Abstract
Background
Neonatal hypoxic ischemic encephalopathy (HIE) is a major cause of mortality, morbidity, and long-term neurological deficits. Despite the availability of neuroimaging and neurophysiological testing, tools for accurate early diagnosis and prediction of developmental outcome are still lacking. The goal of this study was to determine if combined use of magnetic resonance imaging (MRI) and electroencephalography (EEG) findings could support outcome prediction.
Methods
We retrospectively reviewed records of 17 HIE neonates, classified brain MRI and EEG findings based on severity, and assessed clinical outcome up to 48 months. We determined the relation between MRI/EEG findings and clinical outcome.
Results
We demonstrated a significant relationship between MRI findings and clinical outcome (Fisher’s exact test, p = 0.017). EEG provided no additional information about the outcome beyond that contained in the MRI score. The statistical model for outcome prediction based on random forests suggested that EEG readings at 24 hours and 72 hours could be important variables for outcome prediction, but this needs to be investigated further.
Conclusion
Caution should be used when discussing prognosis for neonates with mild-to-moderate HIE based on early MR imaging and EEG findings. A robust, quantitative marker of HIE severity that allows for accurate prediction of long-term outcome, particularly for mild-to-moderate cases, is still needed.
Keywords: electroencephalography, hypoxic ischemic encephalopathy, MRI, outcome
1. Introduction
Neonatal hypoxic ischemic encephalopathy (HIE) occurs in 1–2/1000 live births, accounts for 23% of neonatal deaths, and is the fifth largest cause of death in children under 5 years.1
Brain hypoxia triggers a cascade of events leading to neuronal death 12–36 hours after the initial ischemic insult,2 so prompt recognition is imperative for early, effective interventions that will prevent loss of neurons, including therapeutic hypothermia.3 In addition, identification of HIE and accurate classification of severity are important for reliable prediction of clinical outcome and long-term planning. Routine diagnostic modalities including electroencephalography (EEG) and magnetic resonance imaging (MRI) are somewhat limited tools, particularly in cases of moderate severity.
We investigated the relationship between EEG and MRI findings and outcome in infants with HIE who underwent therapeutic hypothermia. As a secondary investigation, we considered the predictive utility of EEG and MRI findings at 24 hours and 72 hours for determining the clinical outcome.
2. Methods
This study was approved by the West Virginia University Institutional Review Board (Morgantown, WV, USA). All newborns with HIE who underwent therapeutic hypothermia (total body cooling) at West Virginia University Hospital (WVUH) between 2009 and 2013 were included in this retrospective study. Clinical data were obtained from electronic medical records (Table 1).
Table 1.
Summary of clinical data.
| Pt | Sex | GA (wk) | Maternal history (age) | Delivery | Complications at delivery | HIE severity* |
Birth Wt (g) |
APGAR 0 min, 5 min, 10 min |
ABG pH |
Postnatal support | Sz | AED or sedative |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 38 | (35) | SVD | Shoulder dystocia | Moderate | 3800 | 0,0,2 | 6.97* | Ventilator and pressors | Y | Y |
| 2 | M | 37 | (23) anemia | SVD | Meconium aspiration | Moderate | 3033 | 2,4,7 | 6.98 | Ventilator | Y | Y |
| 3 | F | 41 | (34) | SVD | Meconium aspiration | Moderate | 3500 | 3,7 | 6.94 | Ventilator and pressors | N | N |
| 4 | F | 39 | (36) | SVD | DIC, PPHN, ARF | Moderate | 3000 | 6,8 | 6.8 | Ventilator and pressors | N | Y |
| 5 | F | 38 | (17) | SVD | Abnormal fetal HR | Severe | 3000 | 0, 0, 0 | 7.39 | Ventilator and pressors | Y | N |
| 6 | M | 40 | (29) tobacco | ECS | Fetal deceleration. Meconium aspiration |
Moderate | 3300 | 3, 7, 8 | 7.04 | Ventilator | N | Y |
| 7 | F | 38 | DM, preeclampsia hypothyroidism, (27) |
ECS | Fetal decelerations | Severe | 4180 | 1, 4, 8 | 6.8 | Ventilator | N | Y |
| 8 | F | 37 | tobacco (18) | SVD | Fetal decelerations | Moderate | 2520 | 1, 1, 4 | 7.25 | Ventilator | Y | Y |
| 9 | M | 36 | HELLP, polyhydramnios, tobacco, (30) |
CS | Hyaline membrane disease, RDS | Moderate | 3500 | 1, 2, 6 | 6.84 | Ventilator and pressors | N | N |
| 10 | M | 39 | (34) | ECS | Fetal decelerations cord prolapse | Moderate | 4325 | 0, 3, 6 | 7.07 | Nasal oxygen | Y | N |
| 11 | M | 37 | (20) | ECS | Poor BPP, fetal decelerations | Moderate | 3033 | 0, 0, 3 | 7.39 | Ventilator and pressors | Y | N |
| 12 | M | 38 | (18) | SVD | Abnormal HR, cord encirclement | Severe | 2466 | 1, 1, 2 | 7.08 | Ventilator and pressors | Y | Y |
| 13 | F | 41 | (28) | ECS | Fetal decelerations, cord prolapse | Severe | 3790 | 0, 0, 5 | 6.96* | Pressors | Y | Y |
| 14 | F | 40+ | (40) | SVD | Cord wrapped around body | Moderate | 3250 | 2, 4, 6 | 7.32 | Ventilator | N | N |
| 15 | M | 36 | (18) | SVD | Delivered at local restaurant | Severe | 3500 | 0, 0 | 7.45 | Ventilator and pressors | Y | Y |
| 16 | M | 36 | DM, HTN, (28) | SVD | Fetal decelerations | Severe | 3200 | 1, 4, 4 | 7.24 | Pressors | Y | Y |
| 17 | M | 36 | TBI, (31) | ECS | Fetal decelerations | Severe | 2500 | 2, 3, 3 | 7.29 | Ventilator and pressors | Y | Y |
ABG = arterial blood gas; AED = antiepileptic drugs; BPP = biophysical profile; BWt = birth weight; CS = Cesarean section; DIC = disseminated intravascular coagulation; ECS = emergency Cesarean section; F = female; GA = gestational age; HELLP = hemolysis elevated liver enzymes low platelet count; HR = heart rate; M = male; N = no; PPHN = persistent pulmonary hypertension of the newborn; DM = Diabetes Mellitus; ARF = Acute Renal Failure; HTN = Hypertension; HIE = Hypoxic Ischemic Encephalopathy; Pt = patient; SVD = standard vaginal delivery; SZ = seizures; TBI = traumatic brain injury; UC = umbilical cord; wk = weeks; Y = yes.
Umbilical cord pH.
2.1. Cooling protocol
The decision to cool infants was based on inclusion/exclusion criteria as published (Appendix 1).4 Cooling was achieved by a cooling blanket to an esophageal temperature of 33.5 °C for 72 hours, followed by slow rewarming by 0.5 °C/h.
2.2. Neurodevelopmental outcome
Outcome was monitored at 6 months, 12 months, 24 months, or 36 months by neurologic examination, Denver developmental scales (DDST).7 Pediatric Stroke Outcome Measure (PSOM)9 and, when possible, Bayley Scales of Infant Development (BSID).8 All evaluations were performed by a certified pediatric neurologist, or by neuro-developmental specialists as part of routine clinical assessments for patients followed in the high-risk neonatal clinic at WVU. Most recent assessments are reported for each individual (Table 2). All neurological evaluations were assigned a numeric value of progressive severity (Table 3). DDST scores were as follows: 1, normal; 2, mild delay; 3, moderate delay; and 4, severe delay for each category (personal/social, language, fine motor/adaptive, gross motor). PSOM scale includes sensorimotor, language, and cognitive/behavioral evaluation with a maximum total score of 10. BSID includes scoring for motor development index (MDI) and psychomotor development index (PDI) with 1–4 scoring levels of progressive severity. The above scores were averaged in a Global Outcome Score (Tables 2 and 3). All assessments were performed by a certified pediatric neurologist and/or by a child development specialist.
Table 2.
Summary of electroencephalography (EEG), magnetic resonance imaging (MRI) findings, and clinical outcome.*,†,‡
| Pt | EEG findings | MRI findings | Age of clinical evaluations (mo) |
Neurological exam score |
DDST Score* |
PSOM score† |
Global outcome score |
|---|---|---|---|---|---|---|---|
| 1 | DOL 1–2: Low amplitude, seizures, rare spikes DOL 4–12: Rare SW |
DOL 4: Bilateral restricted diffusion in parietal/occipital/ temporal WM and thalami |
4 | 1 | 1 | 0 | 1 |
| 2 | DOL 1–3: Bilateral epileptiform spikes; seizures |
DOL 4: R PCA stroke | 2 | 1 | 1 | 0 | 1 |
| 3 | DOL 1–4: Normal | DOL 5: No restricted diffusion or HIE findings |
24 | 1 | 1 | 0 | 1 |
| 4 | DOL 1–3: Suppressed DOL 4–5: Normal |
DOL 11: No HIE findings | 13 | 1 | 1 | 0 | 1 |
| 5 | Deceased | Deceased | 5 | 5 | 4 | Deceased | 4 |
| 6 | DOL 1: Spikes; diffuse slowing DOL 2–5: Diffuse slowing on R |
DOL 28: No HIE findings | 14 | 1 | 1 | 0 | 1 |
| 7 | DOL 1–5: Severe suppression |
DOL 3: Occipital cystic encephalomalacia |
12,14 | 4,5 | 4 | 2 | 4 |
| 8 | DOL 1: Seizures. DOL 2: Burst suppression; spikes DOL 3–4: Intermittent SW, background slowing |
DOL 3: No HIE findings | 12 | 1 | 1 | 0 | 1 |
| 9 | DOL 2–4: Very low voltage | No MRI | Deceased | 5 | 4 | 2 | 4 |
| 10 | DOL 1:Minimal background slowing DOL 2: Normal |
DOL 5: Increased T1 signal in putamen and GP 6 months: Normal |
6, 19 | 1,2 | 2 | 0.5 | 2 |
| 11§ | DOL 1: Rare spikes; mild suppression DOL 2–3: Spikes; discontinuous |
DOL 10: Restricted diffusion in bilateral thalami, corona radiata and parietal/occipital cortex |
4,36 | 2,3 | 3 | 3.5 | 3 |
| 12 | DOL 1–5: Severe suppression, no reactivity |
DOL 5: Restricted diffusion in bilateral thalami |
Deceased | 5 | 4 | Deceased | 4 |
| 13 | DOL 1–3: Severe suppression |
No MRI | Deceased | 5 | 4 | Deceased | 4 |
| 14§ | DOL 1–5: Dysmature, asynchronous |
Normal | 12 | 1 | 1 | 0 | 1 |
| 15 | DOL 1: Multiple seizures DOL 2–4: Multifocal SW, asynchrony DOL 5: Prolonged seizures |
Profound HIE in deep gray matter and corpus callosum. Relative sparing of cortex |
6 | 5 | 4 | Deceased | 4 |
| 16 | DOL 1–2: Low voltage background DOL 3–4: Low voltage rare spikes |
DOL 5: Mild restriction diffusion in bilateral posterior frontal and corona radiata |
Too young | — | — | — | — |
| 17§ | DOL 1: Bilateral spikes DOL 2–3: Rare spikes, no seizures |
Moderate HIE with increased signal in BG, thalami and IC. Restricted diffusion in periventricular region |
12 | 2 | 2 | 1.5 | 2 |
bg = background; BG = basal ganglia; DOL = day of life; GP = globus pallidus; HIE = hypoxic ischemic encephalopathy; IC = internal capsule; L = left; PCA = posterior cerebral artery; Pt = patient; R = right; SW = sharp-waves; WM = white matter.
Denotes that these patients had Bayley Scales of Infant Development (BSID) assessmentce.8
Denver Developmental Screening Test Development Scale (DDST)7: 1 = normal; 2 = mild abnormality (≥2 cautions and ≥1 delay); 3 = moderate (≥3 cautions and/or 2 delays); and 4 = severe (≥4 cautions and/or ≥ 3 delays).
Pediatric Stoke Outcome Measure (PSOM)9: 0 = no delay; 0.5 = mild delay; 1 = moderate delay; 2 = severe or profound delay.
Neurological Exam: 1 = normal; 2 = mild delay, but function is unaffected; 3 = moderate delay, function is somewhat affected; 4= severe delay (spasticity, stiffness, barely functional); and 5=deceased global outcome score is a combined numeric value of DDST, PSOM and Neurological exam.
Criteria per Shankaran et al.4
Table 3.
Relationship between electroencephalography (EEG), magnetic resonance imaging (MRI), and clinical outcome.*
| Patient | EEG findings (24 h)† |
EEG findings (72 h)† |
MRI findings‡ |
Global Outcome Score§ |
|---|---|---|---|---|
| 1 | 4 | 3 | 2 | 1 |
| 2 | 2 | 2 | Stroke | 1 |
| 3 | 1 | 1 | 1 | 1 |
| 4 | 4 | 1 | 1 | 1 |
| 5 | No EEG | No EEG | No MRI | 4 |
| 6 | 2 to 3 | 4 | 1 | 1 |
| 7 | 4 | 4 | 3 | 4 |
| 8 | 4 | 2 | 1 | 1 |
| 9 | 4 | 1 | No MRI | 4 |
| 10 | 2 | 2 | 2 | 2 |
| 11 | 2 | 2 | 3 | 3 |
| 12 | 4 | 4 | 3 | 4 |
| 13 | 4 | 4 | No MRI | 4 |
| 14 | 1 | 1 | 1 | 1 |
| 15 | 4 | 3 | 3 | 4 |
| 16 | 4 | 3 | 2 | Too young |
| 17 | 3 | 3 | 2 | 2 |
Patients in italics were not used for statistics due to imminent death.
EEG was classified as: 1 = normal; 2 = rare spikes and/or sharp waves; 3 = moderate slowing with or without epileptiform discharges; and 4 = low voltage or severe suppression, with or without prolonged or multiple seizures.
MRI images were evaluated by board-certified neuroradiologists and classified into three groups: 1 = normal; 2 = mild restricted diffusion in cortical gray matter or deep nuclei; and 3 = severe restricted diffusion with or without cystic encephalomalacia. This classification was based on a previously published scoring method.10
The Global Outcome Score is a combined numeric score of the Denver Development Screen (DDST), Pediatric Stroke Outcome Measure (PSOM), and neurological examination. The Global Outcome Score is as follows: 1 = normal; 2 = mild delay, 3 = moderate/severe delay; and 4 = deceased.
2.3. MRI
Brain MRI (1.5 or 3 Tesla) was obtained within 1 week after birth including T1-and T2-weighted images, Fluid Attenuated Inversion Recovery(FLAIR), diffusion weighted imaging (DWI), and apparent diffusion coefficient (ADC) sequences. Contrast was used in all patients (0.8 mL Magnevist, Bayer, Toronto, Ontario, Canada). MRI images were evaluated by board-certified neuroradiologists and classified into three groups: 1 = normal; 2 = mild restricted diffusion in cortical gray matter or deep nuclei; and 3 = severe restricted diffusion with or without cystic encephalomalacia. This classification was based on a previously published scoring method,10 but given the small number of participants in this report, mild and moderate MRI abnormalities were merged as level 2.
2.4. EEG findings
Continuous electroencephalogram monitoring was obtained for 72 hours. Findings were evaluated by a board-certified epileptologist and classified at 24 hours and 72 hours into four groups of increasing severity: 1 = normal; 2 = rare spikes and/or sharp waves; 3 = moderate slowing with or without epileptiform discharges; and 4 = low voltage or severe suppression, with or without prolonged or multiple seizures. Seizures were defined as electrographic seizures detected on EEG according to the definition of Classen and co-workers5 of “rhythmic discharge or spike and wave pattern with definite evolution in frequency, location, or morphology lasting at least 10 sec”. Electrographic definition of seizures was deemed necessary as it has been reported that only one-third of neonatal EEG seizures displays clinical signs on simultaneous video recordings.5,6
2.5. Statistical analysis
Positive and negative predictive values, sensitivity, and specificity were calculated for EEG and MRI findings and clinical outcome; positive or negative test and normal/abnormal outcome were used for these calculations, without characterization of levels of severity.
We used Spearman’s rank correlation (rs) for a nonparametric assessment of the association between MRI and EEG on day of life (DOL) 1 (24 hours), and DOL 3 (72 hours). Fisher’s exact test was used to assess the relationship between MRI and EEG with the clinical outcome (normal, mild delay, moderate delay, and deceased). Multinomial logistic regression and log-linear regression were used to assess the combined association of MRI and EEG with the clinical outcome.
We explored the predictive ability of EEG and MRI scales using random forests. We performed data preprocessing11 and near zero predictors. Variables with sparse or unbalanced (skewed) distributions were removed, as they contain insufficient information for stable model construction and may cause the algorithms to fail.12,13 Random forests of classification trees in conjunction with Scree plots11 of the variable-importance measure were used to identify variables that could be important for predicting the clinical outcome. R statistical software was used for all analyses.11
3. Results
A total of 17 infants (8 females, 9 males) were identified. Patients’ clinical details including presence or absence of seizures are summarized in Table 1. Fifty-eight percent of patients had moderate HIE, and 41% had severe HIE. A total of 65% received one or more antiepileptic drugs (AED), and 53% needed pressors. One patient was found to have acute ischemic stroke and was excluded from all analyses.
3.1. Clinical outcome
For the majority of patients, the most recent follow-up visit was at age 1 year or 2 years. One patient was last evaluated at 6 months. Seven patients had no delay, six patients were deceased, and three patients had mild delay. DDST was obtained in 10 patients, PSOM in 12 patients, and BSID in three patients (Table 2).
3.2. MRI (n = 14)
On MRI, six patients had no evidence of brain injury (score = 1), four patients had mild restricted diffusion in cortical or deep nuclei (score = 2), and three patients had severe restricted diffusion with or without cystic encephalo-malacia (score = 3). Three patients died prior to MRI (Tables 2 and 3).
3.3. EEG findings (n = 16)
One patient was deceased prior to EEG. Two patients had normal EEG findings. Fourteen patients had abnormal readings in the first 24 hours of life; of these, six had mild EEG abnormalities (score = 2) and eight had severe changes (score = 4). At 72 hours, three of the six patients with mild EEG abnormalities were unchanged, one patient progressed to have severe EEG abnormalities (score = 4), and two patients had moderate abnormalities (score = 3;Tables 2 and 3). We observed a moderate rank correlation between the EEG and MRI scores at 24 hours (rs = 0.53, p = 0.065) and a statistically significant rank correlation at 72 hours (rs = 0.67, p = 0.012).
3.4. Relation between brain MRI and clinical outcome
Three patients died prior to MRI. Five patients had normal MRI and normal developmental outcome. Among four patients with mildly abnormal MRI (score = 2), one had normal development at 6 months, two had mild developmental delay, and one was too young to evaluate. Three patients with severely abnormal MRI findings (score = 3) were deceased. One patient with right posterior cerebral artery (PCA) stroke was excluded from analysis. There was a statistically significant relationship between MRI and the clinical outcome (Fisher’s exact test, p = 0.0174). MRI obtained at 72 hours of life demonstrated Positive Predictive Value (PPV); PPV = 1, Negative Predictive Value (NPV); NPV = 0.83, sensitivity was 100%, and specificity was 83%.
3.5. Twenty-four hours EEG-outcome relation
The relation between EEG recording within the first 24 hours of life was as follows: two patients with normal EEG had normal outcome (score = 1); and of two patients with mild EEG abnormalities (score = 2), one had speech delay and one had global developmental delay. Three patients with moderate EEG abnormalities (score = 3) had normal outcome. Among seven patients with severely abnormal EEG (score = 4), five died, and two had good outcomes. One patient was too young to evaluate for neurological outcome (Table 3). EEG at 24 hours demonstrated PPV = 0.5, NPV = 1, sensitivity = 100%, and specificity = 50%; EEG at 72 hours demonstrated PPV = 0.8, NPV = 0.85, sensitivity = 80%, and specificity = 70% (total of 15 patients). There was a statistically significant relationship between EEG severity scale at 24 hours and clinical outcome (Fisher’s exact test, p = 0.024), but no significant relationship at 72 hours (Fisher’s exact test, p = 0.273).
An AIC stepwise multinomial logistic regression was used to investigate the relationship between the EEG severity scale at 24 hours, MRI severity scales, and the clinical outcome. We observed that EEG was not retained in the final model, thus yielding confirmatory evidence of the MRI-outcome relationship as previously noted. We used a log-linear model to further investigate the combined relationship of EEG and MRI with the clinical outcome and found no evidence to support the combined relationship (likelihood ratio test p = 0.349).
3.6. Relation between MRI-plus-EEG at 24 hours or 72 hours of life and clinical outcome
Three patients died prior to testing. Among the remaining 14 patients, two had normal EEG and MRI and normal clinical outcome.
3.7. Severe MRI findings (4 patients)
Three patients had severe EEG abnormalities and are deceased; one had moderate EEG abnormalities at both time points and had mild delay.
3.8. Mild MRI findings (5 patients)
Two patients with mild EEG abnormalities at 24 hours and 72 hours had variable outcomes (one was developmentally appropriate and had speech delay); three patients had abnormal EEG at 24 hours and 72 hours and had variable outcome.
3.9. Normal MRI findings (5 patients)
Two patients had normal EEG findings at 24 hours and 72 hours and were clinically intact. EEGs of the remaining three patients demonstrated abnormalities at 24–72 hours, but the patients had no developmental problems (Tables 2 and 3).
The EEG severity scale measure was not retained in the AIC selected model, so there is no evidence that the EEG at 72 hours provided additional information about the outcome above that which is contained in the MRI. This was confirmed by the log-linear model results (likelihood ratio test p = 0.209).
We additionally considered the effect of the change in EEG severity scale between 24 hours and 72 hours. Given that the EEG severity scale is ordinal, we categorized the change between the 24 hours and 72 hours EEG measurements as an improvement, no change or a worsening in condition. There was no statistically significant relationship between a change, in EEG scale of severity, and clinical outcome (Fisher’s exact test, p = 0.828). For the multinomial logistic regression model that included the change in the EEG scale and the MRI scale, AIC model selection removed the categorized change in EEG reading from the model and retained only the MRI scale. This was confirmed by the log-linear model results (likelihood ratio test p = 0.589).
For this study, the statistical evidence suggests that EEG and MRI data contain similar information. The AIC-selected multivariable multinomial logistic regression model highlights the MRI-outcome relationship by selecting out the EEG information.
3.10. Outcome prediction
Taking a different perspective from the previous analysis, we explored the ability of EEG and MRI to predict outcomes. We used random forests and the resulting scree plots of variable-importance to identify variables that could play an important role in predicting the developmental outcome status of the children. Identified key predictors include the EEG readings at 24 hours and 72 hours as well as the MRI assessment for normal readings (yes/no). Although the ranking of the predictors should not be heavily interpreted, the MRI scale was a highly ranked predictor amongst the classification trees in the random forest.14
Focusing on the important predictors (EEG at 24 hours, EEG at 72 hours, and MRI findings), random forests yield evidence of the combined predictive utility of early EEG and MRI for the clinical outcomes (normal, delayed, deceased). Although the classification error for the three groups suggests an overly optimistic estimate of the error (out of bag error–0.0%), the structure of the trees in the random forest suggests that prediction using nonparametric classification trees may be a useful approach (median tree depth −3, minimum tree depth −2, maximum tree depth −7).
4. Discussion
Despite continued progress in diagnostic modalities, accurate prediction of outcome for neonates with HIE remains a great challenge, particularly for neonates with mild-to-moderate injury. This study confirms that MRI can reliably predict outcome only in severe HIE cases. Early EEG findings (24 hours) do not provide additional support for outcome prediction and are nonspecific, possibly in relation to medication use.15,16
Given its sensitivity in detecting ischemic lesions, MRI is currently the standard of care imaging modality for diagnosis of HIE, although there is an ongoing debate regarding imaging time, as it is possible to underestimate the severity of lesions during the first few days of life.17
MRI sensitivity of 91% and specificity of 51% in detecting HIE, when performed between 1 day and 30 days, has been reported, but statistical heterogeneity was observed when the study was subgrouped into early and late MRI, with late MRI having a higher sensitivity and specificity (99% and 53%, respectively), when compared to early MRI (sensitivity 85%; specificity 86%).18 Severe abnormalities on T1 and T2 weighted images can predict severe disability at 18–24 months of age (PPV = 0.76 in cooled, and PPV = 0.74, in noncooled neonates).19 However, conflicting results have been reported by a recent study showing a similar adverse developmental outcome in infants with either normal or abnormal signal intensity in posterior limb of internal capsule.18 In 167 term infants with severe HIE, abnormalities in the basal ganglia/thalami had a high predictive value for death or cerebral palsy (PPV = 0.88, NPV = 0.86, sensitivity = 0.71, and specificity = 0.94).20-22 By contrast, 108 infants with mild-moderate lesions (water-shed lesions, punctuate white matter lesions, abnormal signal intensities in the basal ganglia/thalami) did not develop cerebral palsy.18 Hence, it appears that although traditional MRI can be an effective biomarker of disease and treatment efficacy in severe cases, it is not sensitive and specific for detection of mild-to-moderate lesions.
DWI and ADC, both measures of magnitude of water diffusion in cerebral tissue, are considered to be the most sensitive sequences for early detection of ischemic lesions.21 Signal abnormalities occur within minutes after ischemia due to the shift of water from the extracellular to intracellular space resulting in a slowing of water diffusion. High ADC values indicate disorganization of white matter tracts, and unfavorable outcome.23 The value of DWI/ADC changes in neonates is less certain; diffusion characteristics are different in newborns because of incomplete myelination, immature blood brain barrier, and poorly developed autoregulation of cerebral blood flow. Hence, abnormal ADC values—evident in adults 1–7 days after a stroke—may not be apparent in neonates until later.24-28
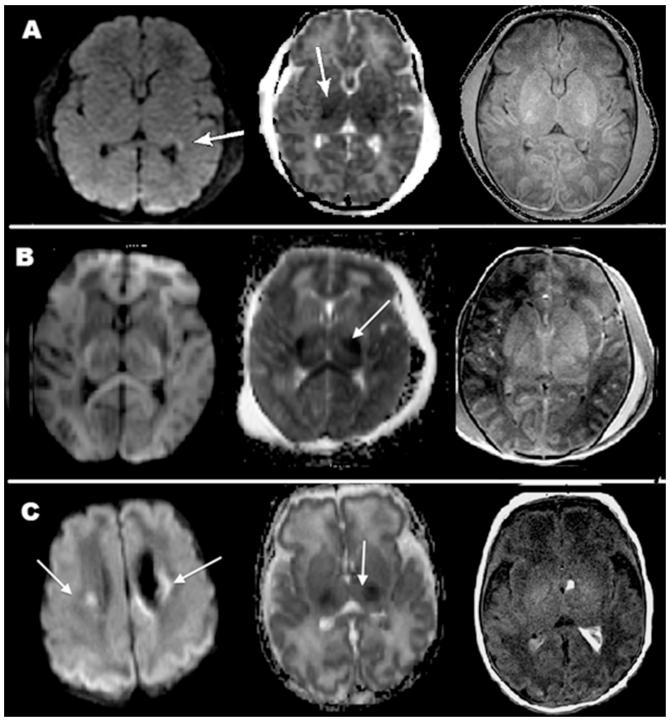
In our case series, MRI demonstrated a very high sensitivity and a good specificity, but the limited number of patients, and particularly the small number of patients with mild-to-moderate findings, raises a question about the significance of this finding for the intermediate group. In addition, when a different statistical approach was undertaken (random forests model) our data demonstrated a correlation between MRI findings and clinical outcome, and they suggested that MRI could be an important predictor of outcome in relation to absence/presence of abnormal imaging findings. Accurate prediction of degree of severity appears less certain, particularly in the mild-to-moderate group; these patients had either normal outcome or various degrees of developmental delay, and similar MRI findings were associated with different outcome (Figure 1).
Figure 1.
Magnetic resonance imaging (MRI) images in three patients showing similar findings despite different clinical outcomes. (A) Patient 1: Restricted diffusion in parietal/occipital/temporal white matter and thalami on day of life (DOL) 4. The patient had normal neurological outcome at 4 months; (B) Patient 12: Restricted diffusion in bilateral thalami on DOL 5. The patient is deceased; (C) Patient 11: Restricted diffusion in bilateral thalami, corona radiata and parietal/occipital cortex. At 36 months the patient had mild developmental and speech delay.
The mortality rate in our patients was 23.5%, higher than the reported 13%.4 This finding is likely to be associated with the higher percentage of severe HIE (41% vs. 32% on admission examination) in this cohort, with a higher number of neonates requiring pressors and AEDs. This severe cohort might have falsely increased the strength of MRI findings for prediction of outcome.
Multiple researchers have investigated the utility of early EEG recordings in predicting outcome in cooled and noncooled infants with HIE.15,16,19,25 Pressler et al15 demonstrated normal neurological outcome in HIE infants with moderate EEG abnormalities in the first 8 hours of life which showed early improvement (12–24 hours). In infants who continued to have severely abnormal EEG findings, death, spastic quadriplegia, and major developmental delay were observed.38,39 There have been conflicting reports regarding EEG findings in outcome prediction.15,16 Studies suggest that EEG can be a useful predictor of outcome in severe HIE injuries, but that it is not a strong predictor of developmental outcome in mild-to-moderate cases.34
Researchers have attempted to use amplitude-integrated EEG (aEEG) to predict outcome in neonates with neuronal injury, but the results are conflicting36,37 and falsely abnormal aEEGs have been reported with shivering in the hypothermic neonate.29,35 Reliable prediction of outcome (PPV = 0.92) by abnormal aEEG background was demonstrated only at 60 hours after HIE, but not at earlier stages.35
Our data demonstrate that early EEG findings may have utility for predicting clinical outcomes despite the lack of associative evidence from the multinomial logistic regression models. Early EEG can be misleading, as some infants demonstrated severely suppressed EEG in the first 24 hours but had a later EEG improvement and ultimately did well. This is supported by the low specificity (50%) of EEG findings at 24 hours. The early EEG suppression could be related to medication administration; hence, caution should be used about utilizing early EEG findings alone to make diagnostic and/or prognostic decisions.
A combination of different diagnostic modalities (MRI plus somatosensory evoked potentials,29,30 MR spectros-copy,32 and aEEG36) to improve prognosis accuracy has been attempted, but MRI still remains the best modality for predictor of motor outcome,31,33 as other tests lack sensitivity or/and specificity particularly in the mild-to-moderate group.26,28,31,34 To our knowledge, only one study has investigated the predictability of outcome based on a combination EEG and MRI, reporting a good correlation between EEG and MRI findings and outcomes in neonates with severe injury.40
Our statistical model using random forests and scree plots suggests that the combination of EEG and MRI may have some role in predicting the clinical outcome. MRI findings are beneficial when these findings are unambiguous; in this instance, MRI continues to be crucial as a predictor of clinical outcome. A larger study could support the development of a model to better explore the predictive power of EEG; in our study EEG findings provided no additional associative information regarding developmental outcome. It is possible that in mild-to-moderate HIE cases, MRI and EEG, even when used in combination, may not be sufficient to predict degrees of severity accurately. Therefore, we advocate the need for the development of precise, quantitative tools for more rigorous evaluation and accurate prognosis of HIE. Recent studies have suggested that use of advanced MRI modalities (diffusion tensor imaging; fractional anisotropy) that allow for quantification of white matter damage and investigation of tissue architecture and organization33 may help define the pattern of lesions characteristic of mild-to-moderate brain injury and possibly allow for accurate prediction of clinical outcome.
5. Conclusion
Accurate prediction of neurodevelopmental outcome in neonates with HIE remains difficult, particularly for mild-to-moderate cases. MRI appears to be the most reliable tool for severe cases. Caution should be used when discussing prognosis for neonates with mild-to-moderate HIE based on early MR imaging and EEG findings.
Acknowledgments
The project described was supported by the National Institute of General Medical Sciences, U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study and all tests were performed at WVUH.
Appendix 1
Cooling protocol
Clinical findings include a cord pH or blood gas at <1 hour of age, with a pH < 7.0 or base deficit >16 mEq/L. If a blood gas is not available or pH is between 7.01 and 7.15 or base deficit of 10–15.9, the infant should have had an acute perinatal event and either an APGAR score <5 at 10 minutes or continued need for assisted ventilation for >10 minutes. If these criteria are met, neurological examination is performed by two independent neonatologists or a pediatric neurologist is obtained, to define moderate and severe encephalopathy according to seizures or presence of one or more signs in three of the six categories as follows:
| Moderate encephalopathy |
Severe encephalopathy |
|
|---|---|---|
| Level of consciousness |
Lethargic | Stupor/coma |
| Spontaneous activity |
Decreased activity | No activity |
| Posture | Distal flexion Full extension |
Decerebrate |
| Tone | Hypotonia Focal, general |
Flaccid |
| Primitive reflexes |
Weak suck Incomplete moro |
Absent suck Absent moro |
| Autonomic system |
Constricted pupils Bradycardia Periodic breathing |
Skew deviation, dilated, nonreactive to light pupils Variable heart rate Apnea |
Therapeutic hypothermia is initiated in eligible infants as soon as possible within 6 hours of birth to achieve and maintain an esophageal temperature of 33.5 °C for a period of 72 hours, followed by gradual rewarming by increasing the core body temperature at the rate of 0.5 °C/h over a period of 6 hours.
The Food and Drug Administration–approved Blanketrol II Hyper/Hypothermia System, (manufactured by Cincinnati Sub-Zero, Cincinnati, OH, USA), was the hypothermia device for thermoregulation. The infant is placed on a cooling blanket in an open warmer with no layers of clothing between the infant and the cooling blanket. The cooling blanket should lie flat, with no kinks in the connecting hoses. The infant should lay supine on the blanket. An esophageal probe will then be used to measure the set point esophageal temperature which should be at 33.5 °C. Fluctuations in this temperature are to be expected, but should not be greater than 1 °C from the set point. Once the set point is reached, a thin layer (such as a receiving blanket) may be placed on the infant. Temperatures from the esophageal probe, infant’s skin and the cooling blanket will be monitored every 15 minutes for the first 4 hours of therapy. Temperatures will then be recorded hourly until 12 hours of cooling and then every 2 hours until the 72 hours of cooling therapy has been completed. Upon completion of 72 the hour cooling period, the infant will be rewarmed gradually so that the core body temperature will increase 0.5 °C each hour over a 6 hour period.
Footnotes
Conflicts of interest
The authors report no conflict of interest.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics 2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol. 2011;2011:609813. doi: 10.1155/2011/609813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammed AT, Woolcott C, Vincer M, Whyte R, Stinson D. Hypothermia for neonatal hypoxic ischemic encephalopathy:an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 6.Murray DM, Boylan GO, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 7.Frankenburg WK, Dodds JB. The Denver developmental screening test. J Pediatr. 1967;71:181–91. doi: 10.1016/s0022-3476(67)80070-2. [DOI] [PubMed] [Google Scholar]
- 8.Black M, Matula K. Essentials of Bayley Scales of Infant Development II Assessment. John Wily; New York: 1999. [Google Scholar]
- 9.Kitchen L, Westmacott R, Friefeld S, MacGregor D, Curtis R, Allen A, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43:1602–8. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 11.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. Available at: http://www.R-project.org. [Google Scholar]
- 12.Kuhn M, Johnson K, editors. Applied predictive modeling. Springer Science and Business Media; New York: 2013. [Google Scholar]
- 13.Kuhn M. Building predictive models in R using the caret package. J Stat Soft. 2008;28:1–26. [Google Scholar]
- 14.Sandri M, Zuccolotto P. Variable selection using random forests. Data Analysis, Classification and the Forward Search; Proceedings of the Meeting of the Classification and Data Analysis Group (CLADAG) of the Italian Statistical Society, University of Parma, June 6-8, 2005; New York: Springer-Verlag Heidelberg. 2006.pp. 263–70. [Google Scholar]
- 15.Pressler RM, Boylan GB, Morton M, Binnie CD, Rennie JM. Early serial EEG in hypoxic ischaemic encephalopathy. Clin Neurohysiol. 2001;112:31–7. doi: 10.1016/s1388-2457(00)00517-4. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe K, Miyazaki S, Hara K, Hakamada S. Behavioral state cycles, background EEGs and prognosis of newborns with perinatal hypoxia. Electroencephalogr Clin Neurophysiol. 1980;49:618–25. doi: 10.1016/0013-4694(80)90402-2. [DOI] [PubMed] [Google Scholar]
- 17.Soul JS, Robertson RL, Tzika AA, Du Plessis AJ, Volpe JJ. Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics. 2001;108:1211–4. doi: 10.1542/peds.108.5.1211. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxicischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leijser LM, Vein AA, Liauw L, Strauss T, Veen S, Wezel-Meijler Gv. Prediction of short-term neurological outcome in full-term neonates with hypoxic-ischaemic encephalopathy based on combined use of electroencephalogram and neuro-imaging. Neuropediatrics. 2007;38:219–27. doi: 10.1055/s-2007-992815. [DOI] [PubMed] [Google Scholar]
- 20.Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB, Chong WK, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–95. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 21.de Vries LS, van Haastert IC, Benders MJ, Groenendaal F. Myth: Cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med. 2011;16:279–87. doi: 10.1016/j.siny.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Azzopardi D, Edwards AD. Magnetic resonance biomarkers of neuroprotective effects in infants with hypoxic ischemic encephalopathy. Semin Fetal Neonatal Med. 2010;15:261–9. doi: 10.1016/j.siny.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Ward P, Counsell S, Allsop J, Cowan F, Shen Y, Edwards D, et al. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics. 2006;117:e619–30. doi: 10.1542/peds.2005-0545. [DOI] [PubMed] [Google Scholar]
- 24.Dağ Y, Firat AK, Karakaş HM, Alkan A, Yakinci C, Erdem G. Clinical outcomes of neonatal hypoxic ischemic encephalopathy evaluated with diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol. 2006;12:109–14. [PubMed] [Google Scholar]
- 25.Van Lieshout HB, Jacobs JW, Rotteveel JJ, Geven W, v’t Hof M. The prognostic value of the EEG in asphyxiated newborns. Acta Neurol Scand. 1995;91:203–7. doi: 10.1111/j.1600-0404.1995.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 26.Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117:S28–33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- 27.Fatemi A, Wilson MA, Johnston MV. Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol. 2009;36:835–58. doi: 10.1016/j.clp.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes KP, Pipe JG, Bird R. Neonatal hypoxic-ischemic encephalopathy: detection with diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2000;21:1490–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Shah DK, Wusthoff CJ, Clarke P, Wyatt JS, Ramaiah SM, Dias RJ, et al. Electrographic seizures are associated with brain injury in newborn undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24. doi: 10.1136/archdischild-2013-305206. [DOI] [PubMed] [Google Scholar]
- 30.Julkunen MK, Himanen SL, Eriksson K, Janas M, Luukkaala T, Tammela O. EEG, evoked potentials and pulsed Doppler in asphyxiated term infants. Clin Neurophysiol. 2014;125:1757–63. doi: 10.1016/j.clinph.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161:799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 32.da Silva LF, Höefel Filho JR, Anés M, Nunes ML. Prognostic value of 1H-MRS in neonatal encephalopathy. Pediatr Neurol. 2006;34:360–6. doi: 10.1016/j.pediatrneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Hüppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–60. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 34.Hagmann CF, Brotschi B, Bernet V, Latal B, Berger TM, Robertson NJ. Hypothermia for perinatal asphyxial encephalopathy. Swiss Med Wkly. 2011;141:w13145. doi: 10.4414/smw.2011.13145. [DOI] [PubMed] [Google Scholar]
- 35.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, Wang A, Cook N, Donnelly M, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8. doi: 10.1177/0883073810390036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marics G, Csekö A, Vásárhelyi B, Zakariás D, Schuster G, Szabó M. Prevalence and etiology of false normal aEEG recordings in neonatal hypoxic-ischaemic encephalopathy. BMC Pediatr. 2013;13:194. doi: 10.1186/1471-2431-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csekö AJ, Bangó M, Lakatos P, Kárdási J, Pusztai L, Szabó M. Accuracy of amplitude-integrated electroencephalography in the prediction of neurodevelopmental outcome in asphyxiated infants receiving hypothermia treatment. Acta Paediatr. 2013;102:707–11. doi: 10.1111/apa.12226. [DOI] [PubMed] [Google Scholar]
- 38.Shankaran S, Pappas A, McDonald SA, Laptook AR, Bara R, Ehrenkranz RA, et al. Predictive value of an early amplitude integrated electroencephalogram and neurologic examination. Pediatrics. 2011;128:112–20. doi: 10.1542/peds.2010-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasiljević B, Maglajlić-Djukić S, Gojnić M. The prognostic value of amplitude-integrated electroencephalography in neonates with hypoxic-ischemic encephalopathy. Vojnosanit Pregl. 2012;69:492–9. [PubMed] [Google Scholar]
- 40.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163:465–70. doi: 10.1016/j.jpeds.2013.01.041. [DOI] [PubMed] [Google Scholar]