Abstract
Objective
Brain-computer interfaces (BCIs) aimed at restoring communication to people with severe neuromuscular disabilities often use event-related potentials (ERPs) in scalp-recorded EEG activity. Up to the present, most research and development in this area has been done in the laboratory with young healthy control subjects. In order to facilitate the development of BCI most useful to people with disabilities, the present study set out to: (1) determine whether people with amyotrophic lateral sclerosis (ALS) and healthy, age-matched volunteers (HVs) differ in the speed and accuracy of their ERP-based BCI use; (2) compare the ERP characteristics of these two groups; and (3) identify ERP-related factors that might enable improvement in BCI performance for people with disabilities.
Methods
Sixteen EEG channels were recorded while people with ALS or healthy age-matched volunteers (HVs) used a P300-based BCI. The subjects with ALS had little or no remaining useful motor control (mean ALS Functional Rating Scale-Revised 9.4(±9.5SD) (range 0–25)). Each subject attended to a target item as the items in a 6×6 visual matrix flashed. The BCI used a stepwise linear discriminant function (SWLDA) to determine the item the user wished to select (i.e., the target item). Offline analyses assessed the latencies, amplitudes, and locations of ERPs to the target and non-target items for people with ALS and age-matched control subjects.
Results
BCI accuracy and communication rate did not differ significantly between ALS users and HVs. Although ERP morphology was similar for the two groups, their target ERPs differed significantly in the location and amplitude of the late positivity (P300), the amplitude of the early negativity (N200), and the latency of the late negativity (LN).
Conclusions
The differences in target ERP components between people with ALS and age-matched HVs are consistent with the growing recognition that ALS may affect cortical function. The development of BCIs for use by this population may begin with studies in HVs but also needs to include studies in people with ALS. Their differences in ERP components may affect the selection of electrode montages, and might also affect the selection of presentation parameters (e.g., matrix design, stimulation rate).
Significance
P300-based BCI performance in people severely disabled by ALS is similar to that of age-matched control subjects. At the same time, their ERP components differ to some degree from those of controls. Attention to these differences could contribute to the development of BCIs useful to those with ALS and possibly to others with severe neuromuscular disabilities.
Keywords: Brain-computer interface (BCI), alternative and augmentative communication (AAC), event-related potentials (ERP), amyotrophic lateral sclerosis (ALS), brain-machine interface (BMI), electroencephalography (EEG)
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive disease of the lower and often upper motor neurons that usually leads to complete paralysis within 2–5 years. Each year, approximately 5,600 people in the United States are diagnosed with ALS at a mean age of 55 years. About 30,000 people are living with ALS in the United States alone (ALS Association (AA), 2013). As the disease progresses, individuals may use their remaining muscle control to operate a variety of assistive communication devices for interacting with the world (Doyle and Phillips, 2001). However, as their disease progresses these devices may become ineffective. Brain-computer interface (BCI) technology allows people with severe motor disabilities (i.e. ALS) to use brain signals, rather than muscles, to communicate and to control their environments (Wolpaw and Wolpaw, 2012). Most BCIs use electrical signals recorded from the scalp, the cortical surface, or within the brain. These signals are analyzed and translated into control commands for an output device (e.g., mouse, keyboard, robotic arm).
Use of the P300 event-related evoked potential (ERP) (also called the oddball response) as the basis for a BCI was first demonstrated by Farwell and Donchin (1988). Since this initial description, visual P300-based BCIs have been extensively studied primarily in the laboratory (See Sellers, et al. 2012 for review). For example, in the P300-based BCI speller, a 6×6 matrix of 36 items (i.e., characters and numbers) is presented on a screen; and the user attends to the desired item (i.e., the target) while different groups of items flash rapidly. About 300 msec after the target item flashes, a positive deflection occurs in the EEG; this is the P300 ERP. By flashing each item a number of times, and averaging the EEG following each flash (i.e., stimulus), the BCI can usually identify the target item (i.e., the item that evokes a P300 ERP). P300 BCI requires little user training, and can provide to most people slow but reliable communication.
While most P300-based BCIs have been studied mainly in healthy volunteers (HVs), their usage has been explored in small numbers of people with ALS or other severe neuromuscular disabilities (Escolano et al., 2010, Hoffmann et al., 2008, Ikegami et al., 2014, Kaufmann et al., 2013a, Kubler and Birbaumer, 2008, Kubler et al., 2009, Manyakov et al., 2011, McFarland et al., 2011, Nijboer et al., 2008, Pires et al., 2011, Pires et al., 2012, Sellers and Donchin, 2006, Sellers et al., 2010, Silvoni et al., 2009, Spuler et al., 2012, Townsend et al., 2010). Visual problems can occur in people with advanced ALS (e.g. Cooperman, 1974, Hall, 2003, Hayashi and Oppenheimer, 2003, Murguialday et al., 2011) and could impair use of visual BCIs in some people (McCane et al., 2014). Tactile and auditory P300 BCIs have been explored for those individuals with vision problems (Hill et al., 2014, Severens et al., 2014). All of these studies indicate that P300 BCIs might be useful to people with ALS as their disease advances and they lose the ability to use conventional assistive communication devices, all of which require some measure of muscle control.
However, it is not clear whether P300-based BCIs can function as well in people with severe disabilities as in HVs, nor is it clear whether specific disorders are associated with ERP differences that may affect the design of optimal BCI systems. The present study sought to address these issues for people with ALS. The P300-based BCI performance and ERP characteristics of people severely disabled by ALS were compared to those of an age-matched group of HVs. We expected to find that HVs and people with ALS and no visual problems would not differ in BCI performance and would have similar ERP components. The results are both encouraging in regard to the potential value of P300-based BCIs for people with severe disabilities and instructive in regard to how BCIs might be most effectively developed and optimized for use by those who need them. The study is part of a program to enable the clinical dissemination of BCIs for independent use by people with severe neuromuscular disabilities (Vaughan et al., 2006).
Methods
Subjects
The study was approved by the Institutional Review Boards of Helen Hayes Hospital (subjects with ALS) and the New York State Department of Health (HVs). All subjects provided informed consent (or assent) for the study. The 14 subjects (2 women and 12 men) with ALS were referred for BCI evaluation by the Center for Rehabilitation Technology (CRT) at Helen Hayes Hospital or were self-referred; and they were studied in their homes (N=12) or at the CRT (N=2). They were medically stable and: had little or no remaining useful motor control; had no major visual impairments; had stable physical and social environments; had elected to accept artificial ventilation when and if it became necessary; and had an interest in using BCI technology for communication on a daily basis (Vaughan et al., 2006). Their mean age was 55.9(±9.4SD, range 41–72) and their mean ALS Functional Rating Scale-Revised, ALSFRS-R, (Cedarbaum et al., 1999) was 9.4(±9.5SD, range 0–25). Seven (50%) were completely dependent on mechanical ventilation (MV). Data from 13 of these subjects were included in a prior study that showed no relationship between ALSFRS-R score and BCI accuracy (McCane et al., 2014). The fourteen age-matched HVs (2 women and 12 men) were evaluated at the Wadsworth Center. Their mean age was 55.8(±9.0SD, range 39–69).
Evaluation Protocol
All aspects of BCI operation and data collection were controlled by the BCI2000 software platform (Schalk et al., 2004, Schalk and Mellinger, 2010) on a Lenovo T61 laptop (Intel Core2 Duo CPU, 2 Ghz, 1.9 GB of RAM, Windows XP SP3). The subject viewed a separate 50-cm Dell monitor at a distance of about 1 meter.
The subject sat in a comfortable chair or in his/her own wheelchair or bed facing the monitor. The monitor was on a rolling stand or an over-the-bed table; and its position was adjusted for each user. The evaluation comprised 9 copy-spelling runs (i.e., the subject was told which item to select, i.e., the target item). Each run included one word (i.e., The/quick/brown/fox/jumps/over/the/lazy/dog), for a total of 35 letters (i.e., trials) that encompassed the entire English alphabet. For each trial, the subject was asked to attend to the target character and to count the number of times it flashed. These instructions were carefully explained and illustrated; and the first run did not begin until the subject indicated full understanding of the task. After each run, each subject was asked if s/he wished to continue. The entire evaluation (i.e., consenting process, electrode cap application, task instructions, 9 runs, and cap removal) lasted 60–90 min.
The user’s monitor displayed 36 items (i.e., English characters and numbers) arranged in a 6×6 matrix (target ratio 1:36; 2.8% chance accuracy). The items were light gray and the background was black. In the evaluation (i.e., copy-spelling) protocol, the subject was asked to spell (i.e., select) 35 letters comprising 9 words. The “text–to–spell” bar above the matrix displayed the word to be spelled (i.e., copied) (Figure 1A). At the beginning, the words “Waiting to start” were displayed over the matrix and the first target item (i.e., the first letter of the first word to be spelled) was shown in parenthesis at the end of the word. After 4 sec, items began to flash in groups of 4, 5 or 6 with no two items in the group adjacent to each other (i.e., the checkerboard (CB) format, Townsend et al., 2010). A group flashed every 250 ms (i.e., 4-Hz flash rate). For each selection by HVs, the flashes continued until each item had flashed 14 times (a total of 490 target and 3920 non-target flashes for the 35 copy-spelling selections); for each selection by subjects with ALS, the flashes continued until each item had flashed 14, 20 or 24 times depending on the subject (i.e., 490–840 target and 3920–6720 non-target flashes for the 35 selections). The 35 selections were separated by 4-sec breaks. When each letter of the word had served as the target, the phrase “Time Out” was shown and the run was over. Several minutes later the next run began.
Figure 1.
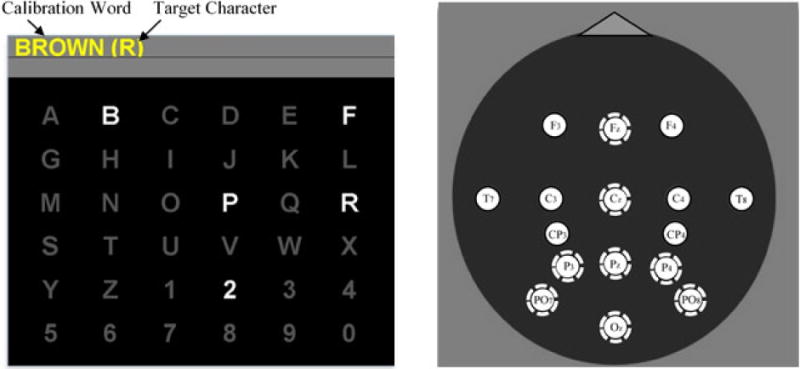
A. The BCI user’s monitor displayed a 6×6 matrix with the calibration word in a gray text-to-spell bar across the top. Target characters were cued one at a time in parenthesis after the word. A set of characters in the pseudo-random fashion of the CB mode is illuminated (flashed). Here, the user is asked to count the flashes of the target character “R” in the calibration word “BROWN.” B. The 16 channels of EEG collected during the evaluation and the 8-channel standard subset used for classification (dashed circles).
A 16–channel electrode cap (Electro-Cap International, Inc.) with tin electrodes at locations F3, Fz, F4, T7, C3, Cz, C4, T8, CP3, CP4, P3, Pz, P4, PO7, PO8 and Oz (Sharbrough et al., 1991) (Figure 1B) was used; and electrode impedance was kept below 20 kΩ (Kappenman and Luck, 2010). All electrodes were referenced to the right mastoid and grounded to the left mastoid. EEG activity was amplified by a 16-channel g.USBamp biosignal amplifier (i.e., from g-tec), sampled at a rate of 256 Hz, high-pass filtered at 0.5 Hz and low-pass filtered at 30 Hz, and stored. The noisy home environment and the close proximity of life-support equipment often necessitated use of the 58–62 Hz notch filter.
Data Analysis
A stepwise linear discriminant function (SWLDA) was applied to the standard P300 8-channel montage (Fz, Cz, P3, Pz, P4, PO7, PO8, and Oz) previously defined in order to select and weight the EEG features (i.e., voltages at specific EEG electrodes in specific time segments in the 800 msec after the items flashed) that were used to classify the subject’s response to each item and to thereby determine which item was the target (i.e., the desired selection). (See Krusienski, et al., 2008, for full description of analysis).
The first five runs (21 trials) were used as the calibration set. The EEG data from this set were used to determine the SWLDA coefficients, i.e., up to 60 features (Krusienski et al., 2008). These coefficients were applied to the test set: the last four runs of 14 trials. The accuracies reported here refer to the test set. We calculated for each subject the accuracy for each number of flashes up to the total number used, and determined the maximum accuracy (MA) and the least number of flashes that provided the MA. Based on these results, we calculated for each subject the theoretical communication rate (characters/min) and bit rate (bits/min). Characters/min was defined as the total correct characters minus total incorrect characters divided by the total time for each trial (McFarland et al., 2011). Bit rate was calculated as reported in Wolpaw et al. (2002). These calculations took into account: the time it took for all the characters in the matrix to flash for each trial; the time needed to correct an error; and the time between trials (Table 1).
Table 1.
Average (± SE) accuracy and rate for subjects with ALS and age-matched healthy volunteers (HVs). There were no significant differences between the groups.
| Maximum Accuracy (MA) (%) | 95.7 ± 2 | 98.8 ± 1 |
| Flashes required for MA | 11.6 ± 5 | 8.7 ± 2 |
| MA characters/min | 2.1 ± 0.3 | 2.6 ± 0.2 |
| MA bits/min | 11.2 ± 1.3 | 13.7 ± 1.1 |
We used the data from all 9 runs (i.e., 35 trials) to compute for each electrode of each subject the average ERPs for the target item and for the non-target items. The N200 component was defined as the most negative point between 80 and 240 ms, the P300 component as the most positive point between 230 and 450 ms, and the LN (late negativity) as the most negative point between 400 and 800 ms. For the target ERPs, component amplitude and latency measures were determined for the four central locations (Fz, Cz, Pz, Oz) for each subject and then averaged across each subject group (Table 2); grand average waveforms at Fz, Cz, Pz, and Oz were computed for each group (Figure 2); and 16-channel topographies at each subject’s component latencies were averaged across each group (Figure 3).
Table 2.
The average peak locations, latencies (± SD), and amplitudes (± SD) of the target ERP components for the ALS group and the HV group.
| N200 | Cz | Amplitude (μv) | −1.56 ± 0.8 | −0.73 ± 0.4 |
| Latency (ms) | 137 ± 38 | 123 ± 24 | ||
| P300 | Cz | Amplitude | 3.54 ± 2.4 | 3.21 ± 0.8 |
| Latency | 283 ± 56 | 260 ± 19 | ||
| LN | Fz | Amplitude | −4.41 ± 3.6 | −3.55 ± 2.5 |
| Latency | 627 ± 81 | 598 ± 121 |
Figure 2.
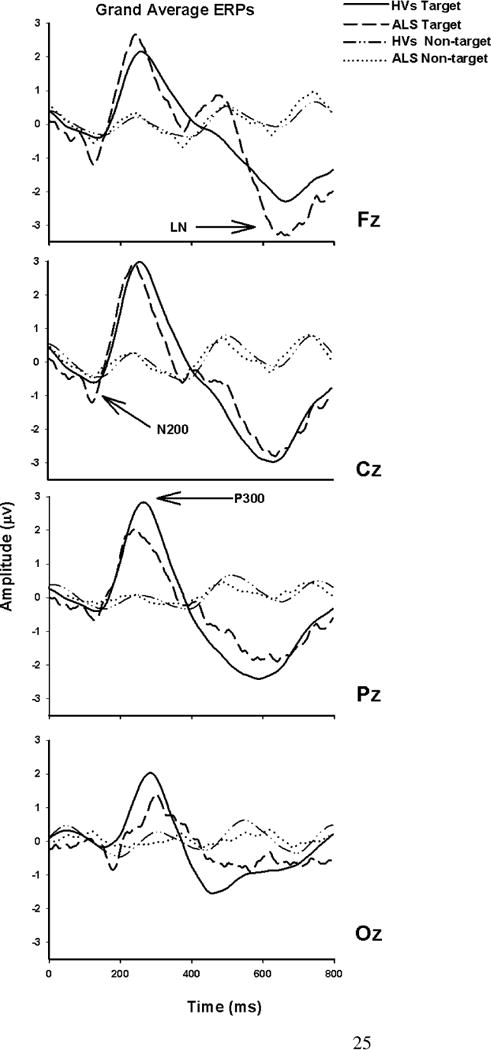
Grand-average ERP waveforms for the fourteen subjects in each group at Fz, Cz, Pz and Oz (all 35 trials). Target ERPs are solid for the HV group and dashed for the ALS group. Non-target ERPs are dot-dash (HV) and dotted (ALS). The three ERP components (N200, P300, and LN (late negativity)) are indicated with arrows.
Figure 3.
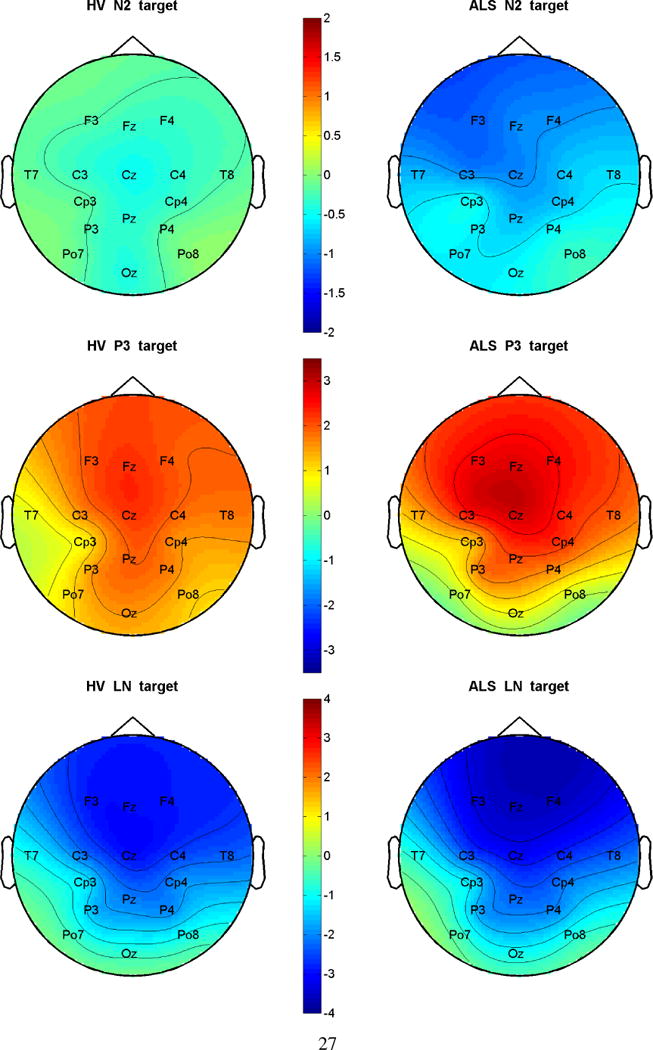
Spatial distributions of the three ERP components in the target condition for each group. The scale is in μv. Red is more positive and blue the more negative.
Statistical Methods
Measures that were normally distributed (Shapiro-Wilk) were evaluated by t-test (i.e., demographics, rates, and MA flashes). Measures that were non-normally distributed were evaluated by the Mann-Whitney Rank Sum Test or Spearman-Rank Order correlation (i.e., accuracies and online flashes). Analysis of variance (ANOVA) was performed for each component of the target ERPs with group as a between-subjects factor and electrode location (Fz, Cz, Pz, Oz) as a within-subjects factor.
Results
Accuracy and Rate
The 14 subjects with ALS and the 14 HVs did not differ significantly in maximum accuracy (MA) (95.7(±2SE)% and 98.8(±1), respectively, Table 1). The average number of flashes needed to achieve the MA was greater for ALS users than for HVs, but the difference did not reach significance (11.6(±1SE) and 8.7(±0.6), respectively; p=0.08). HVs had a slightly higher communication rate (and bit rate) than the subjects with ALS, but the difference was not significant (Table 1, p=0.15 and p= 0.16 respectively).
Event-related potentials
The morphologies of the grand-average target and non-target waveforms were similar for the two groups (Figure 2). Table 2 shows for each group and each component of the target ERPs the location at which the component was largest and its amplitude and latency at that location. In both groups, N200 and P300 were largest at Cz and LN was largest at Fz. The latencies of all three ERP components were longer in the ALS group than in the HV group.
The topographical distributions of the three ERP components for the two groups in the target condition are shown in Figure 3. In the HV group, N200 was located centrally while P300 and LN were located anteriorly. In the ALS group, N200 and P300 were located more anteriorly than in the HV group, while LN was located similarly to the HV group. N200 was larger in the ALS group than in the HV group, and P300 was more focused topographically.
Analysis of variance (ANOVA) revealed a significant effect of group (df=1,26, F=16.35, p<0.004) and electrode (df=3,78, F=5.48, p<0.0018) for N200 amplitude. The larger N200 peak in the ALS group compared to the HV group can be seen in Figures 2 and 3. The significant effect of electrode location for N200 latency (df=3,78, F=14.72, p<0.0001) indicates individual differences for this metric. There was a significant effect of electrode (df=3,78, F=18.62, P<0.0001) and the group × electrode interaction (df=3,78, F=4.25, p<0.0078) for P300 amplitude, indicating that the group difference is dependent on the electrode location. Posterior attenuation of the P300 component in the ALS group compared to the HV group was evident in the grand average waveforms (Figure 2) and 16-channel topographies (Figure 3). The effect of electrode location was significant for P300 latency (df=3,78, F=5.52, p<0.0017), but not the location × group interaction (df=3,78, F=1.78, p=0.16). The effect of electrode location was significant for LN amplitude (df=3,78, F=12.69, p<0.0001); while the effect of group (df=1,26, F=4.38, p<0.0462) was significant for the LN latencies. The LN latency difference between the groups was not channel-dependent.
Impact of Disability
The ALSFRS-R did not correlate with BCI accuracy nor with the latency or amplitude of any component of the target ERP.
Effects of Age
There was no significant difference in age between the two groups (p=0.89). Age at time of evaluation correlated negatively with MA in the HV group (p=0.05). Bit rate increased slightly with age in the ALS group and decreased slightly in the HV group, but these effects were not significant. P300 amplitude measured at Fz decreased with age in both groups, but the decrease did not reach significance. Age did not correlate with N200 or LN amplitude, nor with the latency of any target ERP component.
Discussion
This study set out to address two questions: whether the P300-based BCI performance of people severely disabled by ALS differed from that of age-matched healthy control subjects (HVs); and whether these two groups differed in the amplitudes, latencies, or topographies of P300 and other ERP components during BCI use. These questions are important for the development and potential efficacy of these BCIs as alternative communication and control devices for people with severe disabilities.
BCI Performance
Our results revealed no significant difference in BCI performance (i.e., assessed as accuracy, communication rate, or bit rate) between very disabled ALS users (50% MV dependent, mean ALSFRS-R=9.4) and age-matched HVs. At the same time, it is important to note that subjects with ALS who had visual impairments were excluded from the study. Visual problems can occur in advanced ALS (Cooperman, 1974, Hall, 2003, Hayashi and Oppenheimer, 2003, Murguialday et al., 2011) and the exclusion of these subjects may help to explain the superior performance reported here compared to previous studies in people with ALS or other severe neuromuscular disabilities where visual problems were not documented. (Ikegami et al., 2014, Kaufmann et al., 2013b, Ortner et al., 2011, Piccione et al., 2006, Sellers et al., 2006). In addition, differences in target presentation parameters (i.e., flash rate, number of target flashes, data used for the classifier, target ratio, etc.) could have contributed to the better performance in this study. While the present results are from a single evaluation, recent studies demonstrating consistent BCI performance over several years for subjects with ALS (Nijboer et al., 2010, Sellers et al., 2010, Silvoni et al., 2013) suggest that the present results are representative.
Offline analysis indicated both HVs and subjects with ALS could have performed just as well with fewer flashes than were actually presented online. The ALS users needed more flashes to reach maximum performance and had slightly lower bitrates on average than the HVs, however, these differences were not statistically significant (Table 1). Accuracies were higher for ALS subjects in this study than in other P300 communication device studies of subjects with motor impairments and control subjects. However, the average bit rate for the ALS users here (11.2 bits/min) was within the range reported in those studies (range 6.7 to 20 bits/min) (Hoffmann et al., 2008, Kaufmann et al., 2013b, Piccione et al., 2006, Pires et al., 2011, Pires et al., 2012, Silvoni et al., 2009). The similar bit rates of the subjects with ALS in this study when compared to other studies with lower accuracies may have been due to the accuracy versus speed protocol parameters chosen here. These settings were designed to capture P300 BCI speller capability in a wide range of potential users. Communication rate can be improved with stimuli optimization after initial evaluation with the BCI speller system (McFarland et al., 2011). The HV bit rate here (13.7 bits/min) was below the average of five studies where bit rate was reported for younger control subjects (range of average ages 29–54 years; range of average bit rates 6.7–32 bits/min) (Hoffmann et al., 2008, Piccione et al., 2006, Pires et al., 2011, Pires et al., 2012, Silvoni et al., 2009). These results indicate the importance of age-matched controls when comparing BCI communication rates in people with disabilities to those in healthy control subjects.
The study did not reveal any strong correlations between age and BCI performance, either in people with ALS or in HVs. Silvoni et al. (2013) found a positive correlation between BCI accuracy (four-choice task) and age at study entry in 24 more able-bodied people with ALS (mean ALSFRS-R= 32, age= 56). The difference from the present study could be due to the marked difference in level of disability and/or to differences in methods.
Event-Related Potential (ERP) Components
As expected (Brunner et al., 2010, McCane et al., 2014, Sellers et al., 2006, Townsend et al., 2010), the target ERPs had three major components, N200, P300, and the late negativity (LN). We compared the two subject groups in regard to the amplitudes, latencies, and topographies of these components.
Delayed P300 latencies and reduced amplitudes (at Pz) for ALS patients when compared with HVs have previously been documented (Ogawa et al., 2009, Paulus et al., 2002) and were also noted in this study (though the latency differences were not significant here). The significant effects of channel and group × channel for P300 amplitudes found here may reflect the more anterior focus of this component in subjects with ALS. This location shift of the P300 seen in the ALS group (mean age 56, with only one person over 65 years) is similar to that reported for older subjects (i.e., age 65–79) (Fjell and Walhovd, 2001, Friedman et al., 1997, Pato and Czigler, 2011, Riis et al., 2008). Thus, the P300 topographies of our subjects with ALS appear to be similar to those of older healthy control subjects. This more anterior distribution should be taken into account in developing electrode montages for use by people with ALS.
The target ERPs of the ALS group displayed an N200 component that was focused over frontal areas and was significantly larger than the N200 of the HV group. Several previous studies in healthy subjects have indicated the importance of early (i.e., <200 ms) components for the performance of comparable BCI systems (Bianchi et al., 2010, Brunner et al., 2010, Halder et al., 2013, Mak et al., 2011). The present results suggest that montages that include additional frontal electrodes could improve BCI performance in people with ALS.
The prominent LN component in the present data is consistent with previous data for the same BCI paradigm (Townsend et al., 2010). The longer latency for the HVs of the present study compared to the earlier study may reflect the greater age of the present subjects. As found for the N200 and P300 latencies, LN latency was longer, but not significantly, in the subjects with ALS than in the HVs (Figure 2). The prominence of this component in people with ALS implies that it can contribute substantially to BCI performance; and it suggests that BCI development should seek recording and presentation methods that enhance it (e.g., the CB paradigm Townsend et al., 2010 or the Faces paradigm Kaufmann et al., 2013b).
All the subjects understood the task and could maintain their attention well enough to produce excellent BCI performance (i.e., accuracy >95%). Nevertheless, the observation that all three target ERP components had longer latencies in the ALS group imply changes in cortical function that could conceivably affect cognition. Cognitive changes often accompany ALS (Abrahams et al., 2014, Goldstein and Abrahams, 2013); and further study of the relationships of such changes with ERP components and BCI performance is needed. Recent studies have shown that cognitive training can increase ERP amplitudes in elderly subjects (O’Brien et al., 2013) and that mindfulness training can improve BCI performance in healthy subjects (Lakey et al., 2011).
Conclusions
P300-based BCI performance in people severely disabled by ALS who do not have significant visual impairments is similar to that in age-matched healthy control subjects. In subjects with ALS compared to age-matched control subjects, the N200 and P300 components of the target ERP were located more anteriorly, the N200 component was larger, and the N200, P300, and LN components tended to have longer latencies. Recognition and engagement of such differences from control subjects could contribute to the development of BCIs useful to those with ALS and possibly to others with severe neuromuscular disabilities.
Highlights.
Communication rate and accuracy with a P300-based BCI speller did not differ significantly between people severely disabled by ALS who have adequate vision and age-matched control subjects.
The two groups differed significantly in P300 amplitude and location, N200 amplitude, and latency of the late negativity (LN).
P300-based BCI studies and usage in people with ALS may benefit from use of additional ERP components and alternative montages.
Acknowledgments
We thank all the people with ALS and their caregivers who donated their valuable time to participate in this study; and we thank William Baxter for assistance in creating the topographies.
This work has been supported by the National Institutes of Health (NIH) (Grants HD30146 (NCMRR/NICHD) and EB00856 (NIBIB & NINDS)), the James S. McDonnell Foundation, the NEC Foundation, the Altran Foundation, the ALS Hope Foundation, and the Brain Communication Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None of the authors have potential conflicts of interest to be disclosed.
References
- ALS Association (AA) Augmentative Communication [Online] ALS Association Website. 2013 Available at: http://www.alsa.org [Accessed September 2013].
- Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:9–14. doi: 10.3109/21678421.2013.805784. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Sami S, Hillebrand A, Fawcett IP, Quitadamo LR, Seri S. Which physiological components are more suitable for visual ERP based brain-computer interface? A preliminary MEG/EEG study. Brain Topogr. 2010;23:180–185. doi: 10.1007/s10548-010-0143-0. [DOI] [PubMed] [Google Scholar]
- Brunner P, Joshi S, Briskin S, Wolpaw JR, Bischof H, Schalk G. Does the ‘P300’ speller depend on eye gaze? J Neural Eng. 2010;7:056013. doi: 10.1088/1741-2560/7/5/056013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Cooperman R. Letter: Decreased Lacrimation in Amyotrophic Lateral Sclerosis. JAMA. 1974;230:536. [PubMed] [Google Scholar]
- Doyle M, Phillips B. Trends in augmentative and alternative communication use by individuals with amyotrophic lateral sclerosis. Augment Altern Comm. 2001;17:167–178. [Google Scholar]
- Escolano C, Murguialday AR, Matuz T, Birbaumer N, Minguez J. A telepresence robotic system operated with a P300-based brain-computer interface: initial tests with ALS patients. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:4476–4480. doi: 10.1109/IEMBS.2010.5626045. [DOI] [PubMed] [Google Scholar]
- Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988;70:510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topogr. 2001;14:25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephalogr Clin Neurophysiol. 1997;104:498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–380. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- Halder S, Hammer EM, Kleih SC, Bogdan M, Rosenstiel W, Birbaumer N, Kubler A. Prediction of auditory and visual p300 brain-computer interface aptitude. PLoS One. 2013;8:e53513. doi: 10.1371/journal.pone.0053513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V. Applying holism in the home care environment for clients with adavanced ALS: a toolkit for practice. Top Adv Pract Nur eJour. 2003;3 Retrieved from: http://www.medscape.com/viewarticle/452535. [Google Scholar]
- Hayashi H, Oppenheimer EA. ALS patients on TPPV: totally locked-in state, neurologic findings and ethical implications. Neurology. 2003;61:135–137. doi: 10.1212/01.wnl.0000069925.02052.1f. [DOI] [PubMed] [Google Scholar]
- Hill NJ, Ricci E, Haider S, McCane LM, Heckman S, Wolpaw JR, Vaughan TM. A practical, intuitive brain-computer interface for communicating ‘yes’ or ‘no’ by listening. J Neural Eng. 2014;11:035003. doi: 10.1088/1741-2560/11/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann U, Vesin JM, Ebrahimi T, Diserens K. An efficient P300-based brain-computer interface for disabled subjects. J Neurosci Methods. 2008;167:115–125. doi: 10.1016/j.jneumeth.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Takano K, Kondo K, Saeki N, Kansaku K. A region-based two-step P300-based brain-computer interface for patients with amyotrophic lateral sclerosis. Clin Neurophysiol. 2014;125:2305–2312. doi: 10.1016/j.clinph.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 2010;47:888–904. doi: 10.1111/j.1469-8986.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Holz EM, Kubler A. Comparison of tactile, auditory, and visual modality for brain-computer interface use: a case study with a patient in the locked-in state. Front Neurosci. 2013a;7:129. doi: 10.3389/fnins.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Schulz SM, Koblitz A, Renner G, Wessig C, Kubler A. Face stimuli effectively prevent brain-computer interface inefficiency in patients with neurodegenerative disease. Clin Neurophysiol. 2013b;124:893–900. doi: 10.1016/j.clinph.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM, Wolpaw JR. Toward enhanced P300 speller performance. J Neurosci Methods. 2008;167:15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Birbaumer N. Brain-computer interfaces and communication in paralysis: extinction of goal directed thinking in completely paralysed patients? Clin Neurophysiol. 2008;119:2658–2666. doi: 10.1016/j.clinph.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Furdea A, Halder S, Hammer EM, Nijboer F, Kotchoubey B. A brain-computer interface controlled auditory event-related potential (p300) spelling system for locked-in patients. Ann N Y Acad Sci. 2009;1157:90–100. doi: 10.1111/j.1749-6632.2008.04122.x. [DOI] [PubMed] [Google Scholar]
- Lakey CE, Berry DR, Sellers EW. Manipulating attention via mindfulness induction improves P300-based brain-computer interface performance. J Neural Eng. 2011;8:025019. doi: 10.1088/1741-2560/8/2/025019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JN, Arbel Y, Minett JW, McCane LM, Yuksel B, Ryan D, Thompson D, Bianchi L, Erdogmus D. Optimizing the P300-based brain-computer interface: current status, limitations and future directions. J Neural Eng. 2011;8:025003. doi: 10.1088/1741-2560/8/2/025003. [DOI] [PubMed] [Google Scholar]
- Manyakov NV, Chumerin N, Combaz A, Van Hulle MM. Comparison of classification methods for P300 brain-computer interface on disabled subjects. Comput Intell Neurosci. 2011;2011:519868. doi: 10.1155/2011/519868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCane LM, Sellers EW, McFarland DJ, Mak JN, Carmack CS, Zeitlin D, Wolpaw JR, Vaughan TM. Brain-computer interface (BCI) evaluation in people with amyotrophic lateral sclerosis. Amyotroph Lat Scler Frontotemporal Degener. 2014;15:207–215. doi: 10.3109/21678421.2013.865750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Sarnacki WA, Townsend G, Vaughan T, Wolpaw JR. The P300-based brain-computer interface (BCI): effects of stimulus rate. Clin Neurophysiol. 2011;122:731–737. doi: 10.1016/j.clinph.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murguialday AR, Hill J, Bensch M, Martens S, Halder S, Nijboer F, Schoelkopf B, Birbaumer N, Gharabaghi A. Transition from the locked in to the completely locked-in state: a physiological analysis. Clin Neurophysiol. 2011;122:925–933. doi: 10.1016/j.clinph.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Nijboer F, Birbaumer N, Kubler A. The influence of psychological state and motivation on brain-computer interface performance in patients with amyotrophic lateral sclerosis – a longitudinal study. Front Neurosci. 2010;4:1–15. doi: 10.3389/fnins.2010.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer F, Furdea A, Gunst I, Mellinger J, McFarland DJ, Birbaumer N, Kubler A. An auditory brain-computer interface (BCI) J Neurosci Methods. 2008;167:43–50. doi: 10.1016/j.jneumeth.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JL, Edwards JD, Maxfield ND, Peronto CL, Williams VA, Lister JJ. Cognitive training and selective attention in the aging brain: an electrophysiological study. Clin Neurophysiol. 2013;124:2198–2208. doi: 10.1016/j.clinph.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Tanaka H, Hirata K. Cognitive deficits in amyotrophic lateral sclerosis evaluated by event-related potentials. Clin Neurophysiol. 2009;120:659–664. doi: 10.1016/j.clinph.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Ortner R, Aloise F, Pruckl R, Schettini F, Putz V, Scharinger J, Opisso E, Guger C. Accuracy of a P300 speller for people with motor impairments: a comparison. Clin EEG Neurosci. 2011;42:214–218. doi: 10.1177/155005941104200405. [DOI] [PubMed] [Google Scholar]
- Pato L, Czigler I. Effects of novelty on event-related potentials: aging and stimulus replacement. Gerontology. 2011;57:364–374. doi: 10.1159/000314159. [DOI] [PubMed] [Google Scholar]
- Paulus KS, Magnano I, Piras MR, Solinas MA, Solinas G, Sau GF, Aiello I. Visual and auditory event-related potentials in sporadic amyotrophic lateral sclerosis. Clin Neurophysiol. 2002;113:853–861. doi: 10.1016/s1388-2457(02)00082-2. [DOI] [PubMed] [Google Scholar]
- Piccione F, Giorgi F, Tonin P, Priftis K, Giove S, Silvoni S, Palmas G, Beverina F. P300-based brain computer interface: reliability and performance in healthy and paralysed participants. Clin Neurophysiol. 2006;117:531–537. doi: 10.1016/j.clinph.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Pires G, Nunes U, Castelo-Branco M. Statistical spatial filtering for a P300-based BCI: tests in able-bodied, and patients with cerebral palsy and amyotrophic lateral sclerosis. J Neurosci Methods. 2011;195:270–281. doi: 10.1016/j.jneumeth.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Pires G, Nunes U, Castelo-Branco M. Comparison of a row-column speller vs. a novel lateral single-character speller: assessment of BCI for severe motor disabled patients. Clin Neurophysiol. 2012;123:1168–1181. doi: 10.1016/j.clinph.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR. Compensatory neural activity distinguishes different patterns of normal cognitive aging. Neuroimage. 2008;39:441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- Schalk G, Mellinger J. A Practical Guide to Brain–Computer Interfacing with BCI 2000. London: Springer; 2010. [Google Scholar]
- Sellers EW, Arbel Y, Donchin E. BCIs that use P300 Event-Realted Potentials. In: Wolpaw JR, Wolpaw EW, editors. Brain-computer interfaces: priciples and practice. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Sellers EW, Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clin Neurophysiol. 2006;117:538–548. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Sellers EW, Vaughan TM, Wolpaw JR. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler. 2010;11:449–455. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- Severens M, Van der Waal M, Farquhar J, Desain P. Comparing tactile and visual gaze-independent brain-computer interfaces in patients with amyotrophic lateral sclerosis and healthy users. Clin Neurophysiol. 2014;125:2297–2304. doi: 10.1016/j.clinph.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Picton TW. AEEGS Guidlines for Standard Electrode Postion Nomenclature. J of Clin Neurophysiol. 1991;8:200–202. [Google Scholar]
- Silvoni S, Cavinato M, Volpato C, Ruf CA, Birbaumer N, Piccione F. Amyotrophic lateral sclerosis progression and stability of brain-computer interface communication. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:390–396. doi: 10.3109/21678421.2013.770029. [DOI] [PubMed] [Google Scholar]
- Silvoni S, Volpato C, Cavinato M, Marchetti M, Priftis K, Merico A, Tonin P, Koutsikos K, Beverina F, Piccione F. P300-Based Brain-Computer Interface Communication: Evaluation and Follow-up in Amyotrophic Lateral Sclerosis. Front Neurosci. 2009;3:60. doi: 10.3389/neuro.20.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuler M, Bensch M, Kleih S, Rosenstiel W, Bogdan M, Kubler A. Online use of error-related potentials in healthy users and people with severe motor impairment increases performance of a P300-BCI. Clin Neurophysiol. 2012;123:1328–1337. doi: 10.1016/j.clinph.2011.11.082. [DOI] [PubMed] [Google Scholar]
- Townsend G, LaPallo BK, Boulay CB, Krusienski DJ, Frye GE, Hauser CK, Schwartz NE, Vaughan TM, Wolpaw JR, Sellers EW. A novel P300-based brain-computer interface stimulus presentation paradigm: moving beyond rows and columns. Clin Neurophysiol. 2010;121:1109–1120. doi: 10.1016/j.clinph.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan TM, McFarland DJ, Schalk G, Sarnacki WA, Krusienski DJ, Sellers EW, Wolpaw JR. The Wadsworth BCI Research and Development Program: at home with BCI. IEEE Trans Neural Syst Rehabil Eng. 2006;14:229–233. doi: 10.1109/TNSRE.2006.875577. [DOI] [PubMed] [Google Scholar]
- Wolpaw J, Wolpaw E. Brain-Computer Interfaces: Something New Under the Sun. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]


