Abstract
Purpose
EFdA is a potent nucleoside reverse transcriptase inhibitor (NRTI) with activity against a wide spectrum of wild-type and drug resistant HIV-1 variants. CSIC is a tight-binding non-nucleoside reverse transcriptase inhibitor (NNRTI) with demonstrated anti-HIV properties important for use in topical prevention of HIV transmission. The objective of this study was to develop and characterize film-formulated EFdA and CSIC for use as a female-controlled vaginal microbicide to prevent sexual transmission of HIV.
Methods
Assessments of EFdA- and CSIC-loaded films included physicochemical characteristics, in vitro cytotoxicity, epithelia integrity studies, compatibility with the normal vaginal Lactobacillus flora and anti-HIV bioactivity evaluations.
Results
No significant change in physicochemical properties or biological activity of the combination films were noted during 3 months storage. In vitro cytotoxicity and bioactivity testing showed that 50% cytotoxic concentration (CC50) of either EFdA or CSIC was several orders of magnitude higher than the 50% effective concentration (EC50) values. Film-formulated EFdA and CSIC combination showed additive inhibitory activity against wild type and drug-resistant variants of HIV. Epithelial integrity studies demonstrated that the combination vaginal film had a much lower toxicity to HEC-1A monolayers compared to that of VCF®, a commercial vaginal film product containing nonoxynol-9. Polarized ectocervical explants showed films with drug alone or in combination were effective at preventing HIV infection.
Conclusions
Our data suggest that vaginal microbicide films containing a combination of the NRTI EFdA and the NNRTI CSIC have potential to prevent HIV-1 sexual transmission.
Keywords: HIV prevention, microbicide, vaginal film, EFdA, CSIC
Introduction
Sexually transmitted infections (STIs), such as human immunodeficiency virus (HIV) and herpes simplex virus (HSV), are spread predominantly through unprotected intercourse1. In light of the increased HIV/AIDS pandemic, there is an increasing need to develop effective and novel prevention strategies to control the sexual transmission of HIV-1. With women bearing the greater prevalence of HIV than men, it is imperative to develop female-controlled prevention pharmaceutical strategies such as vaginal applied gels, films and rings to reduce the risk of male to female HIV-1 sexual transmission2–4. Vaginal films offer several advantages over other traditional dosage forms such as gels and creams. These advantages include (1) more accurate dose administration without an applicator; (2) quick drug release upon exposure to vaginal fluid; (3) discreet use and decreased product volume; (4) improved drug stability especially when the drug substance degrades in aqueous environments; (5) convenient transportation and storage; (6) minimized leakage as compared to a gel formulation; and (7) improved acceptability as compared to those of gels, foams, tablets and suppositories5–9. Three vaginal films are presently commercially available: a contraceptive, a lubricant, and a scented film. Vaginal film formulations thus have proven acceptability. While no vaginal microbicide films have been marketed yet, they are being considered for development6, 10.
The use of antiretroviral combinations is standard of care for therapeutic treatment of HIV infection, providing potent and durable suppression of virus replication. Microbicides incorporating antiretroviral drugs are promising strategies to prevent sexual HIV transmission, yet most microbicide products studied to date generally include only a single antiretroviral agent. Microbicides comprising combinations of antiretroviral agents are likely to be superior to those based on a single active agent, by providing an increased barrier to HIV-1 infection due to the different mechanisms of action of the anti-HIV agents and possible reduction in the required dose if the combination provides additive or synergistic antiretroviral activity, thereby diminishing the potential for in vivo tissue toxicity2, 11. However, different antiretroviral agents can have quite different physicochemical properties, which can make co-formulation difficult. Films for vaginal use may provide a formulation platform that enables simpler and stable co-formulation.
The nucleoside reverse transcriptase inhibitor (NRTI) 4’-ethynyl-2-fluoro-2’-deoxyadenosine (EFdA) has a unique mechanism of action and shows exceptional antiviral potency and low toxicity both in vitro and in vivo testing12–17. Therefore, EFdA is a promising anti-HIV agent for use both in HIV therapeutic and prevention modalities. To develop a combination vaginal microbicide with high anti-HIV potency, we chose another antiretroviral drug, the non-nucleoside reverse transcriptase inhibitor (NNRTI) 5-chloro-3-phenylsulfonylindole-2-carboxamide (CSIC), a tight-binding NNRTI which was found to be superior to UC781 (the first NNRTI to provide an extended barrier to HIV infection)18.
The aim of the present work was to develop a vaginal thin film containing both EFdA and CSIC to prevent HIV-1 sexual transmission. A series of in vitro and ex vivo evaluations of vaginal combination films, including physicochemical properties, in vitro cytotoxicity, epithelial integrity, bioactivity, Lactobacillus compatibility and ex vivo assessment of anti-HIV activity, were performed. Our results demonstrated that the vaginal thin film platform delivered EFdA and CSIC and is a promising topical microbicide approach to deliver potent antiretroviral drugs for prevention of HIV-1 sexual transmission.
Materials and methods
Materials
Custom synthesis of EFdA was carried out by Life Chemicals (Burlington, ON, Canada) and CSIC was synthesized by Dalton Laboratories (Toronto, ON, Canada). The drugs were >95% pure as assessed by HPLC/MS and NMR according to the suppliers. DMSO, McCoy’s 5A medium and fetal bovine serum (FBS) were obtained from Fisher Scientific (Pittsburgh, PA, US). PBS (pH 7.4) and penicillin-streptomycin were purchased from Mediatech Inc (Manassas, VA, US). Polyvinyl alcohol (PVA), Pullulan, (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD), glycerin and PEG 400 were purchased from Spectrum (Gardena, CA, US). PEG 4000 and hydroxypropyl methyl cellulose E5 (HPMC E5) were obtained from Dow Chemical Company (Midland, MI, US). MTT, cremophor RH 40, bovine serum albumin (BSA) and methyl-β-cyclodextrin (M-β-CD) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pluronic F127 was kindly provided by BASF Ltd. (NJ, US). All other chemicals were analytical grade. Ultrapure water was obtained in-house from a MilliQ water purification system.
HPLC analysis
An HPLC system (Dionex Ultimate 3000, Thermo Scientific) equipped with an autosampler, a quaternary pump and a diode array detector was used to quantify EFdA and CSIC. Briefly, separation of EFdA was achieved using a Zorbax Eclipse XDB C18 column (3.5µm, 100 × 4.6 mm). The mobile phase consisted of (A) 0.4% phosphoric acid in MilliQ water and (B) methanol using a gradient elution of 10–40% B at 0–5 min, 40–60% B at 5–10 min 60% B at 10–11 min, 60–10% B at 11–13 min, and 10% B at 13–20 min at a flow rate of 0.8 ml/min. EFdA was quantified by UV detection at 260 nm as described before19. For quantification of CSIC, separation was achieved by a Gemini C18 column (5µm, 150 × 4.6 mm) in isocratic mode. The mobile phase consisted of acetonitrile-0.075% (v/v) TFA solution (40:60, v/v), being pumped at a flow rate of 1.2 ml/min. CSIC was quantified by UV detection at 302 nm. All experiments were performed at room temperature and the total area of peak was used to quantify the compound. The linearity of calibration curve of CSIC was measured by linear least squares regression analysis of a plot of peak area versus CSIC concentration. The method was shown to be specifically linear in the range of 0.5–200µg/ml (R2 = 0.9999), precise at the intra-day and inter-day levels as reflected by the relative standard deviation (RSD) values (between 0.27 and 2.78%), accurate (overall recovery rate of 99.43 ± 1.30%). The limit of detection (LOD; 0.17µg/ml) and limit of quantification (LOQ; 0.5µg/ml) for CSIC were estimated at a signal-to-noise ratio (S/N) of 3:1 and 10:1, respectively.
CSIC solubility
An excess amount of CSIC was added to microcentrifuge tubes containing 1.5 ml of the different solubilizer solutions. The samples were mixed using a Multi-Purpose Rotator with moderate rotation speed at ambient temperature for 120 h. The samples were then filtered through a 0.2µm membrane filter and the filtrate was analyzed by HPLC as described above.
EFdA/CSIC compatibility study
The drug-drug compatibility study was conducted at 25°C/65% relative humidity (R.H.) and 40°C/75% R.H. for 3 months. Due to the poor solubility of CSIC, 40% acetonitrile solution was used to dissolve the drug substances to make the initial concentration of EFdA and CSIC at 100µg/ml. The samples were taken at specific time points over the period of 3 months and analyzed by HPLC.
Preparation of EFdA and CSIC combination film
The vaginal polymeric film was prepared by the solvent casting method. Briefly, PVA (7%, w/w) was dissolved in MilliQ water, and to expedite the PVA dissolving process, the mixture was placed in a hot water bath (90°C) until the PVA was completely hydrated. PEG 4000 (0.5%, w/w) and HPMC E5 (3%, w/w) were slowly added into the PVA solution with moderate stirring using an overhead mixer, respectively. After the polymer solution became uniform, CSIC (0.12%, w/w) dissolved in a solution of PEG400 (3%, w/w) and ethanol was added into the polymer solution, and followed by a mixture of EFdA (0.12%, w/w), glycerin (1.5%, w/w) and ethanol. Then, pullulan (4%) was slowly added into the polymer solution, and the mixture was allowed to stir overnight in order to remove the entrapped air bubbles. The final uniform polymer solution was cast onto a polyester substrate attached to the hot surface of an automatic film applicator (Elcometer® 4340) using an 8-inch doctor blade. The film sheet was allowed to dry for 13 min before it was peeled off from the substrate, and then cut into 1 × 2 inch film pieces using a die press. Placebo film was prepared using the same method as described above except that no drug substances were added into the polymer solution.
EFdA/CSIC stability in the polymer solution
The stability of EFdA and CSIC in the film polymer solution was performed at 25°C/65% R.H. and 40°C/75% R.H. for 4 weeks. The drug loaded polymer solutions were prepared as described before. Approximately, 0.5ml drug-loaded polymer solution was transferred to the vial, and the vials were sealed with parafilm and foil to protect against evaporation and light. The samples were placed under the specified conditions. At regular intervals, samples were removed and the EFdA and CSIC drug content was determined by HPLC.
Physicochemical characterization
a. Drug content
The matrix of the drug-loaded film (1 × 2 inch) was dissolved in 15 ml MilliQ water and then 10ml acetonitrile was added to further extract the drug substance. An aliquot was withdrawn from the mixture and centrifuged at 6000 rpm for 3 min. The supernatant was analyzed by HPLC as described above.
b. Dissolution test
To determine the kinetics of drug release from drug-loaded films, an in vitro release study was conducted using a class IV USP apparatus (SOTAX CP7, Horsham, US) using a 70 ml reservoir and a flow rate of 16 ml/min. 1% cremophor were used as the media to meet the sink condition of CSIC. The test was carried out at 37°C for 60 min. At appropriate time intervals, 0.5 ml aliquots were withdrawn and the samples were analyzed by HPLC.
c. Moisture content
Residual water content of the films was measured using a Karl Fisher apparatus (Metrohm, 758 KFD Titrino) in accordance with the titration method specified by the manufacturer.
d. Puncture strength
Puncture strength of placebo and drug-loaded films was measured using a texture analyzer (TA. XT. Plus®, New York, NY). Briefly, the film was fixed in between two steel plates, and the maximum force required to rupture the film using a round compression probe (TA, 8A) was recorded. The equation for calculation was shown below:
| (1) |
e. X-ray diffraction (XRD)
XRD analysis of the films and drug powders was conducted by an X-ray diffractometer (Bruker D8 Discover) equipped with a Cu Kα radiation source (40 kV, 40 mA, λ = 0.15406 nm). Drug powders were pressed onto the sample holder to form a thin layer, while the films were directly placed on the sample holder. The samples were measured from 3.5° to 45° at a rate of 0.04°/sec.
f. Field emission scanning electron microscopy (FESEM)
Surface morphology of the films and drug powders was imaged by field emission scanning electron microscopy system (JEOL, JSM-6335F) at an accelerating voltage of 3kV. Samples for FESEM were mounted on sample holders by double sided tape and silver conductive adhesive, and then coated with a gold palladium layer (5µm thickness) by cressington sputter coater before scanning.
g. Stability assessment
To test physical stability, EFdA and CSIC combination films were stored at 25°C/65% R.H. and 40°C/75% R.H. for 3 months, and the films were tested at specific time points. The puncture strength, water content, drug content and dissolution behavior of the film were evaluated during the storage period.
In vitro cytotoxicity
HEC-1A cell line was obtained from ATCC and the cells were cultured at 37°C with 5 % CO2 under fully humidified conditions19. HEC-1A cells were cultured in McCoy’s 5A modified medium, supplemented with 10% FBS, 100IU/ml penicillin and 100µg/ml streptomycin sulfate. Cells were seeded at the density of 1×104 cells per well in 96-well plates. After 24 h incubation at 37°C with 5 % CO2, the growth medium was replaced with 200 µl medium containing either drug substance with the concentration ranging from 5ng/ml to 50µg/ml (equivalent dose for drug-loaded films) or placebo film at corresponding concentration. After 24h incubation, cell survival was measured using MTT assay. 180 µl of fresh growth medium and 20 µl of MTT (5 mg/ml) solution were added to each well. The plate was incubated for an additional 3 h at 37°C and the media were removed, and then 200µl of DMSO was added to each well to dissolve the purple formazan crystals formed. All the plates were vigorously shaken before measuring the intensity. The absorbance at 595 nm of each well was determined by a microplate reader (Beckman Coulter ® DTX 880, USA).
Epithelial integrity study
For epithelial integrity studies, HEC-1A cells were seeded at a density of 1 × 105 onto per polycarbonate insert (1.12 cm2, 0.4µm) in 12-well cell culture plates. The cells were cultured for 7 days and the cell growth medium was changed every other day. To determine the toxic effect of drug-loaded and placebo films on epithelial integrity, the transepithelial electrical resistance (TEER) values were determined using a Millicell-ERS apparatus (Millipore, Bedford, MA). TEER values were corrected by subtracting the background TEER obtained from inserts containing the same volume of culture medium but in the absence of cells. The combination film solution dissolved in the complete cell culture medium (with the concentration of 10µg/ml CSIC) and the corresponding placebo/EFdA alone/CSIC alone film solutions were added to the apical chamber at t = 0 and resistance readings were measured at 30 min, 1, 2, 4 and 24 h. Additionally, a VCF film (active ingredient: 28% Nonoxynol-9) solution (w/v) prepared based on the same weight of the combination film, and the complete culture medium were used as positive and negative control, respectively. The relative epithelia integrity (%) of each formulation was normalized with respect to the TEER value of negative control3.
Compatibility with the normal vaginal flora Lactobacillus
To assess the compatibility between different vaginal films and the normal vaginal flora Lactobacillus, a standard safety microbicide test was conducted in this study20, 21. Briefly, Lactobacillus crispatus (ATCC 33197) and L. jensenii (LBP 28Ab) suspensions were prepared in N-(2-acetamido)-2-aminoethanesulfonic acid buffer, respectively and the desired amount of films was then dissolved and mixed in the suspensions. The suspensions were then incubated for 30 min at 37°C. Samples were taken at zero time and after 30 min incubation, and the viability test was determined by the standard plate count. A sample was considered to be safe if the reduction in viability was less than one Log difference.
Bioactivity evaluations
Antiviral assays were carried out using HeLa-derived P4R5-MAGI indicator cells (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: p4R5-MAGI from Dr. Nathaniel Landau). Cells were cultured in 96-well microplates (5×103 cells per well and maintained in DMEM/10% FBS supplemented with puromycin (0.5 mg/ml). Immediately prior to use, film segments were dissolved in cell culture medium and serial dilutions of the film solutions were added to the culture wells. Cells were incubated with HIV in the presence or the absence of drug for 48h, and then the extent of infection was assessed using a fluorescence-based β-galactosidase detection assay, as previously described22.
Possible biological drug interaction of EFdA and CSIC was evaluated by determining the antiviral potency of film-formulated EFdA alone, film-formulated CSIC alone, and film-formulated 1:1 molar combination of EFdA and CSIC, in P4R5-MAGI cells as described above. Synergy of the drug combination was assessed by the median-effect principle/combination index approach of Chou and Talalay23 using the program Calcusyn (Biosoft, Cambridge, UK).
Ex vivo efficacy study
The normal human ectocervical tissues (IRB # 0503103) were acquired from pre-menopausal women undergoing hysterectomy. Polarized explant cultures were set-up as previously described24, 25. Briefly, the explant was placed with the luminal side up in a Transwell system. The edges around the explant were sealed with Matrigel™ (BD Biosciences, San Jose, CA). The explants were maintained with the luminal surface at the air-liquid interface. The lamina propria was immersed in complete medium (DMEM containing 10% huAB serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and nonessential amino acids). Cultures were maintained at 37°C in a 5% CO2 atmosphere. Drug-loaded or placebo films were dissolved in 2 ml of complete medium, and 100 µl of dissolved film solution was added to the apical side of the explants followed by adding 100 µl of 5×104 tissue culture 50% infective dose (TCID50) of HIV-1BaL. After culture overnight, the explants were washed and fresh culture medium was applied to the basolateral compartment. Every 3 to 4 days over a 3-week period, supernatant was collected and stored at −80°C. Fresh medium was replaced in the basolateral compartment. At day 21, the explants were fixed in formalin and processed for immunohistochemistry (IHC) to detect HIV p24 expressing cells. The supernatant was tested for HIV-1 replication using the p24gag ELISA (Perkin Elmer Life and Analytical Sciences, Inc., Waltham, MA, USA).
Results
CSIC solubility
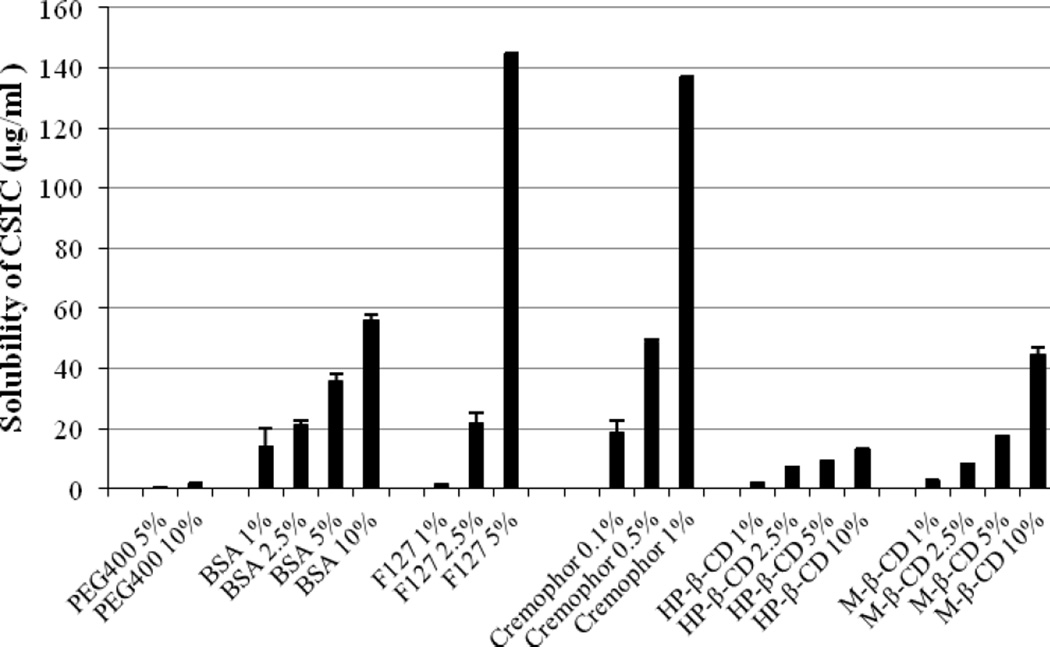
In our preliminary study, the aqueous solubility of CISC was determined to be less than 0.5µg/ml. Thus various solubilizer solutions with different concentrations were applied to increase the CSIC solubility. Addition of solubilizers significantly improved the CSIC solubility, and CSIC provided a concentration-dependent increase in the solubility as the concentration of solubilizers increased (Fig 1). Results showed that the CSIC solubility in 10% BSA, 5% Pluronic F127, 1% cremophor and 10% M-β-CD (w/v) was 55.9µg/ml, 144.59µg/ml, 136.79µg/ml and 45.09µg/ml respectively, which was at least two orders of magnitude higher than the solubility of CSIC in water. Thus, 1% cremophor (w/v) was chosen as the dissolution medium to meet the sink condition because of its high solubilization efficiency.
Fig.1.
Solubility profiles of CSIC in various solubilizer solutions at different concentrations. The experiment was conducted in duplicate.
EFdA/CSIC compatibility study
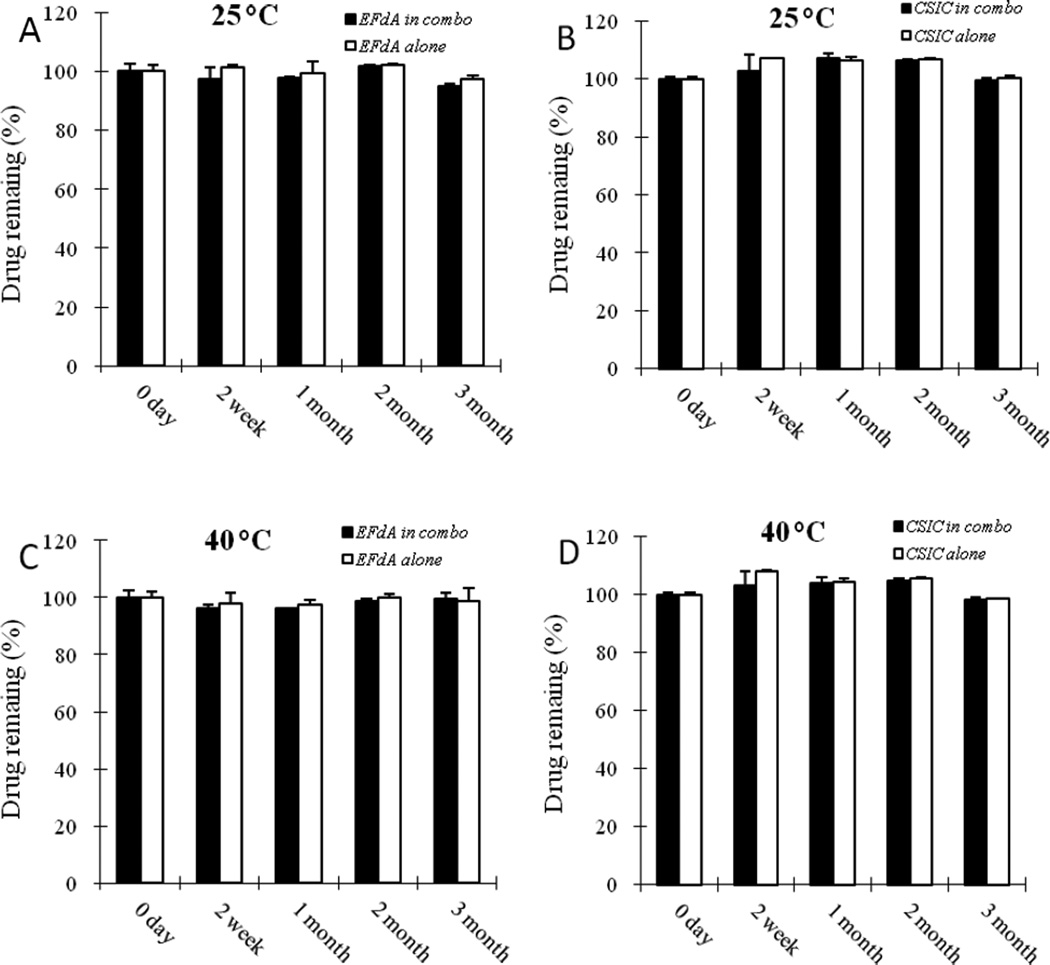
To develop an EFdA and CSIC combination film formulation, a drug-drug compatibility study was done. No drug loss was observed under 25°C/65% R.H. and 40°C/75% R.H. during the storage period (Fig 2), indicating good compatibility between EFdA and CSIC.
Fig.2.
Recovery of EFdA from EFdA/CSIC combination solution and EFdA alone solution at 25°C (A) and 40°C (B); Recovery of CSIC from EFdA/CSIC combination solution and CSIC alone solution at 25°C (C) and 40°C (D). Each point represents mean ± SD (n = 3).
EFdA/CSIC stability in the polymer solution
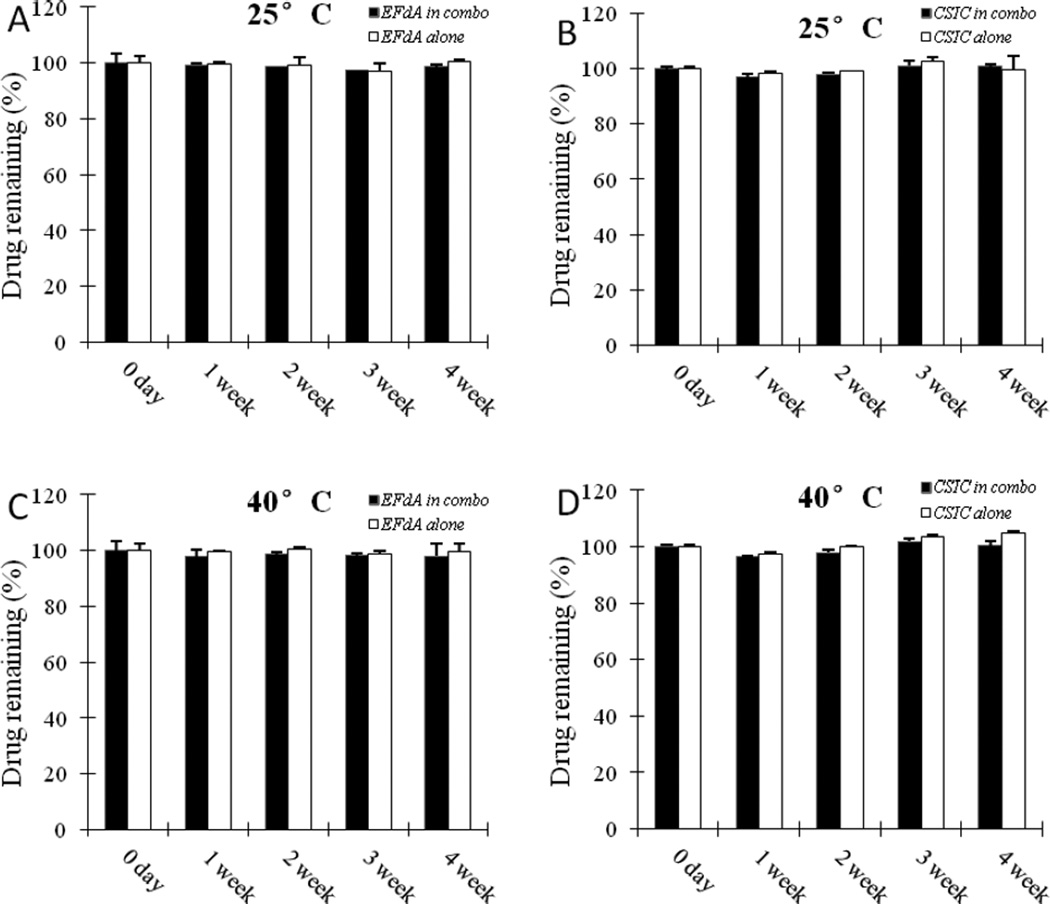
The stability of EFdA and CSIC in the film polymer solution was determined under different temperature and humidity conditions to ensure the stability profile of the drug substances during the manufacturing process. No loss of drug content was noted after 4 weeks under 25°C/65% R.H. and 40°C/75% R.H. (Fig.3), providing confirmatory data for the manufacture of the combination films and chemical compatibility between the drug substances and the film forming excipients used in the formulation.
Fig.3.
Recovery of EFdA from EFdA/CSIC combination polymer solution and EFdA alone polymer solution at 25°C (A) and 40°C (B); Recovery of CSIC from EFdA/CSIC combination polymer solution and CSIC alone polymer solution at 25°C (C) and 40°C (D). Each point represents mean ± SD (n = 3).
Physicochemical characterization of EFdA/CSIC combination vaginal films
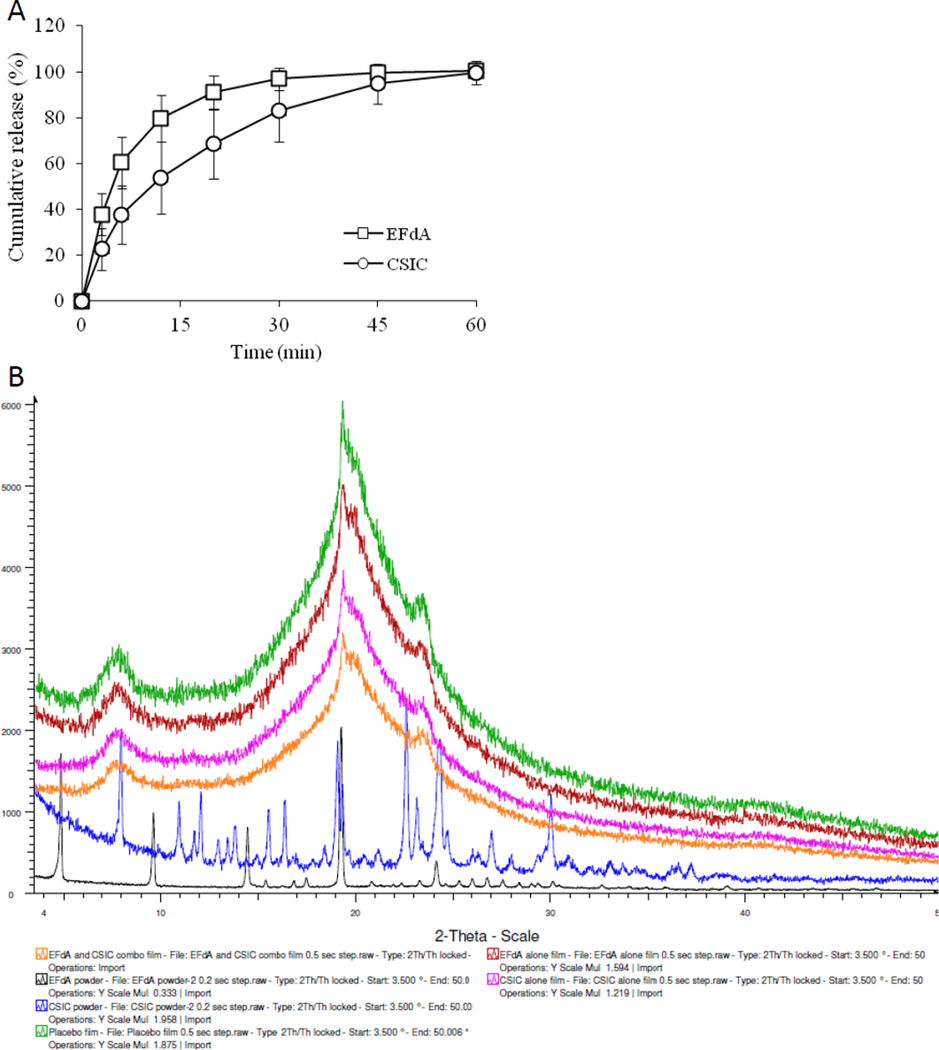
The drug content for EFdA and CSIC in the vaginal film was about 1mg/film, respectively and there was no change in the appearance, water content and puncture strength between placebo and drug-loaded films, suggesting the drug incorporation had little influence on the properties of vaginal polymeric films (Table 1). SEM images of the surface morphologies of EFdA powder, CSIC powder, placebo and drug-loaded combination films showed that EFdA powder exists in planar or flaky shape while CSIC crystals are rod or needle-shaped (Fig 4). Over 95% of EFdA and CSIC were released from the film matrix in 1% cremophor after 60 min which is consistent with the solubility results of CSIC, indicating that 1% cremophor solution is an ideal dissolution medium for the combination film in this study (Fig 5A). In addition, the dissolution rate of EFdA is faster than CSIC, mainly due to the better hydrophilicity of EFdA compared to CSIC. The stability quality control parameters including water content, puncture strength, drug content and cumulative drug release did not change when films were maintained under 25°C/65% R.H. and 40°C/75% R.H. over a 3 month period, indicating the good stability of this vaginal film formulation (Table 2). Importantly, there was also no change in the biological activity of EFdA and CSIC combination films under the different storage conditions (Table 2), further confirming the drug compatibility and stability in the film formulation. The surface of placebo and drug-loaded films are relatively smooth although some small alternating protrusions and depressions can be observed by SEM. It is noteworthy that no drug crystals were observed on the surface of the drug-loaded film. To further elucidate the crystalline state of the drug substances in EFdA and CSIC combination film, XRD analysis was conducted. A number of sharp and intense diffractive peaks at 4.8°, 9.5°, 14.4°, 19.3° and 24.2° for EFdA powder, and 7.9°, 10.9°, 12.0°, 15.5°, 16.4°, 19.3°, 22.6°, 24.3°and 30.0° for CSIC powder were observed, indicating the high crystallinity of the drug substances (Fig. 5B). However, the XRD patterns of EFdA alone, CSIC alone and combination films were almost the same as that of placebo film, suggesting that no drug crystals can be found on the surface of the film and the incorporated drugs were distributed in an amorphous state in the film matrix.
Table1.
Physicochemical characteristics of placebo and EFdA & CSIC combination films.
| Parameter | Placebo film | EFdA & CSIC combination film |
|---|---|---|
| Size (sq. in.) | 2 × 1 | 2 × 1 |
| Appearance | Translucent and flexible | Translucent and flexible |
| Weight (mg) (n = 9) | 173.87 ± 5.40 | 178.74 ± 8.22 |
| Moisture content (% w/w) (n = 3) | 6.4 ± 0.2 | 6.6 ± 1.0 |
| Puncture strength (kg/mm) (n = 3) | 6.5 ± 0.3 | 8.5 ± 1.1 |
| Drug content (mg drug/Film) (n = 3) | N.A. | (1.1± 0.02) for CSIC (1.0± 0.02) for EFdA |
N.A. denotes not applicable
Fig.4.
SEM images of EFdA powder (A and B); CSIC powder (C and D); placebo (E and F) and EFdA and CSIC combination film (G and H) with different magnification scales (100 and 10 µm). For image A, C, E and G, the scale bar was set at 100µm; for image B, D, F and H, the scale bar was set at 10µm.
Fig.5.
(A) In vitro release profiles of EFdA and CSIC from polymeric combination film as a function of time in 1% cremophor; (B) X-ray diffraction images of the drug substances and different vaginal polymeric films.
Table2.
Stability evaluation of EFdA plus CSIC combination filmsa
| Time | Puncture Strength (kg/mm) |
Moisture Content (%) |
Drug Content | Dissolutionb | Antiviral (EC50) (nM) |
||
|---|---|---|---|---|---|---|---|
| EFdA (mg/Film) | CSIC (mg/Film) | EFdA (%) | CSIC (%) | ||||
| 25°C/65% R.H. | |||||||
| 0 day | 8.5 ± 1.1 | 6.6 ± 1.0 | 1.0 ± 0.02 | 1.1 ± 0.02 | 100 ± 2 | 100 ± 5 | 1.8 ± 0.2 |
| 1 M | 9.9 ± 0.3 | 6.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 98 ± 1 | 98 ± 5 | 1.7 ± 0.1 |
| 2 M | 9.5 ± 0.8 | 6.8 ± 0.4 | 1.0 ± 0.1 | 1.1 ± 0.1 | 97 ± 3 | 97 ± 6 | 1.9 ± 0.2 |
| 3 M | 8.4 ± 0.4 | 7.1 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.01 | 100 ± 2 | 94 ± 5 | 2.0 ± 0.5 |
| 40°C/75% R.H. | |||||||
| 0 day | 8.5 ± 1.1 | 6.6 ± 1.0 | 1.0 ± 0.02 | 1.1 ± 0.02 | 100± 2 | 100 ± 5 | 1.8 ± 0.2 |
| 1 M | 8.3 ± 0.1 | 6.4 ± 0.04 | 1.0 ± 0.03 | 1.1 ± 0.02 | 97 ± 0.5 | 102 ± 2 | 1.9 ± 0.3 |
| 2 M | 8.6 ± 1.0 | 7.0 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.06 | 95 ± 3 | 94 ± 4 | 2.5 ± 0.5 |
| 3 M | 8.6 ± 1.0 | 7.3 ± 0.5 | 1.0± 0.04 | 1.1 ± 0.06 | 98 ± 5 | 95 ± 6 | 2.2 ± 0.6 |
: Mean ± SD of three determinations
: Dissolution at 60 min
In vitro cytotoxicity
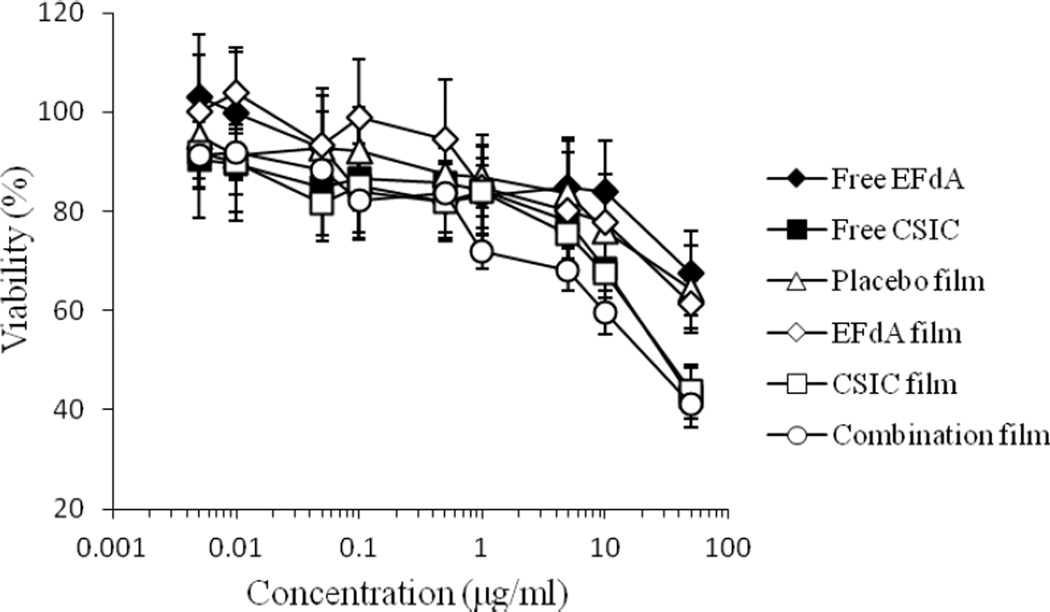
Dilutions of free EFdA, free CSIC, placebo film, and drug-loaded films were cultured with HEC-1A cervical epithelial cell line for 24 h to define any changes in cell viability. The viability of HEC-1A cells after treatment with CSIC and combination films was most affected at the highest concentration suggesting that CSIC could be affecting cell viability (Fig 6). A 5-fold dilution restored cell viability to levels of either drug substance or the placebo and EFdA films. The 50% cytotoxic concentration (CC50) of free EFdA and EFdA-loaded films in HEC-1A cells lines was greater than 50µg/ml, which is over 100,000 times the level of EFdA that provides anti-HIV activity in PBMCs12. The CC50 values of free CSIC, CSIC-loaded film and combination film were (11.4± 2.8) µg/ml, (10.2 ± 0.4) µg/ml and (10.2 ± 0.3) µg/ml, respectively, which are several orders of magnitude higher than the EC50 of CSIC (1.2 nM)18.
Fig.6.
In vitro cytotoxicity of free EFdA, free CSIC, and drug-loaded films at various concentrations of drug against HEC-1A cells after 24 h incubation. For preparation of a series of placebo film solutions for cytotoxicity test, the weight of the placebo film was used the same as that of combination film. Each point represents mean ± SD (n = 8).
Epithelial integrity study
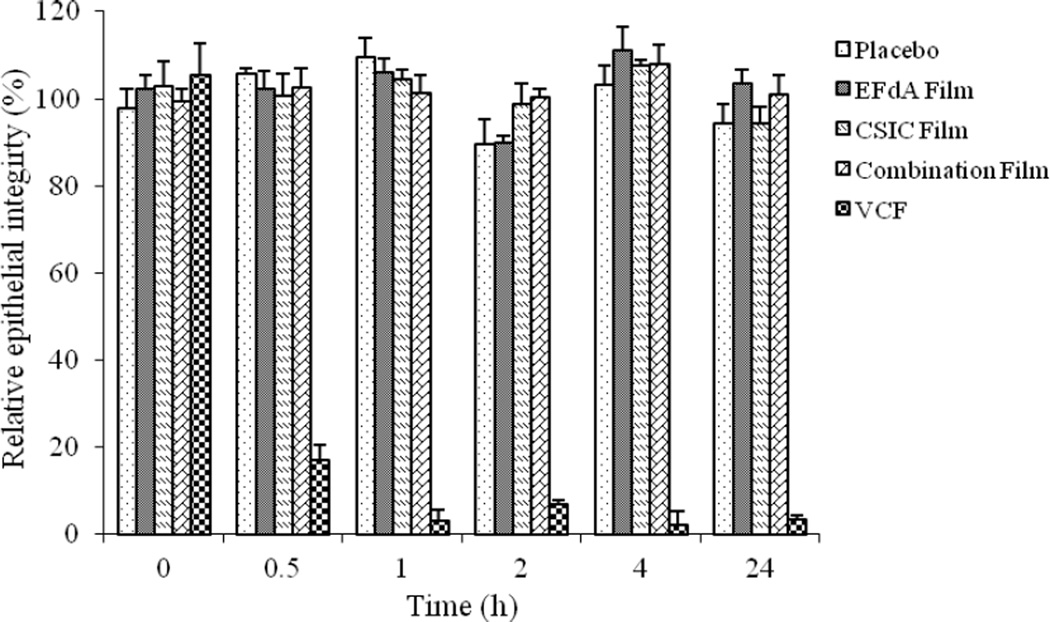
The VCF® film solution, the negative control in this study, reduced the TEER value of HEC-1A cell monolayers by ~ 80% at 0.5h compared to the medium only treatment. The TEER values of VCF® film group did not return to normal levels after 24h (Fig.7), indicating that the high cytotoxicity and the disruption of TEER caused by nonoxynol-9 was irreversible. In contrast, placebo, EFdA alone, CSIC alone and combination film-treated HEC-1A monolayers varied by no more than 12% in TEER values over the incubation period, suggesting the low toxicity of the drug-loaded films to the cell monolayers.
Fig.7.
Relative HEC-1A epithelial cell monolayer integrity of placebo and different drug-loaded films as a function of time. Each point represents mean ± SD (n = 4).
Compatibility with the normal vaginal flora Lactobacillus
The compatibility of different polymeric films with the normal vaginal flora Lactobacillus was evaluated against two Lactobacillus strains (Lactobacillus L. crispatus and L. jensenii). Placebo and drug-loaded vaginal films did not affect bacteria viability (Table 3).
Table3.
The vaginal polymeric film compatibility with the normal vaginal flora Lactobacillus.
| Log difference (T30min plate count –T0 plate count) | ||
|---|---|---|
| L. crispatus | L. jesenii | |
| Placebo | −0.012 | 0.100 |
| EFdA alone Film | 0.260 | −0.025 |
| CSIC alone Film | 0.129 | −0.099 |
| Combination Film | −0.096 | 0.015 |
Bioactivity evaluation
Film-formulated EFdA and CSIC have similar antiviral potency against wild type HIV-1 (EC50 3.7 nM and 1.2 nM, respectively; Table 4). Film-formulated combinations of EFdA plus CSIC showed essentially additive activity against HIV replication, with calculated Combination Indices around 1 (range 0.95 – 1.2). Importantly, the combination film retained additive potency against EFdA-resistant HIV (M184V) and against CSIC-resistant HIV (L100I/K103N), suggesting that this film-formulated combination is likely to be effective at preventing transmission of a broad spectrum of HIV variants.
Table 4.
Inhibitory activity of film-formulated EFdA, CSIC and 1:1 combinations against wild type and drug-resistant HIV variants.
| Virus | EFdA EC50 (nM) |
CSIC EC50 (nM) |
Combination EC50a (nM) |
C.I.50b |
|---|---|---|---|---|
| Wild type | 3.7 ± 0.3 | 1.2 ± 0.1 | 1.7 ± 0.3 | 0.95 (additive) |
| M184V | 18.2 ± 0.3 | 1.6 ± 0.4 | 3.8 ± 0.2 | 1.2 (additive) |
| L100I/K103N | 3.8 ± 0.5 | 435 ± 40 | 9.3 ± 0.6 | 1.2 (additive) |
: Combination films contained EFdA and CSIC at 1:1 molar ratio
: Combination Index at 50% inhibition (mutually non-exclusive) calculated as described23
Ex vivo efficacy study
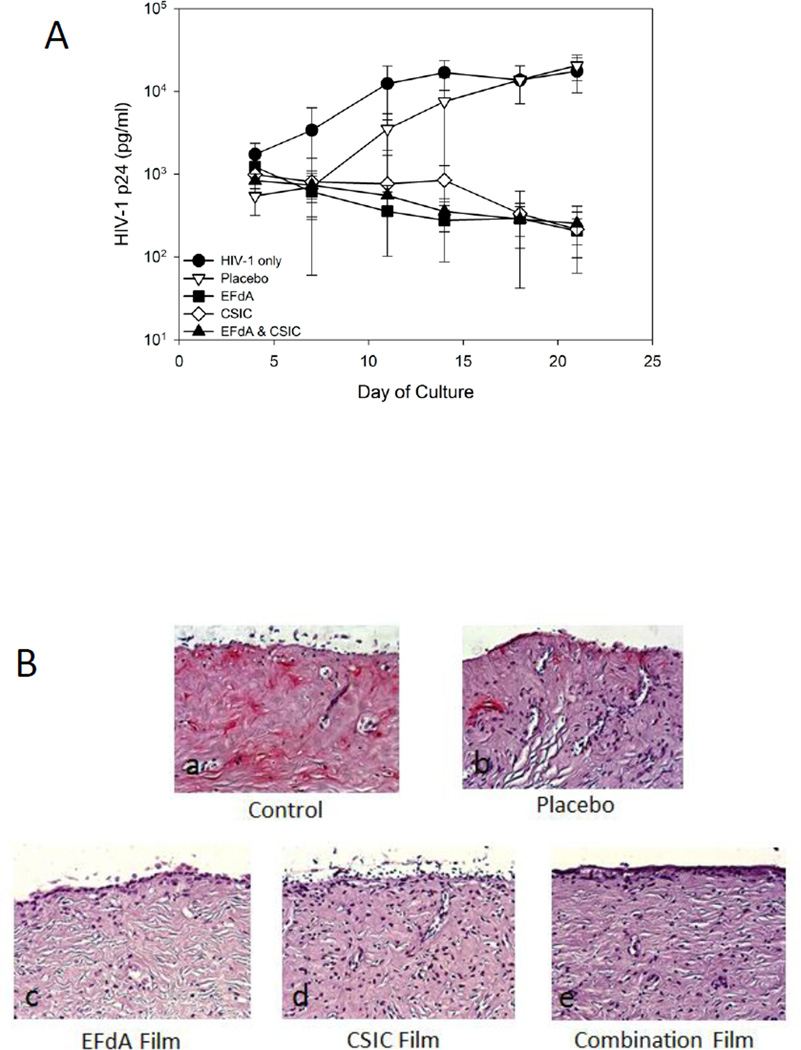
The ex vivo efficacy of EFdA and CSIC combination films was tested in an excised human ectocervical tissue explant model. Results showed that explants treated with drug-containing films either alone or in combination showed a ≥ 1.8 log10 reduction in HIV-1 p24 levels by day 21 as compared with the HIV-1 only treated explants (Fig. 8A). The placebo film did not affect HIV-1 infection of ectocervical explants. These results were confirmed by IHC staining for HIV-1 p24 positive cells in the tissues (Fig. 8B). No HIV-1 infected cells were detected in drug-loaded film treated explants, whereas untreated (HIV-1 only) and vehicle control (placebo film)-treated explants harbored infected cells.
Fig.8.
Ex vivo efficacy of drug-loaded vaginal films using human ectocervical tissue explant cultures. (A) HIV-1 inhibition by drug-containing film formulations. Placebo film did not prevent HIV-1 infection of the ectocervical tissue. (B) Endpoint IHC staining of tissues treated with HIV-1 only (control), placebo film, EFdA film, CSIC film, and combination film. Tissues were exposed to the different film formulations overnight, followed by washing and 21 days of culture. At study end, the tissues were fixed and stained with p24 monoclonal antibody. The red color in the untreated control and placebo-treated tissues indicate p24-positive cells, reflecting the presence of HIV-infected cells. No HIV-infected cells were found in the drug-loaded film formulation-treated tissue samples. The IHC data are representative of 3 independent tissues.
Discussion
Therapeutic combinations of antiretrovirals provide potent and durable viral suppression in HIV-infected individuals. For example, the combination of two NRTIs plus an NNRTI is a preferred regimen for treatment of HIV-1 infection26. Microbicides incorporating antiretroviral drugs are promising strategies to prevent sexual HIV transmission, and the Food and Drug Administration (FDA) recently issued a draft Guidance for Industry for the development of vaginal microbicide products, where the development of suitable vaginal delivery systems for HIV prevention is highly reinforced27. However, despite the success of combination chemotherapy in the treatment of HIV infection, most microbicide products studied to date have included a single antiretroviral drug, in part due to difficulties in incorporating active agents with disparate physicochemical properties into a stable single formulation.
We therefore examined the suitability of the antiretroviral drug combination of the NRTI EFdA (relatively hydrophilic) and the NNRTI CSIC (hydrophobic) for formulation into a vaginal polymeric film. Previous work had shown that combinations of EFdA with a variety of clinically used drugs provided beneficial (generally additive) inhibition of HIV replication in vitro; no antagonism was seen with any combination tested, including EFdA and NNRTI combinations28. In the present work, we found that the combination of EFdA and the NNRTI CSIC in film formulations provided additive antiviral activity, which is beneficial in the context of enhanced protection against HIV transmission. The excipients used in our film formulation such as PVA, HPMC E5, PEG 400, glycerin and PEG 4000 are listed in the FDA inactive ingredients database as excipients for the development of pharmaceutical dosage forms. Although pullulan is not listed in the inactive ingredients database, it is a “generally recognized as safe” (GRAS) polysaccharide widely used in the food industry.
Investigation of drug-drug compatibility is a crucial step in the preformulation stage to ensure the good stability and consistent in vitro and in vivo efficacy of the drug delivery systems. Therefore, it is important to demonstrate that the active pharmaceutical ingredients (APIs) are compatible with each other before incorporation into our combination vaginal film formulation. Our data show that EFdA and CSIC are highly compatible, as no changes in physical parameters, drug composition or biological activity of the film-formulated combination were observed after 3-month short term storage under two different conditions. Moreover, analysis of the stability of EFdA and CSIC drug substances in the film polymer solution indicated excellent chemical compatibility between these APIs and the film-forming excipients, suggesting that EFdA/CSIC combination films can be prepared with relative facility.
Several preclinical testing algorithms of microbicide candidates have indicated that the drug substances and drug delivery systems should be assessed in suitable in vitro systems to determine the effect each candidate on relevant cell/tissue viability before being further evaluated in animal models and clinical settings24, 29and 30. Our in vitro cytotoxicity, epithelial integrity and lactobacillus compatibility studies show that film-formulated EFdA and CSIC combinations minimally impact these in vitro systems, and thus safe.
Additive HIV-1 inhibitory effect was observed for film-formulated EFdA and CSIC combinations against both wild type and drug-resistant HIV variants (including M184V and L1001/K103N). In bioactivity tests against M184V, the potency of the combination film was 4.8-fold higher than that of the EFdA alone film; For L1001/K103N, results showed that the combination film was much more potent (46.8-fold higher) as compared to the CSIC alone film (Table 4). The protective effect of drug-loaded films in the mucosal tissues against HIV-1 infection was also observed in polarized explant model test (Fig. 8). Additionally, as shown in Table 2, the combination film maintained anti-HIV activity throughout the 3-month storage period of time at 25°C/65% R.H. and 40°C/75% R.H.
Conclusion
Vaginal films containing the combination of the NRTI EFdA and the NNRTI CSIC were successfully prepared by the solvent cast technique. The drug-loaded films with desired physicochemical properties were found to be stable, non-toxic and highly potent against both wild-type and drug resistant HIV-1 strains in vitro and ex vivo. Thus, EFdA and CSIC-loaded vaginal microbicide films should be considered as a promising female-controlled pharmaceutical strategy for prevention of HIV-1 sexual transmission.
Acknowledgements
We thank Ryan Winstead for his assistance with SEM study. We also would like to thank Eva Nagy for her help with bioactivity studies. Research reported in this publication was supported in part by grants AI076119 and AI079801 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Abbreviations
- HIV
human immunodeficiency virus
- HSV
herpes simplex virus
- EFdA
4’-ethynyl-2-fluoro-2’-deoxyadenosine
- CSIC
5-chloro-3-phenylsulfonylindole-2-carboxamide
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- FBS
fetal bovine serum
- PVA
polyvinyl alcohol
- HPMC
hydroxypropyl methyl cellulose
- PEG
polyethylene glycol
- RSD
relative standard deviation
- LOD
limit of detection
- LOQ
limit of quantification
- XRD
X-ray diffraction
- FESEM
field emission scanning electron microscopy
- TEER
transepithelial electrical resistance
References
- 1.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010 [Google Scholar]
- 2.Ham AS, Ugaonkar SR, Shi LJ, et al. Development of a combination microbicide gel formulation containing IQP-0528 and Tenofovir for the prevention of HIV infection. J Pharm Sci. 2012;101:1423–1435. doi: 10.1002/jps.23026. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Parniak MA, Sarafianos SG, et al. Development of a vaginal delivery film containing EFdA, a novel anti-HIV nucleoside reverse transcriptase inhibitor. Int J Pharm. 2014;461:203–213. doi: 10.1016/j.ijpharm.2013.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John TJ, Gupta KM, Fabian J, et al. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–212. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11:78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akil A, Parniak MA, Dezzutti CS, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1:511–517. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: where have we been? Where are we going? J Womens Health Gend Based Med. 2001;1:163–173. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- 8.Raymond EG, Chen PL, Condon S, et al. Acceptability of five nonoxynol-9 spermicides. Contraception. 2005;71:438–442. doi: 10.1016/j.contraception.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nel AM, Mitchnick L, Risha P, et al. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Womens Health. 2011;20:1207–1214. doi: 10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]
- 10.Ham AS, Rohan LC, Boczar A, et al. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res. 2012;29:1897–1907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cost MR, Dezzutti CS, Clark MR, et al. Characterization of UC781-tenofovir combination gel products for HIV-1 infection prevention in an ex vivo ectocervical model. Antimicrob Agents Chemother. 2012;56:3058–3066. doi: 10.1128/AAC.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakata H, Amano M, Koh Y, et al. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2007;51:2701–2708. doi: 10.1128/AAC.00277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamoto A, Kodama E, Sarafianos SG, et al. 2′-Deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol. 2008;40:2410–2420. doi: 10.1016/j.biocel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Michailidis E, Marchand B, Kodama EN, et al. Mechanism of inhibition of HIV-1 reverse transcriptase by 4’-ethynyl-2-fluoro-2’-deoxyadenosine triphosphate, a translocation defective reverse transcriptase inhibitor. J Biol Chem. 2009;284:35681–35691. doi: 10.1074/jbc.M109.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori S, Ide K, Nakata H, et al. Potent activity of a nucleoside reverse transcriptase inhibitor, 4′-ethynyl-2-fluoro-2′-deoxyadenosine, against human immunodeficiency virus type 1 infection in a model using human peripheral blood mononuclear cell-transplanted Nod/SCID janus kinase 3 knockout mice. Antimicrob Agents Chemother. 2009;53:3887–3893. doi: 10.1128/AAC.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphey-Corb M, Rajakimar P, Michael H, et al. Efficacy of the novel nucleoside reverse transcriptase inhibitor 4’-ethynyl-2-fluoro-2’-deoxyadenosine (EFdA) in controlling virus burden and treating AIDS-like disease in SIV-infected macaques. Antimicrob Agents Chemother. 2012;56:4707–4712. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michailidis E, Huber AD, Ryan EM, et al. 4’-Ethynyl-2-fluoro-2’-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014;289:24533–34548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motakis D, Parniak MA. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:1851–1856. doi: 10.1128/AAC.46.6.1851-1856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Parniak MA, Mitsuya H, et al. Preformulation studies of EFdA, a novel nucleoside reverse transcriptase inhibitor for HIV prevention. Drug Dev Ind Pharm. 2014;40:1101–1111. doi: 10.3109/03639045.2013.809535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncla B, Hillier SL. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sex Transm Dis. 2005;32:491–494. doi: 10.1097/01.olq.0000170444.13666.e9. [DOI] [PubMed] [Google Scholar]
- 21.Moncla BJ, Pryke K, Rohan LC, et al. Testing of viscous anti-HIV microbicides using Lactobacillus. J Microbiol Methods. 2012;88:292–296. doi: 10.1016/j.mimet.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abram ME, Sarafianos SG, Parniak MA. The mutation T477A in HIV-1 reverse transcriptase (RT) restores normal proteolytic processing of RT in virus with Gag-Pol mutated in the p51-RNH cleavage site. Retrovirology. 2010;7:6. doi: 10.1186/1742-4690-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Rohan LC, Moncla BJ, Kunjara Na, Ayudhya RP, et al. In Vitro and Ex Vivo Testing of Tenofovir Shows It Is Effective As an HIV-1 Microbicide. PLoS ONE. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahalingam A, Simmons AP, Ugaonkar SR, et al. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob Agents Chemother. 2011;55:1650–1660. doi: 10.1128/AAC.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.OARAC. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2014 [Google Scholar]
- 27.FDA. Silver Spring, MD, USA: 2012. Guidance for Industry Vaginal Microbicides: Development for the Pre-vention of HIV Infection (Draft Guidance) ( www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm328834.htm). [Google Scholar]
- 28.Hachiya A, Reeve AB, Marchand B, et al. Evaluation of combinations of 4’-ethynyl-2-deoxyadenosine with clinically used antiretroviral drugs. Antimicrob Agents Chemother. 2013;57:4554–4558. doi: 10.1128/AAC.00283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckheit RW, Jr, Buckheit KW. An algorithm for the preclinical development of anti-HIV topical microbicides. Curr HIV Res. 2012;10:97–104. doi: 10.2174/157016212799304698. [DOI] [PubMed] [Google Scholar]
- 30.Lackman-Smith C, Osterling C, Luckenbaugh K, et al. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52:1766–1781. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]