Abstract
Background
The ventroanterior insula is implicated in the experience, expression, and recognition of disgust; however, whether this brain region is required for recognizing disgust or regulating disgusting behaviors remains unknown.
Methods
We examined the brain correlates of the presence of disgusting behavior and impaired recognition of disgust using voxel-based morphometry in a sample of 305 patients with heterogeneous patterns of neurodegeneration. Permutation-based analyses were used to determine regions of decreased grey matter volume at a significance level p<0.05 corrected for family-wise error across the whole brain and within the insula.
Results
Patients with behavioral variant frontotemporal dementia (bvFTD) and semantic variant primary progressive aphasia (svPPA) were most likely to exhibit disgusting behaviors and were, on average, the most impaired at recognizing disgust in others. Imaging analysis revealed that patients who exhibited disgusting behaviors had significantly less grey matter volume bilaterally in the ventral anterior insula. A region of interest analysis restricted to bvFTD and svPPA patients alone confirmed this result. Moreover, impaired recognition of disgust was associated with decreased grey matter volume in the bilateral ventroanterior and ventral middle regions of the insula. There was an area of overlap in the bilateral anterior insula where decreased grey matter volume was associated with both the presence of disgusting behavior and impairments in recognizing disgust.
Conclusion
These findings suggest that regulating disgusting behaviors and recognizing disgust in others involve two partially overlapping neural systems within the insula. Moreover, the ventral anterior insula is required for both processes.
Keywords: Frontotemporal dementia, Voxel-based morphometry, emotion recognition, insula, disgust, neurodegeneration
Introduction
Disgust likely evolved from gustatory mechanisms that protect organisms from ingesting unsafe foods. Charles Darwin thought that disgust was elicited by “something revolting, primarily in relation to the sense of taste, as...perceived or imagined” (1). Disgust protects the body from infectious (e.g., fungi), inedible (e.g., rotten foods), unclean (e.g., feces), gory (e.g., body deformity), or morally offensive (e.g., incest) phenomena (2). Many sensory domains contribute to disgust, including gustation, olfaction, and interoception (3). The insula integrates information from these multiple sensory modalities and has been implicated in disgust processing (4). However, the functional and anatomical relationships between experiencing, expressing, and recognizing disgust remain unclear.
The anterior insula (AI) has been implicated in experiencing, expressing, and recognizing disgust. For example, the AI is activated in response to viewing disgusting scenes (e.g., cockroaches) (5; 6) and smelling foul odorants (4). Furthermore, trait disgust sensitivity correlates with AI activation during viewing of disgusting images (6; 7). Patients with obsessive compulsive disorder who are preoccupied with contamination show abnormally increased AI activation when viewing disgusting scenes (8). When healthy subjects view disgusted faces, AI activity, as measured by fMRI and depth electrodes, increases significantly more than when viewing faces displaying other emotions (9–12). Additionally, a meta-analysis of 106 imaging studies found that the AI is significantly more activated in response to disgusting stimuli than to other emotional stimuli (13). Furthermore, direct electrical stimulation of the AI evokes “unpleasant feelings” in the throat (12), visceral changes associated with being sick (14), and vomiting (15). Yet, prior studies have been limited to interrogation of healthy systems or investigations with epileptic patients, who have substantial neural reorganization that makes brain-behavior mapping problematic. Lesion studies offer a unique opportunity to delineate the clinical correlates of individuals in whom loss of disgust appears to drive behavioral abnormalities and to facilitate understanding of brain regions necessary for disgust processing.
Few studies have investigated the effects of insular lesions on disgust. One patient with a left-hemisphere infarction involving the insula had selective deficits in recognizing disgust in scenes and faces and decreased subjective reports of disgust, even though he could accurately recognize other emotions and could discuss the logical aspects of disgust without difficulty (16). Another patient with bilateral insular (but also temporal and frontal) lesions showed a general deficit in recognizing emotional facial expressions from static pictures, but when dynamic facial signals were used, he had selectively impaired disgust recognition (17). Both patients’ lesions were not restricted to the insula, let alone the AI, allowing for the possibility that insular lesions were not solely responsible for their disgust processing deficits. Selective disruption in disgust recognition has also been reported in patients with Huntington's Disease (HD), a neurodegenerative disease that affects the insula and striatum (18–20), and a single, small study of HD patients directly linked these recognition deficits to AI atrophy (21). Additionally, selective deficits in recognition of disgust have been found in patients with Parkinson's disease (22). One large study found that vascular damage to right somatosensory cortices, including the insula, was associated with impaired ability to recognize emotions, though it did not investigate disgust specifically (23). Finally, we found that behavioral, physiological, and subjective responses were all reduced in bvFTD patients compared to controls while watching a disgust-eliciting film (24). Although the AI is a common early target of neurodegeneration in bvFTD, this study did not report the anatomy of these deficits. In sum, existing links between insular lesions and disgust recognition deficits are imprecise, and there has been limited investigation into the effects of insular lesions on the experience of disgust or on the regulation of disgusting behavior.
We investigated the neural correlates of patients’ increased tendencies to engage in disgusting behaviors and disrupted recognition of disgust in a large sample of patients with heterogeneous patterns of brain damage. We aimed to determine whether neurodegeneration of the insula results in loss of the experience of disgust as indexed by the emergence of behaviors that are typically prevented by feelings of disgust. We hypothesized that neurodegeneration of the insula, a key hub in visceromotor disgust reactivity and subjective emotional experience, would be associated with the presence of disgusting behaviors. We further hypothesized that the tendency to engage in disgusting behaviors and the inability to recognize disgust would correspond to distinct, but partially overlapping, patterns of AI atrophy.
Methods
Assessment
We analyzed the charts of 305 consecutive patients in our research project between 1999 and 2010 diagnosed with one of seven neurodegenerative diseases as well as 25 asymptomatic first-degree relatives of bvFTD patients (FM). Patients were evaluated by a multidisciplinary team and had laboratory screening and brain MRI. For neuropsychological analyses, data from a control group of 90 healthy older subjects (HS, mean age: 69.4 SD: 7.0) were included for comparison. Neuropsychological testing was conducted on 287 of the 305 patients, all FM, and all HS and included the Clinical Dementia Rating Scale (CDR) and the Mini-Mental State Examination (MMSE), both measures of dementia severity (Table 1).
Table 1.
Demographic and clinical parameters of patients with neurodegenerative diseases and healthy subjects. For TASIT, the F-statistic and p-values are for overall diagnostic group differences controlling for age, gender and MMSE. For other measures, no confounding covariates were included.
| AD (N=104) |
bvFTD (N=79) |
bvFTD/A LS (N=22) |
ALS (N=6) |
CBS (N=24) |
nfPPA (N=18) |
PSP (N=31) |
svPPA (N=21) |
FM (N=25) |
HS (N=90) |
F statistic(df) |
P value |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 61.5(8.1)* | 60.7(8.7)* | 61.4(8.5)* | 52.5(8.5)* | 66.4(7.3) | 67.7(8.8) | 66.9(7.8) | 62.8(6.7)* | 48.2(12.4)* | 69.4(7.0) | 18.8(410,9) | <0.001 |
| Gender (M/F) | 64/40 | 44/35 | 15/7 | 3/3 | 11/3 | 5/13 | 19/12 | 11/10 | 17/8 | 43/47 | χ2 = 13.7 | n.s. |
| MMSE | 20.0(7.3)* | 20.7(9.2)* | 23.4(7.6)* | 29.4(0.9) | 19.0(8.4)* | 23.1(6.5)* | 26.7(2.4) | 19.1(8.2)* | 29.4(0.7) | 29.4(0.9) | 18.5(388,9) | <0.001 |
| CDR | 1.0(0.5)* | 1.6(0.8)* | 1.3(0.9)* | 0.3(0.3) | 1.0(0.8)* | 0.4(0.4) | 1.0(0.6)* | 0.8(0.6)* | 0.1(0.3) | 0.0(0.1) | 43.0(375,9) | <0.001 |
| CDR-box | 5.4(3.1)* | 8.8(3.8)* | 7.1(4.1)* | 1.2(1.7) | 5.4(4.0)* | 2.8(2.4)* | 6.0(3.3)* | 4.6(3.4)* | 0.4(0.9) | 0.0(0.2) | 51.3(375,9) | <0.001 |
| GDS | 7.9(5.6)* | 6.4(5.7)* | 7.9(4.9)* | 6.5(3.7) | 6.9(4.2)* | 7.4(6.5)* | 13.3(6.7)* | 10.0(5.9)* | 5.1(4.3) | 2.4(3.4) | 13.0(320,9) | <0.001 |
| w/disgusting behavior | 21.2% | 68.4% | 22.7% | 0.0% | 4.2% | 22.2% | 35.5% | 42.9% | 4.0% | NA | χ2 = 517.2 | <0.001 |
| TASIT | (N=50) | (N=41) | (N=11) | (N=0) | (N=12) | (N=9) | (N=20) | (N=6) | (N=12) | (N=90) | ||
| Revolted (max=2) | 1.1(0.1)* | 0.8(0.1)* | 0.6(0.2)* | NA | 1.2(0.2) | 1.0(0.2) | 1.1(0.2)* | 0.7(0.3)* | 1.2(0.2) | 1.6(0.2) | 8.8(239,11) | <0.001 |
| Other sum (max=12) | 7.9(0.3)* | 6.5(0.3)* | 6.7(0.6)* | NA | 8.7(0.6) | 8.9(0.6) | 8.1(0.4)* | 7.2(0.8)* | 8.5(0.6) | 9.8(0.2) | 12.4(239,11) | <0.001 |
denotes p<0.05 as compared to HS.
a denotes a significantly (p<0.05) higher proportion of patients with bvFTD have disgusting behaviors compared to patients with AD, bvFTD/ALS, ALS, CBS, nfPPA, and PSP, and FM.
b denotes a trend (p<0.1) for a higher proportion of patients with svPPA having disgusting behaviors compared to FM.
* differs from HS p < 0.05
Disgusting Behaviors
Charts, including both patient and caregiver reports as well as clinician observations, were reviewed by two raters for evidence of disgusting behaviors. Behaviors were recorded that fit into any of the categories of disgust derived from the Disgust Scale (25). Number or intensity of disgusting behaviors could not be accurately coded from retrospective chart review, so these variables were not quantified (i.e., a single episode of disgusting behavior was coded identically as multiple episodes). As not all patients with chart data had emotion recognition or neuroimaging data, sub-groups with these data were analyzed to further explore the nature of these behavioral deficits. Studies of these rare neurodegenerative disorders are chronically underpowered. Therefore, we included all valid data to maximize power.
Emotion recognition
149 patients, 12 FM, and 90 HS were administered the Emotion Evaluation subtest of The Awareness of Social Inference Test (TASIT-EET) (26). Subjects watched 14 brief (20-30 second) videos of actors displaying one of 6 emotions: disgust, happiness, sadness, fear, anger, surprise, or no emotion using facial expressions, body language, and vocal tones. The perceived emotion was then selected from a list displayed on the screen without any time limit for responding. Importantly, patients with svPPA are not mute and are able to label basic emotions even late into the illness (27).
Behavioral Data Statistical Analysis
MMSE, CDR, and TASIT-EET score differences between patients with and without disgusting behaviors were analyzed using general linear models (Proc GLM) in SAS. To examine disgust-specific associations, we divided the TASIT-EET into two scores: the TASIT-EET disgust sub-score and the sum of the subscores of the other emotions plus neutral.
Voxel-based morphometry
MRI scans of 231 of the 305 patients and all FM in the study were of sufficient quality for analysis within 6 months of disgust assessment. VBM is a technique for the detection of regional brain volume by voxel-wise comparison of combined gray and white matter volumes between groups of subjects. For the whole brain analysis, the Anatomical Automatic Labeling atlas was used to name the regions with significantly less grey matter as determined by permutation-based thresholding (p<0.05 FWE). For the region of interest (ROI) analysis, we generated masks of the bilateral insular cortices using MARINA (28). The same permutation-based method was used to determine the p<0.05 FWE threshold within these insular ROIs.
Main effects analyses
We performed three VBM analyses: 1: To determine brain areas where less grey matter volume was associated with the presence of disgusting behavior, the presence of a disgusting behavior was the variable of interest. This whole-brain analysis across all subjects was followed by an ROI analysis only looking within two disorders with the highest number disgusting behaviors (bvFTD and svPPA) to investigate whether the same brain-behavior relationships also held true within diagnostic groups. 2: To determine where decreased grey matter volume was associated with impaired disgust recognition, the Revolted sub-score of TASIT-EET was the variable of interest. 3: To determine where decreased grey matter volume was associated with impaired disgust recognition but not recognition of other emotions, we looked for voxel volumes that correlated with disgust recognition accuracy, controlling for recognition accuracy for all the other emotions plus neutral. In order to account for the reduced power in this dysjunction analysis, we accepted a significance level at p < 0.005 uncorrected for FWE. Analyses for 1 and 2 were considered significant only if they met a FWE threshold of p < 0.05. Age, gender, MMSE, total intracranial volume (TIV), and scanner type were entered as nuisance variables in all three analyses; scanner type was included, since a previous study showed that considering scanner type as a nuisance variable effectively accounts for variability introduced by multiple scanners in VBM (29).
Error check-linear regression comparison of significant peak voxels
Regional atrophy in neurodegenerative disease is not randomly distributed across diagnostic categories. Instead, patterns of atrophy are similar within and, to some degree, across the categories, with groups of sometimes anatomically distant structures atrophying at a similar rate in each disease. As a result, main effects analyses using neurodegenerative disease patients are likely to demonstrate some degree of co-atrophy effects, in which areas of the brain unrelated to the behavior of interest will appear significant because they atrophy simultaneously with another region directly associated with the primary behavior of interest. Thus, to further isolate the independent contributions of each brain region identified in the main effects analyses, we performed linear regressions combining voxel values of each peak for each main effect analysis (for analysis 3, we only included significant peak voxels if they survived this error check in analysis 2). Voxel intensities were extracted from the smoothed, warped, modulated, grey plus white matter images of each subject at each peak voxel within the significant clusters in the main effects analysis. These voxel intensity values were then analyzed together as predictors in linear regression analyses, including age, gender, MMSE, scanner-type (1.5, 3, or 4T) and TIV as confounding covariates, and the behavior of interest as the outcome variable (30).
Peak-voxel comparison
A meta-analysis by Kurth et al. delineated 47 peak values in the insula involved in different domains such as emotion, empathy, interoception and pain (31). To compare these peaks to peaks found in our study, we calculated the Euclidean distance of our insula peak voxels with the peak voxels reported in the meta-analysis. Kolmogrov-Smirnov tests at an alpha level of 0.05 revealed non-normal distributions of the distances. As a result, we constructed kernel-smoothed density estimates for the 47 Euclidean distances to each peak voxel. Subsequently, we reported the smallest distances up to the 5th percentile.
For further methodological details, see Supplementary Materials.
Results
Demographic & behavioral data
There were significant diagnostic group differences in age, CDR, CDR-box, and Geriatric Depression Scale (GDS) (32) scores (Table 1). Significantly more patients with bvFTD had disgusting behaviors (68.4%, Table 1) than other diagnostic groups except for svPPA. Furthermore, 42.9% of patients with svPPA and 21% of patients with AD had disgusting behaviors. For examples of disgusting behaviors, see Supplementary Materials. For emotion recognition, bvFTD, svPPA, AD, bvFTD/ALS, and PSP patient groups were impaired at recognizing disgust and other emotions compared to HS. Subjects with disgusting behaviors were significantly more impaired at recognizing disgust and other emotions compared to patients without disgusting behaviors (Table 2). Subjects with disgusting behaviors were also more likely to have lower MMSE, CDR, and CDR-box scores.
Table 2.
Characteristics of subjects with neurodegenerative diseases by presence or absence of disgusting behaviors.
| Disgust (N=107) | No Disgust (N=223) | F-statistic(df) | p-value | |
|---|---|---|---|---|
| Age | 61.3(9.1) | 61.3(9.9) | 0.0(328,1) | n.s. |
| Gender (M/F) | 66/41 | 123/100 | χ2 = 1.3 | n.s. |
| MMSE | 19.6(9.2) | 23.1(7.0) | 14.1(306,1) | <0.001 |
| CDR | 1.5(0.7) | 0.8(0.7) | 59.1(294,1) | <0.001 |
| CDR-box | 8.3(3.7) | 4.6(3.6) | 64.7(294,1) | <0.001 |
| GDS | 8.0(6.4) | 7.8(5.7) | 0.4(239,1) | n.s. |
| TASIT | (N=48) | (N=113) | ||
| Revolt (max = 2) | 0.7(0.1) | 1.0(0.1) | 3.3(156,4) | <0.05 |
| Other sum (max = 12) | 6.5(0.3) | 7.9(0.2) | 5.02(156,4) | <0.001 |
Main effects analyses
Analysis 1
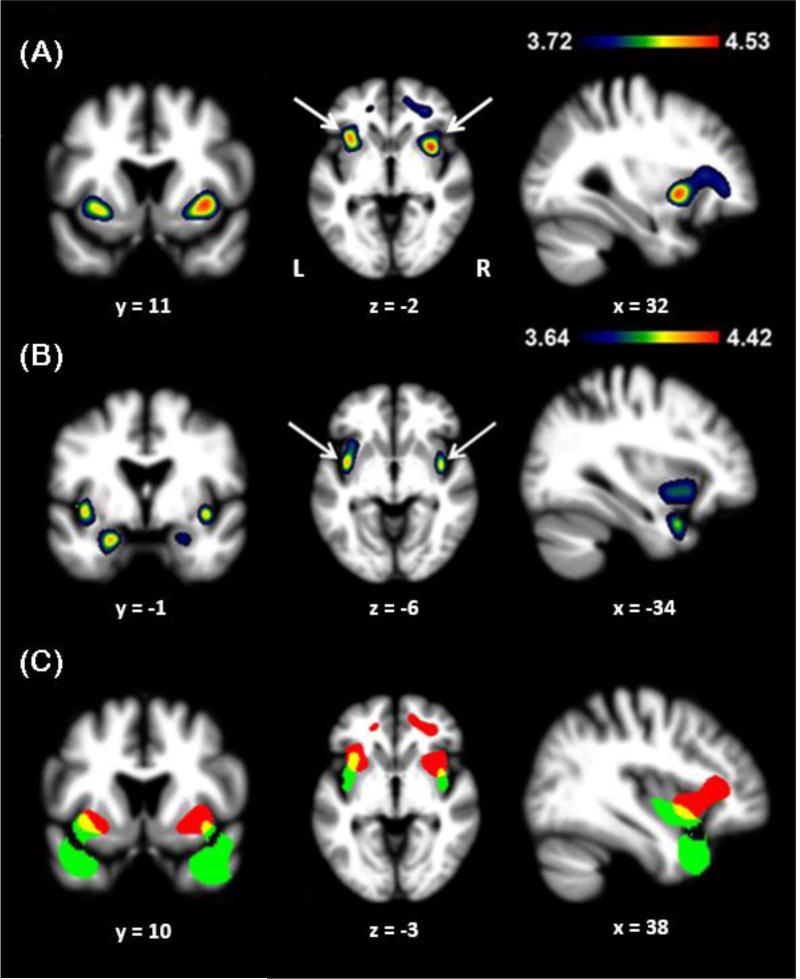
Disgusting behaviors were associated with significantly less grey matter bilaterally in the ventral AI, left cingulate cortex, and white matter tracts near the cingulate cortices (p<0.05 FWE, Fig 1a, Table 3). In order to ensure that decreased grey matter volume was not simply due to the regional atrophy associated with bvFTD or svPPA, we performed ROI analyses of the insula with each group separately. We found that within both diagnostic groups, disgusting behaviors remained associated with reduced ventral AI volumes (p<0.05 FWE).
Figure 1.
Sagittal, coronal, and axial sections representing the results of the main effects analyses, including (a) engagement in disgusting behaviors, (b) disgust recognition, and (c) the overlap in yellow between (a) in red and (b) in green. The regions indicated by the arrows in (a) also remained significant in an ROI analysis of the insula restricted to patients with bvFTD and svPPA. The regions indicated by arrows in (b) remained significant after controlling for the recognition scores of the other emotions (happiness, sadness, fear, anger, surprise) and neutral. X, Y, and Z coordinates for each section are presented below the image, and the left-right orientation of the images is denoted by “L” and “R”.
Table 3.
VBM summary of main effects and regression error check.
| Anatomic Region | Cluster size (mm3) | x | y | z | t-score |
|---|---|---|---|---|---|
| (a) | |||||
| R ventroanterior insula | 11699 | 34 | 10 | −2 | 4.44* |
| L ventroanterior insula | “ | −34 | 18 | −6 | 4.50* |
| L cingulate cortex | “ | −12 | 42 | 4 | 3.79 |
| R white matter tract | “ | 30 | 42 | 0 | 4.06 |
| R white matter tract | “ | 38 | 30 | 10 | 4.03 |
| (b) | |||||
| L ventroanterior insula | 5745 | −38 | 2 | −6 | 4.30* |
| L temporal pole | “ | −42 | 12 | −26 | 4.34* |
| L amygdala | “ | −22 | −2 | −24 | 4.32 |
| R ventroanterior insula | 3390 | 40 | 0 | −6 | 4.28* |
| R temporal pole | “ | 40 | 16 | −30 | 4.40* |
| R amygdala | “ | 24 | −2 | −20 | 3.84 |
Peak voxels are displayed where grey or white matter tissue density correlated with (a) the presence of disgusting behaviors (Analysis 1, lower t-threshold −3.72 FWE p<0.05), and (b) disgust recognition scores as measured by the TASIT EET Revolted score (Analysis 2, lower t-threshold −3.64 FWE p<0.05). The regions in bold remained significant after controlling for the recognition scores of the other emotions and neutral (Analysis 3, lower t-threshold −2.62, uncorrected p<0.005). NB. Given the nature of structural VBM, the output is typically comprised of large clusters of voxels above the correction threshold that encompass multiple neurologically distinct anatomical structures with multiple peaks. Our VBM results show that a single cluster extends through the frontal lobe, which is why “cluster size” refers to the same cluster across multiple structures.
denote regions that survived the error check.
Analysis 2
Two clusters positively correlated with disgust recognition scores were identified in the left and right side of the brain. Inside these clusters, there were peak voxels bilaterally in the ventral medial insula, amygdala, and temporal pole (Fig 1b).
Analysis 3
The clusters in the left and right ventral medial insula found in Analysis 2 remained significant when controlling for the sum of the other TASIT-EET recognition scores. This highlights the importance of these regions in disgust recognition despite the decreased power obtained from controlling for recognition of several other emotions.
While comparing grey matter volumes related to disgusting behaviors and disgust recognition via a unified design matrix would be ideal, this was not possible due to the degree of non-overlap between samples having the two data types. No HS had a chart review, as they did not have clinical charts available. Also, due to the expected high correlation between variance associated with disgusting behaviors and disgust recognition scores, this analysis would be underpowered given the smaller sample size. Thus, we represented each main effect in separate design matrices, making use of the fully powered datasets available to predict anatomic correlates, and superimposed the resulting T-maps on a single template for interpretive purposes (Fig 1c).
Error check
Linear regressions were performed on all peak values in order to remove variables with no independent relationship to the variable of interest. For Analysis 1, peak voxels in the insula bilaterally remained significantly able to predict the presence of disgusting behaviors when entered into regression models with the other regions found to be significant in the main effect analysis. For Analysis 2, the bilateral ventromedial insula and temporal poles remained significant independent predictors of disgust recognition (Table 3).
Peak-voxel comparison
Peak voxels associated with disgusting behaviors were closer to areas associated with attention, gustation, and social functions as determined by the Kurth meta-analysis (31) while the voxels associated with deficits in disgust recognition were associated with insular areas associated with social and hedonic conditions (Table 4).
Table 4.
Relationships between peak voxels identified in the current study and those identified by Kurth et al. with associated domains (31). Peak voxels associated with disgusting behavior are closer to areas implicated in attention, gustation, and social functions while the voxels associated with impaired recognition of disgust are associated with insular areas implicated in social and hedonic conditions.
| Peak voxel | Kurth et al. (2010) peak voxel | Domain | Euclidean distance |
|---|---|---|---|
| Disgusting Behavior | |||
| (36,10,0) | (39,7,0) | Emotion | 4.24 |
| (42,13,−4) | Empathy | 7.81 | |
| (40,12,−6) | Gustation | 7.48 | |
| (−34,18,−6) | (−33,18,−5) | Attention | 1.41 |
| Impaired Recognition of Disgust | |||
| (40,0,−6) | (39,7,0) | Emotion | 9.27 |
| (46,−6,−1) | Empathy | 9.85 | |
| (41,2,3) | Interoception | 9.27 | |
| (−38,2,−6) | (−39,0,−4) | Somatosensation | 3.00 |
| (−38,−4,1) | Somatosensation | 9.22 |
Discussion
We found distinct patterns of decreased bilateral insula grey matter volume associated with an increased tendency to engage in disgusting behaviors and with impaired ability to recognize expressions of disgust in others. Decreased grey matter volume at the transition from frontal insula to the dorsal AI, an area that is involved in integrating socio-emotional with visceral information (31), was associated with both the presence of disgusting behaviors and impaired recognition of disgust. In addition to this shared region, the presence of disgusting behaviors was predominantly associated with decreased grey matter volume in more dorsal AI regions that have been previously associated with cognition (31). Furthermore, even when restricting our analysis to individuals with bvFTD or svPPA, this relationship remained significant. Deficits in recognizing disgust in others were predicted primarily by decreased grey matter volume in more ventral anterior and central insula regions involved in chemical sensory processing such as olfaction and gustation (31), as well as in bilateral amygdala and anterior temporal regions.
While our study did not directly investigate the link between AI damage and alterations in the ability to experience disgust, this link can be logically inferred from patients’ new willingness to spontaneously engage in behaviors that are considered disgusting, and are seldom engaged in by healthy individuals. This link is also supported by our recent study demonstrating that FTD-spectrum patients have reduced subjective and physiological responses while watching disgusting videos (24). The overlapping anatomy between behavior and perception supports the hypothesis that the border area between the frontal and dorsal AI is required for disgust processing (31). This also suggests that the neural substrate allowing recognition of disgust expressions in others is partially involved in the prevention of behaving in ways others find disgusting. However, our results also suggest that additional, distinct, non-overlapping brain regions are required to successfully avoid engaging in disgusting behaviors oneself, or to identify disgust in others.
The AI is implicated in both subjective feeling and recognition of emotion (33–35), and has been proposed as a neuroanatomic substrate for conscious awareness in general and for awareness of feeling disgusted in particular (36). The AI is activated when subjects inhale foul odorants or when they view others inhaling foul odorants (4). Similarly, when subjects view an actor becoming disgusted, read and imagine scenarios that involve disgust, or taste a bitter liquid and become disgusted themselves, the AI becomes activated (37). Furthermore, trait disgust sensitivity correlates with ventral AI activation in response to pictures of disgusting foods (38) and disgusting scenes (39). Taken together with the electrophysiological and few lesion studies, these data support the hypothesis that the AI is involved in both subjective feelings and recognition of disgust.
Our results support the hypothesis that the AI underpins not only disgust perception, but also the real-life behavioral response to disgusting stimuli. The insula is thought to be an integrative hub, receiving sensory, somesthetic and interoceptive inputs from cortical areas including the medial temporal lobe and the amygdala, the basal ganglia, and the thalamus (40–44). Kurth's meta-analysis of 1,768 functional neuroimaging experiments revealed four functionally distinct regions in the human insula (31). Social-emotional tasks activated the anterior-ventral insula, sensorimotor tasks activated the mid-posterior insula, olfacto-gustatory stimuli activated the central insula, and cognitive tasks activated the anterior-dorsal insula. Furthermore, a conjunction analysis across these four domains revealed an area of functional overlap that includes the dorsal AI region identified in our study (Table 4). This convergence suggests that this dorsal AI region might provide functional integration between these systems and may explain why patients with damage to this region can neither recognize disgust in others nor properly regulate their own disgusting behavior probably due to an inability to adequately feel disgusted. Additionally, because our results are derived from lesion-behavior mapping rather than patterns of functional activation in healthy individuals, our study suggests that this AI integrative region is not only functionally involved in, but is actually required for normal disgust processing.
Outside of the key region of functional overlap in the insula, we found additional regions that correlated with either diminished perception of disgust or engagement in disgusting behavior. First, our patients’ ability to recognize and correctly name a disgusted emotional expression correlated with decreased grey matter volume in the central and anterior insula, as well as in the amygdala and temporal poles bilaterally. The central insula peaks in our study were most near regions associated with somatosensory and chemical perception (taste and smell) according to the Kurth meta-analysis (31), suggesting that access to representations of sensory experiences may have played a role in the ability to discriminate among emotions, and specifically to discern disgusted expressions in others. The role of the amygdala in emotional signal detection is well-established (45; 46), and the temporal regions found in our study have been widely associated with both socioemotional (R>L) and object-related (L>R) semantic knowledge (47; 48) as well as the ability to access the lexical names of emotions (49–52).
Regional decreased grey matter volumes associated with patients’ tendency to engage in disgusting behaviors were more dorsal and anterior to regions associated with impaired recognition of disgust, extending rostrally into the frontal lobe, and including the ACC. The functional domain determined by the Kurth et al. meta-analysis most closely related to these AI peaks was attention, particularly on the left, followed by other aspects of cognitive processing (top-down error monitoring, working memory, speech, and language), and emotion processing (i.e., imagining or recalling emotion) on the right (31). These findings are consistent with the known anterior to posterior functional gradient within the brain in which more posterior structures generally are involved in processing sensory inputs, while anterior structures such as the ACC are involved in behavioral response initiation and maintenance of task set (36; 53). Functionally, the ACC is downstream of the sensory representations generated in the insula, thus our patients’ behavioral responses to disgusting stimuli were likely predicated upon their visceroceptive experience of the stimuli. Additionally, the ACC is the primary effector of autonomic response and diminished output from this region may have dampened the individual's ability to stimulate the visceral responses associated with disgust.
Our analysis demonstrated that decreased grey matter volume in the central insula correlated with accuracy of disgust recognition over and above the recognition of other emotions. Whether there are emotion specific (e.g., fear or disgust) neural circuits is an area of dispute (46; 54–60). Because the AI is involved in disgust, pain, and other emotion-related processes, a disgust-specific functional hypothesis for this brain region is untenable. This has led to the hypothesis that the AI may play a broader role in emotion processing by translating what we perceive into visceral responses that color our subjective feelings, and that any disgust-specific relationships are due to disgust's relatively greater dependence on visceral feelings (61). This explanation is consistent with our findings that patients who exhibited disgusting behaviors had a deficit in recognizing disgust as a group, but not every subject who exhibited disgusting behaviors had a deficit in disgust recognition. One possible interpretation is that being able to translate disgusted faces into visceral feelings of disgust is helpful but not required for recognition of disgust in others. Indeed, our imaging and behavioral results suggest that some subjects may be able to recognize disgust in others using purely cognitive strategies without feeling the emotion themselves.
Clinical implications
Patients with bvFTD and to a lesser extent svPPA were more likely to demonstrate disgusting behaviors than other diagnostic groups. BvFTD and svPPA are associated with dramatic behavioral symptoms including disinhibition, loss of insight and empathy, and socially inappropriate behavior (62; 63). In bvFTD, these symptoms often are the first and sole symptoms leading to frequent diagnostic confusion with primary psychiatric disorders (64). While disgusting behaviors have frequently been described in bvFTD and svPPA (65) and decreased sensitivity to disgusting stimuli has been found in patients with bvFTD (24), this association has not previously been systematically quantified or localized to a specific anatomic substrate.
In the earliest clinical phases of bvFTD, atrophy can be seen within the AI, the ACC, and a network of subcortical and thalamic regions (66), a spatial pattern similar to the intrinsically connected salience network that processes diverse homeostatically relevant stimuli (65; 67; 68). In svPPA, initial symptoms are typically loss of knowledge of semantic meaning (69) and behavioral changes akin to those seen in bvFTD depending upon the degree to which the disease has advanced into the right hemisphere (69–71). This symptom progression pattern correlates well with the pattern of neural atrophy spread from temporal to frontal regions in svPPA (72). Thus, bvFTD and svPPA are associated with damage to the AI and the salience network, which likely explains the high prevalence of disgusting behaviors in these disorders (for further discussion, see Supplemental Materials).
Limitations
Our initial sample consisted of all patients with high quality chart-based data. Only a subset of this sample had neuroimaging or emotion recognition data requiring us to perform sub-sample analyses to maximize power. Furthermore, there were discrepancies in cognitive impairment between diagnostic groups and between individuals with and without disgusting behaviors. This is a standard limitation in such observational studies of heterogeneous patient groups, and as a precaution we have co-varied all analyses using a measure of disease severity (MMSE). Disgusting behaviors were determined by chart review, raising the question of the reliability of the data. For example, disgusting behaviors might have been present that were unreported and subtle clinician bias may have been present. Furthermore, severity and frequency of disgusting behaviors could not be adequately assessed by chart review, raising the possibility of significant differences in severity between patient groups that went unmeasured. Cultural factors can also contribute to what is classified as disgusting raising concern for cultural relativism. However, all of the behaviors classified as disgusting were new and distressing to the patient's family suggesting that cultural factors could not solely explain our findings.
Additionally, because patients’ sensory or subjective experiences of disgust were not directly measured, we do not have direct evidence that the disgusting behaviors demonstrated by our patients represent a failure to appropriately feel disgusted. Alternatively, disgusting behaviors may be due to more general disinhibition or impulsivity in spite of experiencing normal feelings of disgust. However, this explanation appears inadequate because disinhibition and impulsivity are common in multiple disorders, including Williams disease, suicidality, obesity, and substance abuse (73–78). Despite this, disgusting behaviors such as those found in the current study are rare in these other conditions, and thus are unlikely to be simply due to general disinhibition or impulsivity. Additionally, we could not elucidate the factors that might contribute to disgusting behaviors. For example, loss of awareness of social conventions or of response inhibition could both contribute to disgusting behavior and these contributing factors could be disease-specific. Future studies should investigate whether AI lesions lead to decreased subjective and physiological responses to disgusting stimuli and should also investigate the specificity between AI lesions and disgust.
Conclusion
Ours is the first large-scale lesion study to demonstrate that disruption of partially overlapping neural circuits within the AI are associated with increased tendency to engage in disgusting behaviors and impaired ability to recognize disgust in others. These findings complement the extant literature linking disgust processing with the insula that have primarily used functional imaging techniques.
Supplementary Material
Acknowledgments
We would like to thank Dr. A.D. (Bud) Craig for his insightful input when preparing this paper. Also, we would like to thank the staff of the Memory and Aging Center, Program Project Grant (PPG), Alzheimer's Disease Research Center (ADRC), and General Clinical Research Center (GCRC), and Clinical and Translational Science Institute (CTSI) for their contributions in time and resources to this article. As always, we are grateful to our patients and their families, because without their generosity we would never be able to do this work.
J. Woolley is funded by a Career Development Award grant from the San Francisco VA Medical Center (SFVAMC) 1IK2CX000758-01A1. V. Sturm is funded by NIA grant 1K23AG040127 and the Larry L. Hillblom Foundation grant 2013-A-029-SUP. R. Levenson is funded by NIH grants NIA P01 AG019724 and NIA R01 AG041762. W. Seeley received consulting fees from Biogen Idec. B. Miller received grant support from the NIH/NIA and the Centers for Medicare & Medicaid Servicces (CMS). As an additional disclosures, Dr. Miller serves as Medical Director for the John Douglas French Alzheimer's Foundation; Scientific Director for the Tau Consortium; Director/Medical Advisory Board of the Larry L. Hillblom Foundation; and Scientific Advisory Board Member for the National Institute for Health Research Cambridge Biomedical Research Centre and its subunit, the Biomeidcla Research Unit in Dementia (UK) K. Rankin is funded by NIH grants NIH/NIA 1R01AG029577, FTD PPG grant AG019724, ADRC grant P50AG023501, as well as the Larry L. Hillblom Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Darwin C. Expr Emot Man Anim. 1st ed. John Murray; London: 1972. Chapter XI.—Disdain—Contempt—Disgust—Guilt—Pride, Etc.— Helplessness—Patience—Affirmation and Negation. p. 254. [Google Scholar]
- 2.Rozin P, Haidt J, McCauley CR. Disgust. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handb Emot. 3rd ed. The Guildford Press; New York: 2008. pp. 757–776. [Google Scholar]
- 3.Miller S. Disgust: The Gatekeeper Emotion. The Analytic Press, Inc.; Hillsdale, New Jersey: 2004. [Google Scholar]
- 4.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 5.Stark R, Schienle A, Walter B, Kirsch P, Sammer G, Ott U, et al. Hemodynamic responses to fear and disgust-inducing pictures: an fMRI study. Int J Psychophysiol. 2003;50:225–234. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 6.Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- 7.Mataix-Cols D, An SK, Lawrence NS, Caseras X, Speckens A, Giampietro V, et al. Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. Eur J Neurosci. 2008;27:3050–3058. doi: 10.1111/j.1460-9568.2008.06311.x. [DOI] [PubMed] [Google Scholar]
- 8.Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, et al. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol Psychiatry. 2003;54:751–756. doi: 10.1016/s0006-3223(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 9.Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 10.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krolak-Salmon P, Hénaff M-A, Isnard J, Tallon-Baudry C, Guénot M, Vighetto A, et al. An attention modulated response to disgust in human ventral anterior insula. Ann Neurol. 2003;53:446–453. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- 13.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 14.Penfield W, Faulk ME. The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- 15.Fiol ME, Leppik IE, Mireles R, Maxwell R. Ictus emeticus and the insular cortex. Epilepsy Res. 1988;2:127–131. doi: 10.1016/0920-1211(88)90030-7. [DOI] [PubMed] [Google Scholar]
- 16.Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- 17.Adolphs R, Tranel D, Damasio AR. Dissociable neural systems for recognizing emotions. Brain Cogn. 2003;52:61–69. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 18.Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, et al. Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain. 1996;119(Pt 5):1647–1665. doi: 10.1093/brain/119.5.1647. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Hoosain R, Yang R-M, Meng Y, Wang C-Q. Impairment of recognition of disgust in Chinese with Huntington's or Wilson's disease. Neuropsychologia. 2003;41:527–537. doi: 10.1016/s0028-3932(02)00171-9. [DOI] [PubMed] [Google Scholar]
- 20.Gray JM, Young AW, Barker WA, Curtis A, Gibson D. Impaired recognition of disgust in Huntington's disease gene carriers. Brain J Neurol. 1997;120(Pt 11):2029–2038. doi: 10.1093/brain/120.11.2029. [DOI] [PubMed] [Google Scholar]
- 21.Kipps CM, Duggins AJ, McCusker EA, Calder AJ. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. J Cogn Neurosci. 2007;19:1206–1217. doi: 10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Hoshino T, Shigemasu K, Kawamura M. Disgust-specific impairment of facial expression recognition in Parkinson's disease. Brain J Neurol. 2006;129:707–717. doi: 10.1093/brain/awl011. [DOI] [PubMed] [Google Scholar]
- 23.Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci Off J Soc Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckart JA, Sturm VE, Miller BL, Levenson RW. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia. 2012;50:786–790. doi: 10.1016/j.neuropsychologia.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidt J, McCauley C, Rozin P. Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personal Individ Differ. 1994;16:701–713. [Google Scholar]
- 26.McDonald S, Flanagan S, Rollins J, Kinch J. TASIT: A new clinical tool for assessing social perception after traumatic brain injury. J Head Trauma Rehabil. 2003;18:219–238. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, Vaitl D. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses [abstract].. Presented at the 9th International Conference on Functional Mapping of the Human Brain, NeuroImage.2003. [Google Scholar]
- 29.Stonnington CM, Tan G, Klöppel S, Chu C, Draganski B, Jack CR, Jr, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. NeuroImage. 2008;39:1180–1185. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage. 2009;47:2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 33.Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 37.Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PloS One. 2008;3:e2939. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- 39.Schafer A, Leutgeb V, Reishofer G, Ebner F, Schienle A. Propensity and sensitivity measures of fear and disgust are differentially related to emotion-specific brain activation. Neurosci Lett. 2009;465:262–266. doi: 10.1016/j.neulet.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage. 2012;61:1129–1142. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 42.Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci Off J Soc Neurosci. 2009;29:14223–14235. doi: 10.1523/JNEUROSCI.3398-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 44.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 45.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 46.Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Soc Cogn Affect Neurosci. 2009;4:127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gesierich B, Jovicich J, Riello M, Adriani M, Monti A, Brentari V, et al. Distinct neural substrates for semantic knowledge and naming in the temporoparietal network. Cereb Cortex. 2012;22:2217–2226. doi: 10.1093/cercor/bhr286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mesulam MM. From sensation to cognition. Brain J Neurol. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 49.Wildgruber D, Ackermann H, Kreifelts B, Ethofer T. Cerebral processing of linguistic and emotional prosody: fMRI studies. Prog Brain Res. 2006;156:249–268. doi: 10.1016/S0079-6123(06)56013-3. [DOI] [PubMed] [Google Scholar]
- 50.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 51.Skipper LM, Ross LA, Olson IR. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49:3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiethoff S, Wildgruber D, Kreifelts B, Becker H, Herbert C, Grodd W, Ethofer T. Cerebral processing of emotional prosody--influence of acoustic parameters and arousal. NeuroImage. 2008;39:885–893. doi: 10.1016/j.neuroimage.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 55.Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72:70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 56.Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cogn Neuropsychiatry. 2006;11:219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- 57.Atkinson AP, Heberlein AS, Adolphs R. Spared ability to recognise fear from static and moving whole-body cues following bilateral amygdala damage. Neuropsychologia. 2007;45:2772–2782. doi: 10.1016/j.neuropsychologia.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 59.Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci. 2002;14:709–720. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- 60.Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural Netw. 2005;18:317–352. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 62.Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Assoc Disord 19 Suppl. 2005;1:3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- 63.Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4:771–780. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- 64.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72:126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seeley WW. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct. 2010;214:465–475. doi: 10.1007/s00429-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain J Neurol. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 72.Fletcher PD, Warren JD. Semantic dementia: a specific network-opathy. J Mol Neurosci. 2011;45:629–636. doi: 10.1007/s12031-011-9586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris CA. The behavioral phenotype of Williams syndrome: A recognizable pattern of neurodevelopment. Am J Med Genet C Semin Med Genet. 2010;154C:427–431. doi: 10.1002/ajmg.c.30286. [DOI] [PubMed] [Google Scholar]
- 74.Gvion Y, Apter A. Aggression, impulsivity, and suicide behavior: a review of the literature. Arch Suicide Res. 2011;15:93–9112. doi: 10.1080/13811118.2011.565265. [DOI] [PubMed] [Google Scholar]
- 75.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes Rev. 2008;9:409–419. doi: 10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 76.Leeman RF, Grant JE, Potenza MN. Behavioral and neurological foundations for the moral and legal implications of intoxication, addictive behaviors and disinhibition. Behav Sci Law. 2009;27:237–259. doi: 10.1002/bsl.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 78.Aragues M, Jurado R, Quinto R, Rubio G. Laboratory paradigms of impulsivity and alcohol dependence: a review. Eur Addict Res. 2011;17:64–71. doi: 10.1159/000321345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.