Abstract
Background
Based on rodent studies, Group II metabotropic glutamate receptors (mGluR2 & 3) were suggested as targets for addiction treatment. However, LY379268 and other Group II agonists do not discriminate between the mainly presynaptic inhibitory mGluR2 (the proposed treatment target) and mGluR3. These agonists also produce tolerance over repeated administration and are no longer considered for addiction treatment. Here, we determined the effects of AZD8529, a selective positive allosteric modulator (PAM) of mGluR2, on abuse-related effects of nicotine in squirrel monkeys and rats.
Methods
We first assessed modulation of mGluR2 function by AZD8529 using functional in-vitro assays in both membranes prepared from a cell line expressing human mGluR2 and in primate brain slices. We then determined AZD8529 (0.03-10 mg/kg, i.m.) effects on intravenous nicotine self-administration and reinstatement of nicotine seeking induced by nicotine priming or nicotine-associated cues. We also determined AZD8529 effects on food self-administration in monkeys and nicotine-induced dopamine release in accumbens shell in rats.
Results
AZD8529 potentiated agonist-induced activation of mGluR2 in both the membrane-binding assay and in primate cortex, hippocampus, and striatum. In monkeys, AZD8529 decreased nicotine self-administration at doses (0.3-3 mg/kg) that did not affect food self-administration. AZD8529 also reduced nicotine priming- and cue-induced reinstatement of nicotine seeking after extinction of the drug-reinforced responding. In rats, AZD8529 decreased nicotine-induced accumbens dopamine release.
Conclusions
Our results provide evidence for efficacy of PAMs of mGluR2 in non-human primate models of nicotine reinforcement and relapse. We propose that this drug class should be considered for nicotine addiction treatment.
Keywords: allosteric modulation, glutamate, nicotine, relapse, self-administration, smoking cessation
Introduction
Tobacco smoking, the leading cause of preventable death, is primarily driven by nicotine (1, 2). There are currently several medications for smoking cessation (varenicline, bupropion, nicotine replacement ) but high relapse rates are observed both during and after treatment (3-5). Thus, novel treatments to prevent relapse are needed.
In rodents, manipulations of metabotropic glutamate receptors (mGluRs) decrease nicotine-induced potentiation of brain stimulation reward and nicotine withdrawal symptoms (6). Based on these and related findings, Group II mGluRs have been considered potential targets for nicotine addiction treatment (7). Group II mGluRs consists of mGluR2 and mGluR3 subtypes (8). mGluR2s are expressed primarily on presynaptic glutamate neurons and their activation leads to decreased evoked glutamate release (8, 9). mGluR3s are expressed on postsynaptic and presynaptic neurons and on glia (8, 10). The prototype drug used to assess the function of Group II mGluRs is LY379268, an orthosteric agonist that binds to both mGluR2s and mGluR3s (8, 11),
In rats, systemic injections of LY379268 or related mGluR2/3 agonists decrease discriminative-cue-induced reinstatement of cocaine seeking and context-induced reinstatement of heroin seeking (12, 13), decrease discrete cue-induced reinstatement of heroin seeking (14), cocaine priming-induced reinstatement (15), spontaneous recovery of alcohol seeking (16), cue-induced cocaine seeking (17), and discrete cue- and drug priming-induced reinstatement of methamphetamine seeking (18). LY379268 injections also decrease cocaine priming-induced reinstatement in squirrel monkeys (19). Finally, LY379268 injections decrease nicotine self-administration and discrete cue-induced reinstatement of nicotine seeking (20).
However, from a medication development perspective there are limitations with LY379268 and related agonists. LY379268 has low bioavailability (21) and tolerance develops to its effects (20). Additionally, LY379268 activates the mGluR3 subtype whose physiological functions are unknown (8). These limitations led to development of selective positive allosteric modulators (PAMs) of mGluR2 (22-24). PAMs of mGluR2 bind to an allosteric site of the receptor and only activate these receptors in the presence of glutamate (25, 26).
Markou's group recently reported that a selective PAM of mGluR2, BINA, decreases cocaine self-administration and cue-induced reinstatement (27). They also reported that a BINA analogue with superior pharmacokinetic properties and brain penetration decreases nicotine self-administration in rats (21). Based on these studies, we used our squirrel monkey model (28, 29) to determine the effects of AZD8529, a selective PAM for mGluR2 (30), on nicotine self-administration and relapse to nicotine seeking, as assessed in the reinstatement procedure (31). We also provide results from in vivo and in vitro assays on the selectivity of AZD8529 to mGluR2 and results on the drug's effect on nicotine-induced dopamine release in nucleus accumbens shell.
Methods
Subjects
For the autoradiography experiment, we used three male 5-6 year old cynomolgus monkeys (Macaca fasciculari, Covance). AstraZeneca ACUC approved the experiment and procedures were performed in accordance with the AstraZeneca Global R&D animal-care standards.
For the behavioral experiment, we used 9-13 years old male squirrel monkeys (Saimiri sciurea), weighing 750-1050 g. Monkeys had been trained to self-administer nicotine or food prior to the study and had no self-administration history with other drugs. We implanted the intravenous catheters as previously described (32). The monkeys wore nylon-mesh jackets to protect these catheters. Each weekday, we flushed the catheters, refilled them with saline, and sealed them with obturators. For microdialysis, we used male Sprague Dawley rats (Charles River, 300-350 g). Squirrel monkeys were housed individually and rats were group-housed at the IRP/NIDA facility (regular 12-h light/dark cycle). NIDA-IRP ACUC approved the experiments, which were carried out in accordance with the 2003 National Research Council Guidelines.
Functional mGluR2 assays
Receptor selectivity assay
To determine the selectivity of AZD8529 within the mGluR family, we used fluorescence-based assays (33, 34) and HEK 293 cell-lines expressing human mGluR constructs. The cell lines expressed chimeric fusion constructs hmGluR2/hCaR*, hmGluR1/hCaR*, hmGluR3/hCaR*, hmGluR4/hCaR*, hmGluR5/hCaR*, hmGluR6/hCaR*, hmGluR7/hCaR*, hmGluR8/hCaR*, each comprising the extracellular domain and transmembrane domain of human mGluR, and the intracellular domain of the human calcium receptor fused to the promiscuous chimeric protein Gqi5 as described previously (35).
Receptor screening
We evaluated AZD8529 at 10 μM for off-target effects using radioligand binding assays (MDS Pharma) based on published methods. We ran reference standards for each assay. We determined IC50 values using non-linear, least squares regression analysis of the Data Analysis Toolbox (MDL Information Systems).
[35S]GTPγS binding human mGlu2-CHO membranes
We used membranes prepared from a CHO cell line expressing the human mGluR2 and performed the assay in a scintillation proximity assay (SPA) format. We grew Chinese hamster ovary (CHO) cells expressing the human mGluR2 to approximately 80% confluence, washed the cells in ice-cold phosphate-buffered saline, and stored them frozen until membrane preparation. Assay buffer contained 0.05 M HEPES, 0.10 M NaCl, 0.01 M MgCl2, pH 7.4 plus 100 M dithiothreitol and 3 M guanosine diphosphate. We started the assay by adding a mixture of wheat germ agglutinin SPA beads (0.75 mg/ml) and membranes (6 g/ml) in assay buffer containing AZD8529 or vehicle. After 15-min incubation, we added a solution containing the [35S]GTPγS and L- glutamate (final concentrations 100 pM [35S]GTPγS and 0-100 M glutamate). Following incubation at room temperature (60 min), we centrifuged the assay plates and read them on the TopCount™ scintillation counter. We determined [35S]GTPγS binding in the absence of glutamate and in the presence of 100- M glutamate as 0% and 100% levels, respectively. We estimated the modulator activity of AZD8529 on mGluR2 activation from the concentration response curves of AZD8529 fitted with a 4-parameter logistic equation to calculate the apparent potency (EC50) and maximal efficacy (Emax).
[35S]GTPγS autoradiography in cynomolgus monkey brain slices
We anaesthetized the monkey with sodium pentobarbital (100 mg/kg), perfused it with saline, and then removed the brain and froze it in cooled isopentane. We cut 20-μm striatum and hippocampus sections on a cryostat, mounted the sections on glass slides and stored them at 80°C until use. We warmed the sections to room temperature in a vacuum chamber over 3 hr on the day of the experiment. We incubated the sections in 50 mM Tris HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, and 0.2 mM DTT (Tris Assay Buffer, TAB); pH 7.4 at 25°C for 10 min. We then incubated the slides in TAB containing 2 mM guanosine diphosphate (GDP) for 15 min at 25°C. We placed the slides in one of the following four conditions for 2 hr at 25°C: Basal: TAB + 2 mM GDP + 0.04 nM [35S]GTPγ S; Agonist alone: TAB + 2 mM GDP + 0.04 nM [35S]GTPγS + 1 μM LY379268; Modulator alone: TAB + 2 mM GDP + 0.04 nM [35S]GTPγS + 3 μM AZD8529; Modulator + Agonist: TAB + 2 mM GDP + 0.04 nM [35S]GTPγS + 1 μM LY379268 + 3 μM AZD8529; Modulator + Agonist + Antagonist: TAB+2 mM GDP+0.04 nM [35S]GTPγS+1 μM LY379268+ 3 μM AZD8529+1 μM LY341495. We then washed the sections 2 times in 4°C 50 mM Tris HCl, pH 7.4, 5 min each, rinsed them in ice cold H2O and air dried the slides. We then exposed the slides to Biomax MR film for 2 days and developed using standard techniques, digitized, and analyzed.
Behavioral studies in squirrel monkeys
Apparatus
We performed the experiments in sound-attenuating isolation chambers each equipped with a Plexiglas chair, a house light and white noise for masking of external sound. The chair contained a response lever (Med Associates) mounted on a transparent front wall; each press of the lever with a force greater than 0.2 N produced an audible click and was recorded as a response. Pairs of green and amber stimulus lights, mounted behind the transparent front wall of the chair, could be illuminated and used as visual cues. We connected the monkey's catheter to polyethylene tubing, which passed out of the isolation chamber where we attached it to a motor-driven syringe pump. The self-administration chambers were controlled a Med Associates interface and MED-PC software.
Nicotine self-administration
We performed this phase over a period of 14 weeks and it included 1-hr sessions from Monday through Friday. Before the start of each session, we placed the monkeys into the Plexiglas chairs and restrained them in the seated position by waist locks. We first trained the monkeys to lever-press under a fixed-ratio schedule (FR10, timeout 60 s) of intravenous nicotine (30 μg/kg/injection) reinforcement. After flushing the catheters with 1 ml physiological saline, we connected them to a motor-driven syringe. At the start of each session, the white house-light was turned off and the green stimulus light was turned on; 10 lever-presses turned off the green light and produced 2-s amber light paired with nicotine injection (0.2 ml). During the 60-s timeout period the chamber was dark and lever-presses had no programmed consequences. When responses showed <15% variability for at least 5 consecutive sessions, we tested the effect of AZD8529 pretreatment (0.03, 0.3, 1, 3, and 10 mg/kg, i.m., 3 hr before the session) on nicotine self-administration for 3 sessions; we compared these data to three consecutive session of vehicle pretreatment immediately preceding each test session. The 3-hr pretreatment time is based on AstraZeneca Tmax pharmacokinetic studies (data not shown).
Reinstatement of nicotine seeking
We performed this phase of the study over a period of 9 weeks. We first tested the monkeys for nicotine priming-induced reinstatement after extinction of the drug-reinforced responding. We then retrained them to self-administer nicotine over 5 days and then tested them for cue-induced reinstatement after extinction of the drug-reinforced responding. We tested AZD8529 doses of 3 mg/kg or lower, because 3 mg/kg was the highest effective dose that reduced nicotine but not food self-administration.
Nicotine priming-induced reinstatement
We performed tests for nicotine priming-induced reinstatement after the monkeys underwent daily extinction sessions during which lever-presses led to saline infusions plus the visual cues previously paired with nicotine infusions, but not nicotine. We gave a non-contingent saline injection before each extinction session as a vehicle control for the nicotine-priming injections. After at least two extinction sessions, when responding had reached a low, stable level, we determined the effect of pretreatment with AZD8529 (0.3, 1 or 3 mg/kg, i.m.) or its vehicle on nicotine (0.1 mg/kg i.v.)-induced reinstatement. We gave the nicotine priming injections immediately before the start of the test sessions. During testing, lever-presses (FR10) continued to produce only saline injections and the discrete cues. We also tested the effect of 3 mg/kg of AZD8529 on saline priming to determine whether AZD8529 alone would affect nicotine seeking after extinction.
Cue-induced reinstatement
After the completion of nicotine priming tests, we retrained the monkeys to self-administer nicotine for ~10 sessions. We then gave them 3 extinction sessions during which lever-presses had no reinforced consequences (neither nicotine nor cue were available); additionally, we did not inject monkeys with saline priming before these sessions. After extinction, we determined the effect of pretreatment with AZD8529 (0.3, 1 or 3 mg/kg, i.m.) or its vehicle on cue-induced reinstatement. During testing, lever-presses (FR10) produced the i.v. saline infusions and visual cue presentations. We also determined the effect of 3 mg/kg of AZD8529 on extinction responding in the absence of the cue. Each cue-induced reinstatement test was followed by one or two extinction sessions.
Food self-administration
We determined the effect of AZD8529 in a separate group of monkeys that self-administered 190-mg food pellets under reinforcement schedule conditions identical to those we used with nicotine (FR 10, TO 60 s). We restricted food intake to maintain monkeys’ weights at a level that facilitates food-reinforced responding (no more than 10% below free-feeding weight). The number of reinforcers delivered per session, as well as rates of responding, in this group were very similar to the nicotine group (Figure 2). We injected each dose of AZD8529 (3, 10 and 30 mg/kg, i.m.) for three consecutive sessions, which was preceded by three sessions with vehicle injections before the sessions.
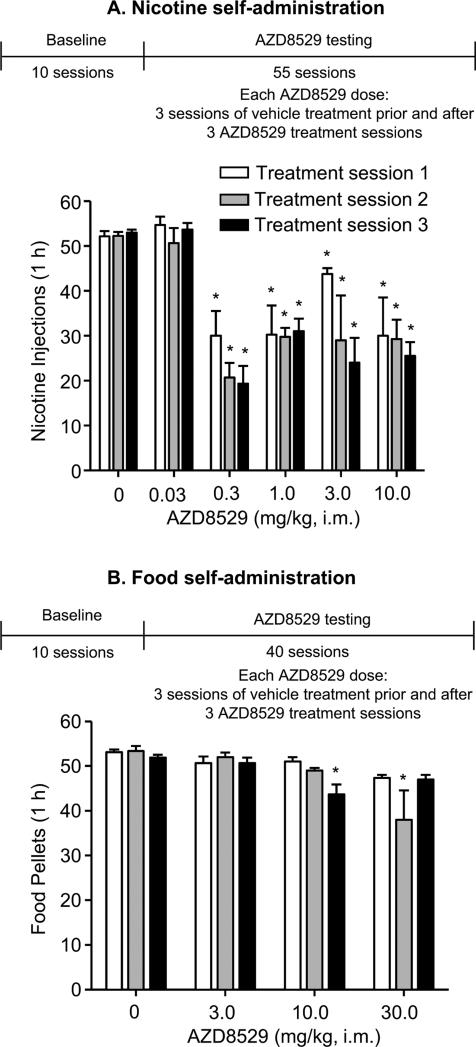
Figure 2. Effect of AZD8529 on nicotine and food self-administration in squirrel monkeys.
Mean±SEM of nicotine injections (30 μg/kg) (A) or food pellets (B) per 1-hr session after pretreatment (3 hr) with vehicle or AZD8529 (0.03 -30 mg/kg) for 3 consecutive sessions. Also shown is the experimental timeline (see Methods). Different from the mean of three sessions of vehicle (0 mg/kg) treatment, *p<0.05, n=3-4.
AZD8529 plasma levels in squirrel monkeys
To determine whether plasma levels during the behavioral experiments reach levels that are well tolerated in humans (per AstraZeneca company information), we injected 3 squirrel monkeys with AZD8529 (1 mg/kg, i.m.) and 3 hr later we collected venous blood samples (approximately 1.5 ml) from the femoral vein under light ketamine (10 mg/kg, i.m.) anesthesia. We rapidly mixed the blood samples and immediately cooled them on ice until centrifugation. Plasma was prepared by centrifugation at 4°C for 10 min at 1500 x g within 30 min of blood sampling. We separated the plasma and transferred it to two 2-ml micro-centrifuge tubes. We stored the plasma samples at −80°C. We shipped the samples on dry ice to AstraZeneca where AZD8529 levels were measured using a standardized LC/MS/MS method.
In vivo microdialysis in rats
The general procedure was described previously (36). We performed microdialysis in Sprague-Dawley rats 20-24 hr after implantation of probes aimed at the accumbens shell (2.0 mm anterior,1.1 mm lateral from bregma, and 8.0 mm below the dura) (37). We collected samples (20 μl) every 20 min (perfusion rate: 1ul per min) and immediately analyzed dopamine levels by HPLC coupled to electrochemical detection. We injected the test drugs or their vehicle after observing stable dopamine levels (<15% variation) in 3 consecutive samples. We injected vehicle or AZD8529 (10 or 30 mg/kg i.p.) 2 hr before vehicle or nicotine (0.4 mg/kg s.c.) injections. We collected dialysate samples for 2 hr after nicotine injections. We based the AZD8529 doses on previous unpublished work of AstraZeneca in rat behavioral models and a recent study on the effect of the drug on ‘incubation’ of methamphetamine craving in rats (38).
Drugs
We dissolved Nicotine [(−)-nicotine hydrogen tartrate] (Sigma) in saline and adjusted the pH of the solution to 7.0 by diluted NaOH. We dissolved AZD8529 (7-methyl-5-(3-piperazin-1-ylmethyl-[1,2,4]oxadiazol-5-yl)-2-(4-trifluoromethoxybenzyl)-2,3-dihydroisoindol-1-one; AstraZeneca) in sterile water (Hospira). We express all nicotine and AZD8529 doses as free-base.
Statistical analysis
We recorded the number of lever-presses and number of injections per sessions. We calculated response rates based on available session time for responding (i.e., timeout time was subtracted). We also recorded timeout responses. We analyzed the nicotine or food self-administration data with repeated measures ANOVAs (SigmaStat), using the within-subjects factors of AZD8529 dose and treatment session (session 1, 2, 3). We analyzed the nicotine priming- and cue-induced reinstatement data with repeated measures ANOVAs, using the within-subjects factor of AZD8529 dose. We express the microdialysis data as a percentage of basal dopamine values; basal values were the mean of three consecutive samples (differing from each other by <15%) taken immediately before the first injection of AZD8529 or vehicle. We analyze these data using repeated measures ANOVA. We followed up on significant main or interaction effects (p<0.05) using Tukey post-hoc tests.
Results
AZD8529 potentiation of mGluR2 receptor function
We assessed the effect of AZD8529 at the human mGluR2 receptor by measuring the potentiation of [35S]GTPγS binding in the presence of increasing concentrations of exogenously applied agonist (L-glutamate). AZD8529 potentiated the effects of glutamate at mGluR2 with an EC50 of 195±62 nM and an Emax of 110%±11% (Figure 1A). In order to assess the selectivity of AZD8529 against the family of mGluRs, we used fluorescence-based assays. AZD8529 potentiated mGluR2 activity with an EC50 of 285±20 nM and did not produce any positive allosteric modulator responses at 20-25 M on the mGluR1, 3, 4, 5, 6, 7, and 8 subtypes (Table 1). In addition, at 25 μM AZD8529 did not elicit antagonist responses on mGluRs. When AZD8529 (10 μM) was studied in a broad receptor screen (Table 2), we observed >50% inhibition of ligand biding at adenosine A3 receptors (51% inhibition) and the norepinephrine transporter (NET, IC50=4.73 μM).
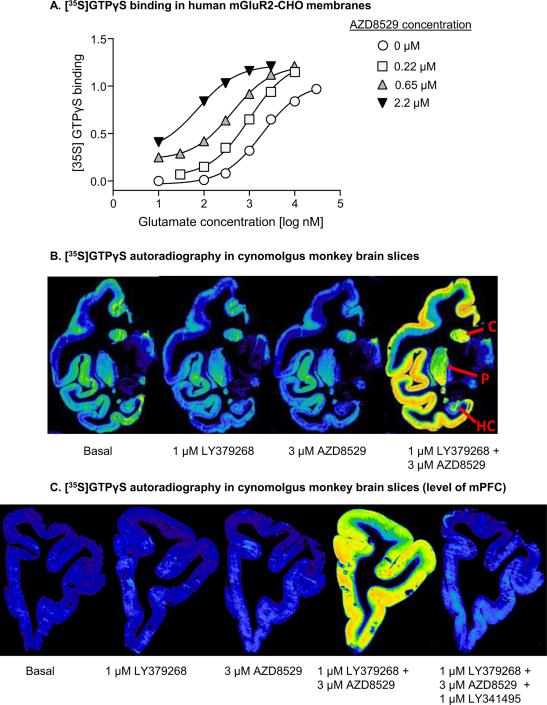
Figure 1. Activity of AZD8529 at mGluRs using functional assays.
(A) Effect of increasing concentrations of AZD8529 on [35S]GTPγS binding to human GluR2s expressed in CHO cells in the presence of increasing concentrations of the agonist L-glutamate. Data are from a representative experiment that was repeated 3 times. AZD8529 potentiated the effects of glutamate at mGluR2 with an EC50 of 195±62 nM and an Emax of 110±11%. (B) Effect of AZD8529 on binding of [35S]GTPγS to cynomolgus monkey brain slices revealed by quantitative autoradiography. Representative digitized autoradiograms are shown from a single experiment repeated 3 times. Basal: [35S]GTPγS binding in the absence of added drug; Agonist alone: [35S]GTPγS binding in the presence of a sub-optimal concentration of mGluR2/3 agonist 1 μM LY379268; Modulator alone: [35S]GTPγS in the presence of 3 μM AZD8529 with no agonist addition; Modulator + Agonist: [35S]GTPγS binding in the presence of both agonist 1 μM LY379268 and modulator 3 μM AZD8529. The caudate nucleus (C), putamen (P) and hippocampus (HC) are annotated on the right panel. (C) Potentiation of agonist-induced activation of mGluR2 by AZD8529 was reversed by the mGluR2/3 antagonist LY341495. Representative autoradiograms are shown from the level of mPFC and the combination of Modulator + Agonis + Antagonist is shown in the right panel: [35S]GTPγS binding in the presence of agonist 1 μM LY379268, modulator 3 μM AZD8529, and antagonist 1 μM LY341495.
Table 1.
The selectivity of AZD8529 at mGluRs was tested in fluorescence-based binding assays using HEK 293 cell-lines expressing human chimeric fusion constructs hmGluR2/hCaR*, hmGluR1/hCaR*, hmGluR3/hCaR*, hmGluR4/hCaR*, hmGluR5/hCaR*, hmGluR6/hCaR*, hmGluR7/hCaR*, and hmGluR8/hCaR*.
| Receptor | Assay | Agonist | Agonist conc. | AZD8529 max conc. | Effect |
|---|---|---|---|---|---|
| mGluR2 | Agonist | 20 μM | NSE | ||
| Positive Modulator | DCG-IV | 0.02 μM | 20 μM | EC50 = 285 ± 20 nM, Emax = 59.9 ± 14% | |
| Antagonist | DCG-IV | 0.2 μM | 20 μM | NSE | |
| mGluR3 | Agonist | NT | NSE | ||
| Positive Modulator | DCG-IV | 0.02 μM | 25 μM | NSE | |
| Antagonist | DCG-IV | 0.2 μM | 25 μM | NSE | |
| mGluRI | Agonist | 20 μM | NSE | ||
| Positive Modulator | 3,5,DHPG | 0.2 μM | 20 μM | NSE | |
| Antagonist | 3,5,DHPG | 1.0 μM | 20 μM | NSE | |
| mGluR5 | Agonist | 25 μM | NSE | ||
| Positive Modulator | 3,5,DHPG | 0.2 μM | 25 μM | NSE | |
| Antagonist | 3,5,DHPG | 1.0 μM | 25 μM | NSE | |
| mGluR6 | Agonist | 20 μM | NSE | ||
| Positive Modulator | L-AP4 | 0.004 μM | 20 μM | NSE | |
| Antagonist | L-AP4 | 0.1 μM | 20 μM | NSE | |
| mGluR7 | Agonist | 20 μM | NSE | ||
| Positive Modulator | L-AP4 | 26 μM | 20 μM | NSE | |
| Antagonist | L-AP4 | 200 μM | 20 μM | NSE | |
| mGluR4 | Agonist | NT | NSE | ||
| Positive Modulator | DL-AP4 | 0.06 μM | 25 μM | NSE | |
| Antagonist | DL-AP4 | 0.4 μM | 25 μM | NSE | |
| mGluR8 | Agonist | NT | NSE | ||
| Positive Modulator | DL-AP4 | 0.06 μM | 25 μM | NSE | |
| Antagonist | DL-AP4 | 0.4 μM | 25 μM | NSE |
NT, not tested
NSE, Non-significant effect
DCG-IV, (2S,2′R,3′R)-2-(2′,3′-Dicarboxycyclopropyl)glycine
3,5,DHPG, (S)-3,5-Dihydroxyphenylglycine
L-AP4, L-(+)-2-Amino-4-phosphonobutyric acid
DL-AP4, DL-2-Amino-4-phosphonobutyric acid
Table 2.
Effects of AZD8529 at 10 μM in a broad receptor screen using radioligand binding assays.
| Target | Source | % inhibition at 10 μM | IC50 μM |
|---|---|---|---|
| Adenosine A1 | Human rCHO cells | −7 | NT |
| Adenosine A2A | Human rHEK-293 cells | 8 | NT |
| Adenosine A3 | Human rCHO-K1 cells | 51 | NT |
| Adrenergic α1A | Rat submaxillary gland | 17 | NT |
| Adrenergic α1B | Rat liver | 13 | NT |
| Adrenergic α1D | Human rHEK-293 cells | 12 | NT |
| Adrenergic α2A | Human rSf9 insect cells | 41 | NT |
| Adrenergic α2C | Human rSf9 insect cells | 34 | NT |
| Adrenergic β1 | Human rRex 16 cells | 10 | NT |
| Adrenergic β2 | Human rCHO cells | 7 | NT |
| Adrenergic β3 | Human rHEK-293 cells | NT | |
| Cannabinoid CB1 | Human rHEK-293 cells | 11 | NT |
| Cannabinoid CB1 | Human rCHO-K1 cells | 26 | NT |
| Dopamine D1 | Human rCHO cells | 7 | NT |
| Dopamine D2 | Human rCHO cells | −4 | NT |
| Dopamine D3 | Human rCHO cells | 14 | NT |
| Dopamine D4 | Human rCHO-K1 cells | −8 | NT |
| Dopamine D5 | Human rCHO cells | 17 | NT |
| GABAA (Agonist) | Rat brain (no cerebellum) | 1 | NT |
| GABAA (BDZ) | Rat brain (no cerebellum) | −15 | NT |
| GABAB1A | Human rCHO cells | 16 | NT |
| GABAB1B | Human rCHO cells | −6 | NT |
| Glutamate, AMPA | Rat cerebral cortex | −19 | NT |
| Glutamate, Kainate | Rat brain (no cerebellum) | 4 | NT |
| Glutamate, NMDA glycine | Rat cerebral cortex | −11 | NT |
| Glutamate, NMDA PCP | Rat cerebral cortex | 10 | NT |
| Glutamate, NMDA Polyamine | Rat cerebral cortex | −14 | NT |
| Glycine, Strychnine sensitive | Rat spinal cord | 8 | NT |
| Histamine H1 | Human rCHO cells | 29 | NT |
| Histamine H2 | Human rCHO-K1 cells | 27 | NT |
| Histamine H3 | Human rCHO-K1 cells | 11 | NT |
| Muscarinic M1 | Human rCHO cells | 33 | NT |
| Muscarinic M2 | Human rCHO cells | 25 | NT |
| Muscarinic M3 | Human rCHO cells | 7 | NT |
| Muscarinic M4 | Human rCHO cells | 21 | NT |
| Muscarinic M5 | Human rCHO cells | 38 | NT |
| Nicotinic α1 | Human RD cells | 9 | NT |
| Nicotinic α7 | Rat brain (no cerebellum) | 4 | NT |
| Opiate DOP | Human rCHO cells | 45 | NT |
| Opiate KOP | Human rHEK-293 cells | 6 | NT |
| Opiate MOP | Human rCHO-K1 cells | 7 | NT |
| Orphanin ORL1 | Human rHEK-293 cells | −7 | NT |
| Serotonin 5-HT1A | Human rCHO cells | 6 | NT |
| Serotonin 5-HT1B | Rat cerebral cortex | 16 | NT |
| Serotonin 5-HT2B | Human rCHO-K1 cells | 19 | NT |
| Serotonin 5-HT2C | Human rCHO-K1 cells | 19 | NT |
| Serotonin 5-HT3 | Human rHEK-293 cells | 9 | NT |
| Serotonin 5-HT5A | Human rCHO-K1 cells | −17 | NT |
| Serotonin 5-HT6 | Human rHeLa cells | 13 | NT |
| Dopamine transporter | Human rCHO-K1 cells | 44 | NT |
| GABA transporter | Rat cerebral cortex | 15 | NT |
| Norepinephrine transporter | Human rMDCK cells | 81 | 4.73 |
| Serotonin transporter | Human rHEK-293 cells | 5 | NT |
NT, not tested
r, recombinant (e.g. rCHO cells – recombinant CHO cells)
IC50 value was determined by a non-linear, least squares regression analysis.
We also determined the ability of AZD8529 to potentiate agonist-induced activation of mGluR2 in the primate brain by using [35S]GTPγS autoradiography on slices prepared from a cynomolgus monkey brain (Figure 1B). This method provides measures of both the efficacy of allosteric modulators at mGluR2 and also provides anatomical localization for the measured activity. AZD8529 (3 μM) significantly potentiated LY379268 (1 μM) activation of the [35S]GTPγS signal in the monkey brain when compared to LY379268 alone. We found that AZD8529 potentiated the [35S]GTPγS signal in the cortex, hippocampus, and striatum. The potentiation of LY379268 activation of the [35S]GTPγS signal by AZD8529 was reversed by the mGluR2/3 antagonist LY341495 (1 μM; Figure 1C, mPFC level shown).
Effect of AZD8529 on nicotine self-administration
Under baseline conditions, nicotine (30 μg/kg/injection) maintained high rates of responding, with significantly more injections per session (mean±SEM: 52.1±1.1) and responses/second (1.3±0.2) than when saline was substituted for nicotine (5.2±0.3 injections per session and 0.02±0.01 responses/second).
AZD8529 at doses of 0.3, 1, 3 and 10 mg/kg (but not 0.03 mg/kg) decreased nicotine self-administration (Figure 2A). The statistical analysis showed a significant effect of AZD8529 dose on both number of infusions (F(4,24)=16.2, p<0.01) and response rate (Table 3; F(4,24)=11.8, p<0.01). The dose × treatment session interaction was not significant (p>0.1) and there was no difference among treatment sessions (p>0.1). The latter finding indicates that tolerance did not develop to AZD8529's effects on nicotine self-administration over repeated testing. Nicotine self-administration behavior rapidly returned to higher levels when treatment with AZD8529 was discontinued.
Table 3.
Effect AZD8529 on response rate during the nicotine or food self-administration sessions (n=4).
| AZD8529 Dose (mg/kg, i.m.) | Nicotine self-administration (Responses/second; Mean±SEM) | Food self-administration (Responses/second; Mean±SEM) |
|---|---|---|
| 0 | 1.39 ± 0.17 | 1.47 ± 0.17 |
| 0.03 | 1.66 ± 0.45* | |
| 0.3 | 0.13 ± 0.04* | |
| 1 | 0.19 ± 0.02* | |
| 3 | 0.31 ± 0.07* | 1.08 ± 0.15 |
| 10 | 0.19 ± 0.04* | 0.74 ± 0.02* |
| 30 | 0.55 ± 0.03* |
The data are means of three sessions of vehicle or AZD8529 treatment.
Significantly different from vehicle pretreatment (0 mg/kg).
Effect of AZD8529 of food-maintained operant responding
The monkeys trained under the FR10 schedule earned 52.8±0.8 pellets/session and lever pressed at a rate of 1.5±0.2 responses/second (Figure 2B). AZD8529 at doses of 10 and 30 mg/kg (but not 3 mg/kg, the highest dose in the reinstatement experiments), decreased the number of pellets earned (AZD8529 dose × treatment session interaction: F(6,12)=3.1, p<0.01) and response rate (Table 3; AZD8529 dose × treatment session interaction: F(6,12)=3.3, p<0.01). This interaction is due to the different effects of the 10-mg/kg and 30-mg/kg doses on food self-administration over repeated testing.
Effect of AZD8529 on reinstatement of nicotine seeking
Nicotine priming-induced reinstatement
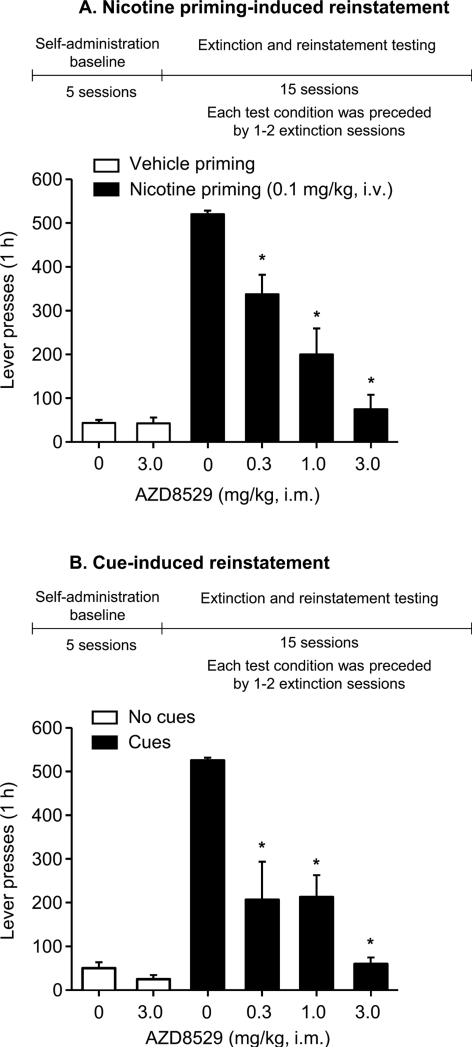
Nicotine priming injections (0.1 mg/kg i.v.) reinstated nicotine seeking (Figure 3A, lever presses: 520.0±8.4 after nicotine priming versus 42.9±7.6 after saline priming; Table 4, response rate: 1.2±0.17 responses/second after nicotine priming versus 0.02±0.01 after saline priming). Pretreatment with AZD8529 dose-dependently decreased nicotine-induced reinstatement (Figure 3A, lever presses: F(5,15)=39.6, p<0.01); Table 4, response rate: F(5,15)=41.9, p<0.01). When the 3 mg/kg dose of AZD8529 was injected prior to vehicle priming, it did not reinstate extinguished drug seeking (p>0.1).
Figure 3. AZD8529 decreased nicotine priming-induced and cue-induced reinstatement in squirrel monkeys.
Mean±SEM number of non-reinforced lever presses during the tests for nicotine priming-induced reinstatement (A) or cue-induced reinstatement (B). Also shown is the experimental timeline (see Methods). During the extinction sessions prior to the tests for nicotine priming-induced reinstatement, saline was substituted for nicotine and lever-presses led to visual cue presentations. During the extinction sessions prior to the tests for cue-induced reinstatement, both intravenous injections and visual cues were discontinued. During the test sessions, AZD8529 (0.3, 1 or 3 mg/kg) or vehicle was injected 3 hr prior to the vehicle or nicotine priming injections (A; 0.1 mg/kg i.v.) or reintroduction of cues (B); lever-presses (FR10) produced intravenous saline injections and the cues in both tests. “Vehicle priming+0 mg/kg” or “No cues +0 mg/kg” represents the mean±SEM of lever-presses of 5 extinction sessions prior to the test sessions. “Nicotine priming+0 mg/kg” or “Cues+0 mg/kg” bars represents mean±SEM of lever presses from 2 tests. Different from “Vehicle priming+0 mg/kg” condition (A) or “Cues+0 mg/kg” condition (B), *p<0.05, n=4.
Table 4.
Effect of AZD8529 on response rates during the extinction sessions and nicotine priming-induced or cue-induced reinstatement tests (n=4).
| AZD8529 Dose (mg/kg, i.m.) | Nicotine priming-induced reinstatement (Responses/second; Mean±SEM) | Cue-induced reinstatement (Responses/second; Mean±SEM) |
|---|---|---|
| 0+ “Vehicle priming” or “No cues” | 0.02 ± 0.01 | 0.02 ± 0.01 |
| 3.0+ “Vehicle priming” or “No cues” | 0.02 ± 0.01 | 0.01 ± 0.01 |
| 0+ “Nicotine priming” or “Cues” | 1.20 ± 0.17 | 1.33 ± 0.14 |
| 0.3+ “Nicotine priming” or “Cues” | 0.25 ± 0.06 | 0.13 ± 0.08 |
| 1.0+ “Nicotine priming” or “Cues” | 0.11 ± 0.06 | 0.11 ± 0.03 |
| 3.0+ “Nicotine priming” or “Cues” | 0.03 ± 0.01 | 0.02 ± 0.01 |
Cue-induced reinstatement
When responding no longer produced nicotine or the interoceptive cues produced by i.v. injection or the visual cues that were previously associated with nicotine, monkeys' response rates decreased to very low levels (Figure 3B, lever presses: 50.0±13.4; Table 4, response rate: 0.02±0.01 responses/second). Reintroduction of the response-dependent, nicotine-associated cues (injection-related and visual) reinstated nicotine seeking (lever presses: 525.0±6.4; response rate: 1.3±0.14 responses/ second). Pretreatment with AZD8529 (0.3, 1.0 or 3 mg/kg) decreased cue-induced reinstatement. The highest AZD8529 dose (3 mg/kg) had no effect on baseline extinction responding without the cues (p>0.1).
Plasma concentrations of AZD8529
In a group of squirrel monkeys (n=3), the plasma concentration of AZD8529 3 hr (the pretreatment time in the self-administration and reinstatement experiments) after drug (1 mg/kg) injections was 112±17 nM. Effect of AZD8529 on nicotine-induced dopamine release in the rat accumbens shell
We determined the effect of systemic AZD8529 injections on nicotine-induced elevations of extracellular dopamine levels in accumbens shell of freely-moving rats. Nicotine (0.4 mg/kg, s.c.) increased extracellular dopamine and this effect was decreased by 30 mg/kg but not 10 mg/kg AZD8529 (Supplementary Figure S1; AZD8529 Dose x Time interaction: F(34,170)=2.24; p<0.001). When given alone, AZD8529 (10 or 30 mg/kg) had no effect on dopamine levels (Supplementary Figure S1).
Discussion
We found that AZD8529, a potent and highly selective positive allosteric modulator of mGluR2 decreased nicotine self-administration and nicotine priming-induced and cue-induced reinstatement in monkeys, and caused these effects at doses 3-10-fold lower than the doses that decreased food self-administration. Our finding that a PAM for mGluR2 decreased nicotine self-administration is consistent with previous findings with the orthosteric agonist LY379268 in rats (20). However, the allosteric modulator AZD8529 exhibited a more promising therapeutic profile, having no effect on food-maintained responding at doses (0.3-3 mg/kg) that had robust effects on nicotine self-administration and reinstatement of nicotine seeking. Furthermore, we did not observe tolerance to the effect of AZD8529 after repeated administration (Figure 2A). Finally, the selective effect of the lower doses of AZD8529 for nicotine self-administration and reinstatement versus food self-administration indicate that it is unlikely that motor deficits or other non-specific behavioral effects of AZD8529 mediate its effect on nicotine-seeking behaviors. We also found that AZD8529 decreased nicotine-induced dopamine release in the rat accumbens shell, an important brain area for drug reward (39). The relevance of these rat results to the AZD8529's effect on nicotine self-administration and reinstatement in monkeys is a subject for future research.
Our data indicate that AZD8529 is selective for mGluR2 over a wide range of targets (Tables 1-2) with very high doses of AZD8529 only causing partial inhibition of radioligand binding at adenosine A3 receptor and the norepinephrine transporter (NET). AZD8529 was also well tolerated in human studies (30) at plasma concentrations that were achieved in monkeys following an effective drug dose in the present study. Moreover, our recombinant and native receptor [35S]GTPγS assay results showed that AZD8529 selectively potentiates agonist-induced activation of mGluR2 in both a cell culture containing the human mGluR2 and in the primate brain. This potentiation was reversed by a selective antagonist at mGluR2/3 in the primate brain. As AZD8529 is inactive at mGluR3 (Table 1), these data indicate that AZD8529's effects are mediated by mGluR2.
The findings that AZD8529 blocked the effects of both nicotine re-exposure and nicotine-associated cues may provide information about the nature of the AZD8529 effect on nicotine self-administration. Drug self-administration involves both the drug's direct reinforcing effects and the conditioned reinforcing effects of drug-associated cues (40). In the case of nicotine self-administration, in particular, it has been shown that drug self-administration also involves nicotine-induced potentiation of the weak reinforcing effects of cues typically associated with nicotine administration (41-43). Thus, there is evidence that nicotine addiction is dependent on the behavioral and psychological effects of internal (interoceptive) and external (exteroceptive) cues associated with cigarette smoking (5).
Using a reinstatement model (44), we found that AZD8529 decreased not only the effects of re-exposure to nicotine (nicotine priming), but also the effects of nicotine-associated cues, which can provoke nicotine craving and relapse in humans (45). These findings are important because relapse is the primary obstacle to successful nicotine addiction treatment (3, 46). Moreover, it has been shown that cue-induced craving can increase with longer abstinence duration in smokers, even as background craving and withdrawal symptoms subside (47). Thus, the ability to block the direct reinforcing effects of nicotine, nicotine's priming effect on cue responding, and the effects of smoking-related cues could make AZD8529 (and related PAMs of mGluR2) more effective than drugs that only block the direct reinforcing effects of nicotine.
In addition to affecting reinstatement of nicotine seeking (20), orthosteric agonism of mGluR2/3 by LY379268 and related drugs have also been shown to decrease different forms of relapse induced by conditioned cues and contexts that were previously associated with the self-administration of alcohol (16), heroin (13, 48), cocaine (12, 15, 17), and methamphetamine (18). To the degree that these results are mediated by mGluR2 (as suggested by the present findings with AZD8529 and the previous findings with BINA and a related PAM of GluR2; (21, 27), these observations may have implications for relapse prevention across drug classes. This is because of the well-established co-morbidity between nicotine addiction and alcoholism (49, 50), as well as addiction to other drugs like THC, methamphetamine, heroin, and cocaine (51-53).
In conclusion, AZD8529 represents a class of orally bioavailable compounds that selectively enhance presynaptic glutamate signaling by binding at an allosteric site of mGluR2s and may have a more desirable and selective therapeutic profile than currently available orthosteric agonists that non-selectively act on both mGluR2 and mGluR3 subtypes. Our results provide novel evidence for the efficacy of PAMs of mGluR2 drugs in non-human primate models of nicotine reinforcement and relapse. We propose that this drug class should be considered for nicotine addiction treatment and potentially to the treatment of other addictions, as AZD8529 was also recently shown to decrease cue-induced methamphetamine seeking after prolonged forced or voluntary abstinence in a rat model of ‘incubation’ of drug craving (38).
Supplementary Material
Acknowledgments
We dedicate this study to our dear mentor, colleague, and friend, Dr. Steven R. Goldberg, who passed suddenly on November 25th, 2014.
Research was supported by the National Institute on Drug Abuse, Intramural Research Program and Division of Pharmacotherapies and Medical Consequences of Drug Abuse, NIH, DHHS. AZD8529 was kindly provided by AstraZeneca.
We thank Drs. Jane Acri and Phil Skolnick for their valuable insights during the planning of the study and preparation of this manuscript. We thank Dr. Ira Baum and Philip White for their excellent veterinary assistance during the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure:
AJC & AM are AstraZeneca employees and LM is a former AstraZeneca employee (Current Address - CHDI Foundation, Princeton, NJ). All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [Google Scholar]
- 2.Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14-20. [DOI] [PubMed] [Google Scholar]
- 3.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33:1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harmey D, Griffin PR, Kenny PJ. Development of novel pharmacotherapeutics for tobacco dependence: progress and future directions. Nicotine Tob Res. 2012;14:1300–1318. doi: 10.1093/ntr/nts201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose JE. New findings on nicotine addiction and treatment. Nebr Symp Motiv. 2009;55:131–141. doi: 10.1007/978-0-387-78748-0_8. [DOI] [PubMed] [Google Scholar]
- 6.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence : implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- 8.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 9.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 10.Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 11.Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2- aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- 12.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- 15.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine-and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 16.Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, et al. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 20.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhanya RP, Sidique S, Sheffler DJ, Nickols HH, Herath A, Yang L, et al. Design and synthesis of an orally active metabotropic glutamate receptor subtype-2 (mGluR2) positive allosteric modulator (PAM) that decreases cocaine self-administration in rats. J Med Chem. 2011;54:342–353. doi: 10.1021/jm1012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Sheffler DJ, Pinkerton AB, Dahl R, Markou A, Cosford ND. Recent progress in the synthesis and characterization of group II metabotropic glutamate receptor allosteric modulators. ACS Chem Neurosci. 2011;2:382–393. doi: 10.1021/cn200008d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C, et al. Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Molecular pharmacology. 2003;64:798–810. doi: 10.1124/mol.64.4.798. [DOI] [PubMed] [Google Scholar]
- 26.Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, et al. Biphenyl indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic-and anxiolytic-like effects in mice. J Pharmacol Exp Ther. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, et al. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology. 2012;37:1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross AJ. AZD8529 – an mGluR2 positive allosteric modulator for the treatment of schizophrenia. Neuropsychopharmacology. 2013;38:S25. [Google Scholar]
- 31.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- 33.Raboisson P, Breitholtz-Emanuelsson A, Dahllof H, Edwards L, Heaton WL, Isaac M, et al. Discovery and characterization of AZD9272 and AZD6538-Two novel mGluR5 negative allosteric modulators selected for clinical development. Bioorganic & medicinal chemistry letters. 2012;22:6974–6979. doi: 10.1016/j.bmcl.2012.08.100. [DOI] [PubMed] [Google Scholar]
- 34.Rosemond E, Wang M, Yao Y, Storjohann L, Stormann T, Johnson EC, et al. Molecular basis for the differential agonist affinities of group III metabotropic glutamate receptors. Molecular pharmacology. 2004;66:834–842. doi: 10.1124/mol.104.002956. [DOI] [PubMed] [Google Scholar]
- 35.Levinthal C, Barkdull L, Jacobsen P, Storjihann L, Van Wagenen BC, Stormann TM, et al. Modulation of Group III metabotropic glutamate receptors by hydrogen ions. Pharmacology. 2009;83 doi: 10.1159/000180124. [DOI] [PubMed] [Google Scholar]
- 36.Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, et al. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 ed. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- 38.Caprioli D, Venniro M, Zeric T, Li X, Marchant NJ, Adhikary S, et al. Effect of the novel positive allosteric modulator of mGluR2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.018. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol Rev. 1976;27:325–340. [PubMed] [Google Scholar]
- 41.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 43.Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiol Behav. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 45.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 46.Herd N, Borland R, Hyland A. Predictors of smoking relapse by duration of abstinence: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104:2088–2099. doi: 10.1111/j.1360-0443.2009.02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context-and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 50.Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- 51.Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, et al. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Drug Alcohol Depend. 1994;2:244–268. [Google Scholar]
- 53.SAMSHA . In: Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings Administration. HHS, editor. Substance Abuse and Mental Health Services; Rockville, MD: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.