able to identify lesions not visible by other imaging modalities [12]. In our study, the principal use of FDG PET CT scans at diagnosis was to provide a baseline assessment of metabolic activity for comparison with studies performed to assess tumor shrinkage in response to treatment effect. The combination of FDG PET CT and diagnostic contrast enhanced CT was used in treatment assessment on an individualized basis.
In studies obtained at our institution after DSRCT resection, PET/CT was particularly useful for assessing the likelihood of tumor recurrence when anatomic imaging findings were inconclusive. In multiple surveillance scans, corresponding anatomic imaging showed areas that might indicate residual or recurrent disease. In each of these cases, the additional information provided by metabolic imaging prevented the misdiagnosis of disease relapse..
There were few “false positive” findings. Patient #2 had changes of radiation pneumonitis. The perihepatic mass of patient #7, is described in Figure 3. False positive findings are not uncommonly seen in FDG PET/CT studies and have been previously reported [28, 29].
Our study has limitations in several aspects. The patient sample was small and may not be representative of the disease at large. Patient therapy was individualized and thus not uniform. PET/CT scans were obtained as part of the clinical management of patients, possibly more frequently acquired in patients believed at higher risk for recurrence. Uptake of FDG between and within tumors was quite variable, potentially limiting the utility of the PET/CT technique, particularly in determining response to therapy in areas of tumor that had low initial metabolic activity.
CONCLUSION
Our review of DSRCT patients showed that FDG PET/CT scans were a useful complement to anatomic imaging for disease assessment. Although there were a limited number of patients in this retrospective review, the data suggest that FDG PET/CT allowed effective identification of DSRCT lesions and accurately depicted the response of tumors to therapy. Overall, these findings indicate that FDG PET/CT imaging is a valuable supplement to anatomic imaging for DSRCT evaluation, and further multiinstitutional studies examining the ability of FDG PET/CT findings to predict prognosis could be valuable for future disease management.
ACKNOWLEDGMENTS
The authors thank Sandra Gaither for secretarial expertise and David Galloway of Scientific Editing for editorial support. The authors are grateful to the Molecular Imaging Research laboratory staff for production of [18F]FDG.
Funded in part by Award Number 5R25CA023944 from the National Cancer Institute (AO) and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatric pathology / affiliated with the International Paediatric Pathology Association. 1989;9:177–183. doi: 10.3109/15513818909022347. [DOI] [PubMed] [Google Scholar]
- 2.Gerald WL, Ladanyi M, de Alava E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:3028–3036. doi: 10.1200/JCO.1998.16.9.3028. [DOI] [PubMed] [Google Scholar]
- 3.Lae ME, Roche PC, Jin L, et al. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. The American journal of surgical pathology. 2002;26:823–835. doi: 10.1097/00000478-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Gonzalez J, Villanueva C, Fernandez-Acenero MJ, et al. Paratesticular desmoplastic small round cell tumor: case report. Urologic oncology. 2005;23:132–134. doi: 10.1016/j.urolonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Tison V, Cerasoli S, Morigi F, et al. Intracranial desmoplastic small-cell tumor. Report of a case. The American journal of surgical pathology. 1996;20:112–117. doi: 10.1097/00000478-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Finke NM, Lae ME, Lloyd RV, et al. Sinonasal desmoplastic small round cell tumor: a case report. The American journal of surgical pathology. 2002;26:799–803. doi: 10.1097/00000478-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Gil A, Gomez Portilla A, Brun EA, et al. Clinical perspective on desmoplastic small roundcell tumor. Oncology. 2004;67:231–242. doi: 10.1159/000081323. [DOI] [PubMed] [Google Scholar]
- 8.Kis B, O'Regan KN, Agoston A, et al. Imaging of desmoplastic small round cell tumour in adults. The British journal of radiology. 2012;85:187–192. doi: 10.1259/bjr/57186741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarville MB, Christie R, Daw NC, et al. PET/CT in the evaluation of childhood sarcomas. AJR American journal of roentgenology. 2005;184:1293–1304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 10.Wegner EA, Barrington SF, Kingston JE, et al. The impact of PET scanning on management of paediatric oncology patients. European journal of nuclear medicine and molecular imaging. 2005;32:23–30. doi: 10.1007/s00259-004-1645-3. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi U, Hosono A, Makimoto A, et al. Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. Journal of pediatric hematology/oncology. 2007;29:608–612. doi: 10.1097/MPH.0b013e318142b5ab. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Sellem D, Liu KL, Cimarelli S, et al. Desmoplastic small round cell tumor: impact of F-FDG PET induced treatment strategy in a patient with long-term outcome. Rare tumors. 2009;1:e19. doi: 10.4081/rt.2009.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrakopoulou-Strauss A, Hohenberger P, Strobel P, et al. A recent application of fluoro- 18-deoxyglucose positron emission tomography, treatment monitoring with a mammalian target of rapamycin inhibitor: an example of a patient with a desmoplastic small round cell tumor. Hellenic journal of nuclear medicine. 2007;10:77–79. [PubMed] [Google Scholar]
- 14.Rosoff PM, Bayliff S. Successful clinical response to irinotecan in desmoplastic round blue cell tumor. Medical and pediatric oncology. 1999;33:500–503. doi: 10.1002/(sici)1096-911x(199911)33:5<500::aid-mpo12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Kushner BH, Laquaglia MP, Gerald WL, et al. Solitary relapse of desmoplastic small round cell tumor detected by positron emission tomography/computed tomography. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4995–4996. doi: 10.1200/JCO.2008.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilera D, Hayes-Jordan A, Anderson P, et al. Outpatient and home chemotherapy with novel local control strategies in desmoplastic small round cell tumor. Sarcoma. 2008;2008:261589. doi: 10.1155/2008/261589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WD, Li CX, Liu QY, et al. CT, MRI, and FDG-PET/CT imaging findings of abdominopelvic desmoplastic small round cell tumors: correlation with histopathologic findings. European journal of radiology. 2011;80:269–273. doi: 10.1016/j.ejrad.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Hayes-Jordan A, Green H, Fitzgerald N, et al. Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. Journal of pediatric surgery. 2010;45:1000–1006. doi: 10.1016/j.jpedsurg.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Saab R, Khoury JD, Krasin M, et al. Desmoplastic small round cell tumor in childhood: the St. Jude Children's Research Hospital experience. Pediatric blood & cancer. 2007;49:274–279. doi: 10.1002/pbc.20893. [DOI] [PubMed] [Google Scholar]
- 20.Evilevitch V, Weber WA, Tap WD, et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:715–720. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- 21.Bajpai J, Kumar R, Sreenivas V, et al. Prediction of chemotherapy response by PET-CT in osteosarcoma: correlation with histologic necrosis. Journal of pediatric hematology/oncology. 2011;33:e271–e278. doi: 10.1097/MPH.0b013e31820ff78e. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins DS, Rajendran JG, Conrad EU, 3rd, et al. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–3284. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 23.Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44:930–942. [PubMed] [Google Scholar]
- 24.Hamada K, Tomita Y, Inoue A, et al. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Annals of nuclear medicine. 2009;23:89–95. doi: 10.1007/s12149-008-0213-5. [DOI] [PubMed] [Google Scholar]
- 25.Arora VC, Price AP, Fleming S, et al. Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol. 2013;43:93–102. doi: 10.1007/s00247-012-2485-0. [DOI] [PubMed] [Google Scholar]
- 26.Fuglo HM, Jorgensen SM, Loft A, et al. The diagnostic and prognostic value of 18F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. European journal of nuclear medicine and molecular imaging. 2012;39:1416–1424. doi: 10.1007/s00259-012-2159-z. [DOI] [PubMed] [Google Scholar]
- 27.Baum SH, Fruhwald M, Rahbar K, et al. Contribution of PET/CT to prediction of outcome in children and young adults with rhabdomyosarcoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:1535–1540. doi: 10.2967/jnumed.110.082511. [DOI] [PubMed] [Google Scholar]
- 28.Asad S, Aquino SL, Piyavisetpat N, et al. False-positive FDG positron emission tomography uptake in nonmalignant chest abnormalities. AJR American journal of roentgenology. 2004;182:983–989. doi: 10.2214/ajr.182.4.1820983. [DOI] [PubMed] [Google Scholar]
- 29.Chang JM, Lee HJ, Goo JM, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean journal of radiology : official journal of the Korean Radiological Society. 2006;7:57–69. doi: 10.3348/kjr.2006.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]