Summary
There is an urgent need to provide effective anti‐HIV microbicides to resource‐poor areas worldwide. Some of the most promising microbicide candidates are biotherapeutics targeting viral entry. To provide biotherapeutics to poorer areas, it is vital to reduce the cost. Here, we report the production of biologically active recombinant cyanovirin‐N (rCV‐N), an antiviral protein, in genetically engineered soya bean seeds. Pure, biologically active rCV‐N was isolated with a yield of 350 μg/g of dry seed weight. The observed amino acid sequence of rCV‐N matched the expected sequence of native CV‐N, as did the mass of rCV‐N (11 009 Da). Purified rCV‐N from soya is active in anti‐HIV assays with an EC50 of 0.82–2.7 nM (compared to 0.45–1.8 nM for E. coli‐produced CV‐N). Standard industrial processing of soya bean seeds to harvest soya bean oil does not diminish the antiviral activity of recovered rCV‐N, allowing the use of industrial soya bean processing to generate both soya bean oil and a recombinant protein for anti‐HIV microbicide development.
Keywords: Cyanovirin‐N, soya bean, anti‐HIV
Introduction
The HIV/AIDS pandemic is one of the largest global health concerns, with over 35 million people worldwide living with HIV and women in developing countries account for more than half of new infections (UNAIDS, 2013). Prevention strategies such as topical microbicides that can be used by women are urgently needed. Currently, no microbicides for HIV are on the market, and most candidates in late‐stage development are formulated with antiretroviral (ARV) drugs that inhibit viral replication (Friend and Kiser, 2013). The potential for viral resistance to ARVs, however, presents a strong challenge to their long‐term use as microbicides, as such resistance could adversely affect current therapeutic options. It is therefore imperative to identify novel non‐ARV microbicide agents to prevent HIV infection.
Cyanovirin‐N (CV‐N), an 11 009 Da protein isolated from cultures of the cyanobacterium Nostoc ellipsosporum, is a potent lectin capable of irreversibly inactivating diverse strains of HIV (types 1 and 2) and simian immunodeficiency virus (Boyd et al., 1997). CV‐N also prevents virus‐to‐cell fusion, virus entry and infection of cells in vitro (Tsai et al., 2003). These properties appear to be mediated through conserved interactions of CV‐N with the viral surface envelope glycoprotein gp120 that are distinct from the interactions of gp120 with the cellular receptor CD4 or with antibodies to known HIV‐neutralizing determinants of gp120 (Boyd et al., 1997).
The antiviral efficacy of CV‐N is coupled with its environmental stability (active over broad pH ranges, organic solvents and temperatures nearing boiling point) and stability in macaque cervical vaginal lavage fluid (Tsai et al., 2003). Recombinant CV‐N (rCV‐N) has been produced in Escherichia coli and is analogous to a natural CV‐N (Mori et al., 1998). The efficacy of gel‐formulated rCV‐N was evaluated in macaques rectally and vaginally challenged against SHIV86.9P: results demonstrated that rCV‐N as a topical microbicide gel can prevent transmission of SHIV in macaques (Tsai et al., 2003, 2004). This result has encouraged further preclinical evaluation of CV‐N to prevent sexual transmission of HIV in humans (Brichacek et al., 2013; Buffa et al., 2009; Lagenaur et al., 2011; Li et al., 2011; Xiong et al., 2010).
Although bioactive rCV‐N can be produced in a bacterial expression system, it is currently considered a nonviable option for large‐scale production due to the higher intrinsic cost. Researchers have therefore tried to produce rCV‐N in other expression systems such as Pichia pastoris (Mori et al., 2002), Nicotiana tabacum (Elghabi et al., 2011; Sexton et al., 2006) and Althaea officinalis (Drake et al., 2013) with the aim of finding the most efficient production system. These candidate expression systems for the practical application of rCV‐N to HIV microbicide development are still limited by their lack of a scalable and economically viable platform to produce bioactive rCV‐N (O'Keefe et al., 2009). Although such a platform exists to recombinantly produce the microbicidal agent griffithsin (GFRT) in Nicotiana benthamiana leaves using a viral‐vector‐based system (O'Keefe et al., 2009), there are no reports on current capacity to produce rCV‐N using a similar approach.
Soya bean protein storage vacuoles (PSVs) are temporal organelles of the endoplasmic reticulum specialized in accumulating and storing seed proteins (Kim and Krishnan, 2004; Mori et al., 2009; Robinson et al., 2005; Takaiwa et al., 2007). The α'‐subunit of the β‐conglycinin promoter and signal peptide is efficient tissue‐specific regulatory sequences that control accumulation of β‐conglycinin, the most abundant seed storage protein in soya bean (Imoto et al., 2008; Ladin et al., 1987; Wilson et al., 1986; Yamada et al., 2008). These seed‐specific regulatory sequences were successfully used to demonstrate the efficacy of accumulating different proteins in soya bean seeds (Cunha et al., 2011a,b; Kim and Krishnan, 2004; Yamada et al., 2008). Here, we report that rCV‐N accumulates to a level of at least 350 mg of protein per kilogram of dry seed when the cv‐n gene is directed to protein storage vacuoles of soya bean seeds via biolistics (Cunha et al., 2011a,b; Rech et al., 2008).
Based on our results on high soya bean seed production and the capacity to produce 350 μg of pure rCV‐N per gram of dry seed, we estimated that at least 1 kg of pure rCV‐N can be produced in a 1524 square metre area of enclosed greenhouse space. We demonstrate that rCV‐N produced in soya bean seeds has potent nanomolar anti‐HIV activity against T‐tropic laboratory strains of HIV‐1, which is comparable to the activity range of native CV‐N and also displays a concentration‐dependent binding to the viral envelope glycoprotein gp120. Furthermore, we also show that soya bean seeds expressing rCV‐N can be processed using the already available soya bean industrial processing system to produce high‐quality raw material ready to enter the purification system as well as soya bean oil indistinguishable from that produced by control soya bean seeds.
Results
Production of rCV‐N in soya bean seeds
Expression of rCV‐N was achieved using specific regulatory sequences within soya bean seed tissues. A co‐bombardment transformation strategy to generate transgenic soya bean plants allowed us to evaluate the pbcongCVN plasmid vector (Figure 1). The cv‐n gene encoding 101 amino acids cloned under control of the α'‐subunit of the β‐conglycinin seed‐specific promoter and 35S terminator was effective in directing CV‐N protein to the PSVs. The selection plasmid carried the herbicide‐resistant ahas gene (under control of the ahas constitutive promoter and terminator) and allowed for the selection of putative transformants on imazapyr, as previously described (Rech et al., 2008). Transgenic soya bean lines were generated following the microparticle co‐bombardment system as previously described (Rech et al., 2008). After co‐bombardment of 10 independent experiments, each with 250 embryonic axes, eight putative transgenic plants containing both cv‐n and ahas genes were obtained. All 8 plants demonstrated biosynthesis of the mature rCV‐N with the expected molecular weight of approximately 11 kDa. Soya bean line CV‐N10 presented the highest expression as determined by ELISA analysis of T1 progeny and was used to advance derived progenies and for all further experiments.
Figure 1.
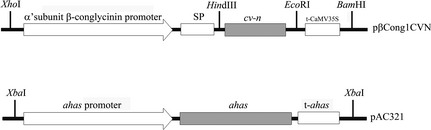
Schematic representation of the expression cassettes of the pβCong1CV‐N and pAC321 plasmids used for particle bombardment transformation of soya bean embryos. The Cyanovirin‐N (cv‐n) gene is under the control of the α'‐subunit of β‐conglycinin promoter and signal peptide, and also the CaMV35S terminator. In the pAC321 plasmid, the ahas gene is controlled by the ahas promoter and terminator (t‐ahas).
Organ‐specific detection and expression kinetics of the recombinant CV‐N
The expression of rCV‐N in different organs of a T3 transgenic soya bean plant line was evaluated by Western blot. As expected, rCV‐N was only detected in protein extracts from seeds, demonstrating that the α'‐subunit of the β‐conglycinin tissue‐specific promoter was efficient in restricting the gene expression to only the soya bean cotyledons. No rCV‐N was detected in roots, leaves, stems or flowers of the transgenic plant, or in nontransgenic seeds (Figure 2a). The kinetics of the CV‐N protein accumulation during seed development was evaluated 2, 4, 6 and 8 weeks after pollination (Figure 2b): rCV‐N protein accumulation increased during seed development. Northern blot analyses indicated there were no detectable transcripts of rCV‐N at 2 weeks after pollination, but we observed an increased accumulation from 4 to 8 weeks (Figure 2c). Western blot analysis revealed that the accumulation of the rCV‐N increased during the development cycle of the seeds, reaching its highest level in the mature seeds 8 weeks after pollination (Figure 2d).
Figure 2.
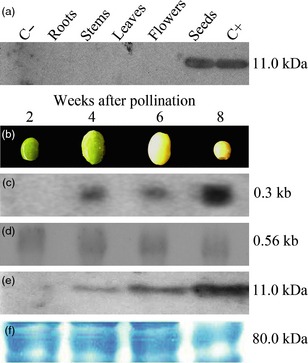
The efficiency of the α'‐subunit of β‐conglycinin promoter to restrict the transgene expression to the transgenic seeds was evaluated by organ‐specific Western blot analysis. (a) Immunoassays of TSP extracts (100 μg) from roots, stems, flowers and seeds of a transgenic a T3 plant from transgenic line CV‐N10 and a nontransgenic plant demonstrated the successful detection of rCV‐N only in transgenic seeds. A total of 100 ng of rCV‐N purified from E. coli (NIH) was properly detected by primary antibody recognition. All molecular weights were estimated with the marker Precision Plus Protein Standards All Blue (Bio‐Rad). (b) The kinetics expression of the cv‐n gene on the transcriptional and translational levels was demonstrated in different phenological stages of T3 soya bean seeds from line CV‐N10. Samples were evaluated after 2, 4, 6 and 8 weeks after pollination. (c) Northern blot detection of primary transcripts of the cv‐n gene 4 weeks after pollination, showing an increase after 8 weeks (above). Ubiquitous elongation factor gene transcripts were detected showing homogeneous mRNAs concentration in all stages of seed development (below). (d) Western blot analysis of transgenic seeds showing the accumulation of the rCV‐N in seeds from 2 to 8 weeks after pollination, with an increase in the last stages of development (above). SDS‐PAGE loading controls of each total soluble protein extracts (approximately 100 μg) were utilized to provide a uniform sample electrophoresis (below).
Localization of rCV‐N in transgenic soya bean seeds
Ultrastructural immunocytochemistry analysis using gold particle visualization showed the accumulation of the rCV‐N in the PSVs of mature seeds (Figure 3, gold particles are seen as dark spots denoted by arrows). Detectable rCV‐N was localized within the PSVs of transgenic soya bean seeds with no significant accumulation inside the oil bodies or in the cell wall (Figure 3a, b) and nor was gold particle accumulation found in the apoplast, starch grains or cytoplasm of transgenic seeds. Nontransgenic seed did not present detectable accumulation of rCV‐N in the PSVs (Figure 3c).
Figure 3.
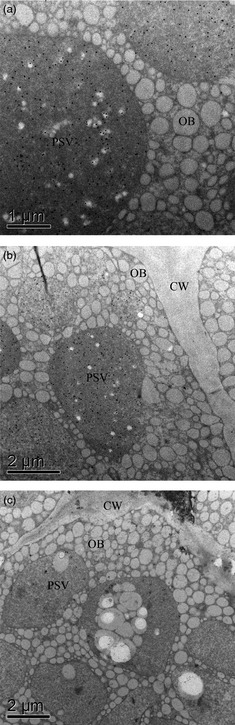
Protein targeting to protein storage vacuoles (PSV) was evaluated by ultrastructural immunocytochemistry of the rCV‐N presence in sections (2 mm) of soya bean cotyledons. (a, b) Subcellular accumulation of the rCV‐N in the PSV of transgenic T3 seeds from line CV‐N10. The accumulation of rCV‐N was restricted to the PSVs (white arrows), with no significant detection of the protein associated to the oil bodies (OB) and the cell wall (CW). (c) The rCV‐N could not be detected in the PSVs of nontransgenic seeds, as shown in the experimental control.
Purification and analysis of rCV‐N from soya bean seeds
Pure, biologically active rCV‐N was isolated from lyophilized soya bean seed powder with a yield of 350 μg/g of dry seed weight by a combination of aqueous extraction, ethanolic precipitation and C‐18 reverse‐phase chromatography. Pure rCV‐N was obtained from the ethanolic precipitate. The soluble fraction following ethanolic precipitation (67% EtOH) also contained rCV‐N; however, purification proved more difficult as other contaminating proteins co‐purified with rCV‐N in almost all fractions. We therefore focused on optimizing purification of rCV‐N from the insoluble fraction. Figure 4(a) shows the Coomassie‐stained SDS‐PAGE analysis of selected fractions during the purification procedure, and purified rCV‐N (lane 6, Figure 4a) migrated in a similar way to the positive control CV‐N (lane 1, Figure 4a). Western blot analysis showed that the purified rCV‐N (lane 2, Figure 4b) was antigenically similar to the positive control (lane 3, Figure 4b, rCV‐N from E. coli), and both were detected as monomers when blots were probed with rabbit anti‐CV‐N antibodies. Other forms of rCV‐N were detected in the soluble fraction as expected (lane 1, Figure 4b); however, these were separated from the active monomer by the reverse‐phase purification protocol. The purified rCV‐N was confirmed to be a monomer of 11 010 Da [M+] by LC‐MS analysis (Figure S1), which is consistent with the expected size of 11 009 Da for native CV‐N. The observed N‐terminal amino acid sequence of rCV‐N also matched exactly with the expected N‐terminal sequence of native cyanovirin (data not shown). Finally, amino acid analysis showed that rCV‐N exhibited 92% purity when compared against the expected composition for native CV‐N.
Figure 4.
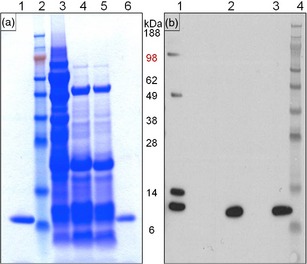
Analysis of the rCV‐N purification process. (a) Coomassie Blue‐stained SDS‐PAGE (reducing conditions) of fractions from the purification procedure. Lanes marked as 1: pure, active CV‐N produced in E. coli (positive control), 2: SeeBlue Plus2 (Invitrogen) molecular weight standard, 3: total soluble protein, 4: supernatant from 67% ethanol precipitation, 5: resuspended pellet from 50% ethanol precipitation and 6: pure rCV‐N isolated in fraction 38 from reverse phase‐HPLC. (b) Western blot (reducing conditions) for detection of CV‐N in selected fractions from the purification. Lanes marked as 1: total soluble protein, 2: pure rCV‐N isolated in fraction 38 from reverse phase‐HPLC, 3: pure CV‐N produced in E. coli (positive control) and 4: SeeBlue Plus2 (Invitrogen) molecular weight standard.
Bioactivity of purified rCV‐N
Anti‐HIV activity analysis of the purified rCV‐N shows it is potently active in a concentration‐dependent manner at protecting against HIV‐1RF‐induced cytopathic effects in CEM‐SS cells with an EC50 of 0.82–2.7 nM (8–30 ng/mL) compared to 0.45–1.8 nM (5–20 mg/mL) for E. coli‐produced CV‐N tested simultaneously (Figure 5). These results compare favourably to the anti‐HIV activity originally reported for native CV‐N (approximately 1 nM) (Boyd et al., 1997). The purified rCV‐N did not show cytotoxicity at the highest concentration tested (1 μg/mL, 90 nM) (Figure 5) which is >30‐fold higher than its EC50. Both rCV‐N and E. coli‐produced CV‐N were also tested against HIV‐1IIIB resulting in similar EC50 values of 0.82 and 0.36 nM, respectively (data not shown). The rCV‐N was further tested for its ability to bind HIV envelope glycoprotein gp120 in an ELISA, and results show that like the control CV‐N sample, rCV‐N bound gp120 in a concentration‐dependent manner with essentially identical affinity (Figure 6).
Figure 5.
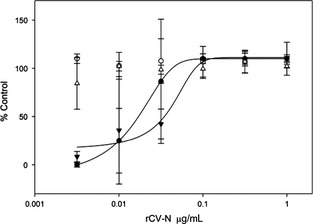
Concentration‐dependent anti‐HIV activity of soya‐produced rCV‐N in HIV‐1RF infected (●) and uninfected (○) CEM‐SS cells assessed after 6 days in culture. The activity of control CV‐N, produced in E. coli, was also tested on infected (▼) and uninfected (▵) CEM‐SS cells. The number of surviving cells was measured by the XTT method and is indicated as percent untreated uninfected cell control. The assay was repeated several times, and soya‐produced rCV‐N displayed EC50 values in the range of 0.82‐2.7 nM, while E. coli‐produced CV‐N showed EC50 values in the range of 0.45‐1.8 nM. Both samples showed no toxicity at the highest tested dose (approximately 90 nM)
Figure 6.
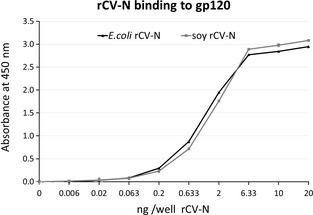
ELISA study of concentration‐dependent binding of pure, active rCV‐N from E. coli (▲) and soya bean seeds (●) to HIV envelope glycoprotein gp120. Points are averages of triplicate samples (corrected for the blocking agent background values).
Stability of rCV‐N after soya bean downstream processing
Soya bean seeds producing rCV‐N protein were tested for the presence of rCV‐N in oil extracted from T3 transgenic soya bean seeds. No rCV‐N was detected in the oil fraction, but a stable fraction of rCV‐N was detected in protein extracts processed for oil removal (Figure S2). A comparison of the fatty acid content in control versus rCV‐N transgenic soya bean seeds showed no significant differences in either the yield or composition of soya bean oil produced (Table S1).
Discussion
Development of a suitable expression source for the manufacture of an anti‐HIV topical microbicide requires a low cost methodology to have the broadest utility in areas of the world most affected by HIV (Essex, 1996; Gartner et al., 1986; O'Keefe et al., 2009). Suitable microbicide candidates must meet an array of criteria including potency; broad‐spectrum activity against different HIV strains; selectivity for viral and/or host cell targets; prevention of cell‐to‐cell transmission; stability both in transit and in vivo; bioavailability in target mucosa; and low toxicity to mucosal surfaces, including direct irritation, immunogenicity and mitogenicity (Essex, 1996; Gartner et al., 1986). Despite the availability of potential microbicides meeting some of these functional criteria, few have been able to be produced at sufficiently low cost. One exception is the recent report of the use of a TMV‐based expression system based on agroinfiltration of Nicotiana benthamiana leaves that demonstrated the capacity to produce rGRFT‐P (O'Keefe et al., 2009) on a scale sufficient to support clinical development.
Here, we report that engineering soya bean seeds to express the anti‐HIV protein CV‐N allowed us to develop a scalable platform to produce biologically active rCV‐N protein, which provides an important alternative for the production of bioactive proteins including microbicides. The cv‐n gene cloned under control of the β‐conglycinin seed‐specific regulatory sequences was effective in directing rCV‐N to PSVs in soya bean seeds. Moreover, this strategy was efficient in producing the monomer, 11 kDa rCV‐N protein. These results are in accordance with previous studies demonstrating that seed‐specific regulatory sequences can be successfully used to express stably accumulated molecules with different structural characteristics, in the PSVs of transgenic soya bean seeds (Cunha et al., 2011a,b; Kim and Krishnan, 2004; Yamada et al., 2008).
We also demonstrate that the kinetic accumulation pattern of rCV‐N during the maturation cycle of soya bean seeds was temporally modulated. Its peak was reached approximately 4 weeks after pollination and maintained steadily until the dry seed maturation stage. These results are in agreement with our previous findings expressing human growth hormone (Cunha et al., 2011a) and human coagulation factor (Cunha et al., 2011b) in soya bean seed, which could be correlated with the kinetics pattern of the PSV organelles' availability (Yoo and Chrispeels, 1980). Localization of rCV‐N in the seeds was indicated by ultrastructural immunocytochemistry analysis. The α'‐subunit of the β‐conglycinin promoter and signal peptide was effective in directing rCV‐N protein accumulation in mature soya bean seeds. The accumulation profile indicated that rCV‐N protein was restricted to the PSVs, and absent in the apoplast and oil bodies. Our results are in agreement with previous findings (Hohl et al., 1996; Muntz, 1998; Vitale and Raikhel, 1999).
We successfully purified rCV‐N from the insoluble fraction of transgenic soya bean seeds by several ethanolic precipitation steps followed by a final separation with reverse‐phase chromatography. We obtained a calculated yield of 350 μg/g of dry seed weight with 92% purity. The predominant contaminants were low molecular weight. However, they can be easily removed by an additional gel filtration step. The soluble fraction and other nonpure fractions of the final purification step also contained bioactive rCV‐N; however, these often contained other contaminating proteins. This indicates a higher yield of purified rCV‐N is possible, and future work will focus on optimizing purification conditions to increase the total yield of pure rCV‐N.
Once purified, the rCV‐N was characterized as a monomer of 11 009 Da, with its N‐terminal sequence identical to native CV‐N. Though denatured during purification, rCV‐N refolded correctly after reverse‐phase HPLC, as confirmed by its potent anti‐HIV activity and binding to gp120, which closely correlate to that of native CV‐N (Boyd et al., 1997). Active at low nanomolar concentrations against HIV‐1RF and HIV‐1IIB, the rCV‐N was active in the same range as CV‐N produced in E. coli (Boyd et al., 1997) and tested simultaneously. The facile refolding of rCV‐N is similar to that previously reported (Boyd et al., 1997; Mori et al., 1998) and speaks to the physiochemical stability of this protein, an important advantage for both production and shipping.
As a potential scalable and low‐cost platform for production of rCV‐N, transgenic soya bean may constitute one of the least expensive systems for its large‐scale production. Under greenhouse conditions, the soya bean plant has a high biomass capacity and it is photoperiod‐sensitive, which means that increasing the length of the light period will induce a delay in flowering and, consequently, high vegetative growth (Cavazzoni et al., 1999; Kantolic and Slafer, 2007). This photoperiod sensitivity, together with the intrinsically high protein content (40%) in the seeds makes soya bean an attractive system for the production of recombinant proteins. Soya bean is a short‐day plant, and its developmental responses are regulated by phytochrome photoreceptors (Watanabe et al., 2009; Wu et al., 2013). Under field and greenhouse conditions, each soya bean plant under a 10‐14 h photoperiod produces on average 100 seeds in its progeny. An increase in the photoperiod to 23–24 h induces a strong vegetative growth of the plant. After a period of 3 months of vegetative growth, the readjustment of photoperiod to 10–14 h induces flowering and large‐scale seed production. This procedure allows production of more than 1000 seeds per plant. Based on our results on soya bean seed production (1000 seeds per plant) and the capacity to produce 350 μg of pure rCV‐N per gram of dry seed, it is possible to estimate the production of at least 1 Kg of pure rCV‐N in an area of 1524 square metres of enclosed greenhouses. Previous results on the production of rGRFT microbicide, using a Nicotiana benthamiana‐based viral expression system (O'Keefe et al., 2009) demonstrated a higher protein expression level of 1 g rGRFT per kg in leaf material and further production of 60 g of pure rGRFT in 1524 square metres. Using a soya bean seed‐based expression system is estimated to produce 15 times as much rCV‐N in the same area.
Another significant aspect of this research is the viability of multiple use components from this production stream. The PSVs of soya bean seeds are an attractive option for the production of recombinant proteins because simple plasmid construction and protein engineering allow for the targeting and accumulation of recombinant proteins in seed storage compartments, which are separate from the oil storage compartments. Using standard downstream processes to generate raw soya bean material ready to go through both oil extraction and rCV‐N purification, this method is rapidly scalable. Furthermore, due to the inherent physical stability of CV‐N (Boyd et al., 1997), soya bean oil could be extracted from seed material while retaining bioactive rCV‐N in the resulting lyophilized soya powder. We observed that a stable fraction of recombinant CV‐N could be detected in protein extracts that were processed for oil removal (Figure S2), indicating that transgenic soya bean seeds can be processed under a standard industrial manufacturing protocol while retaining properly folded rCV‐N. In addition, the oil produced from the transgenic seeds was found to be both free of rCV‐N (Figure S2) and of similar fatty acid composition to oil extracted from control seeds (Table S1). This dual use of the soya bean, both for microbicide production and for soya bean oil production, provides further cost savings for this expression system. Though the scale at which CV‐N would be produced will not result in the scale of production normally used for oil extraction efforts such as biodiesel production, the oil recovered from rCV‐N processing could find additional uses as a secondary product as lecithins (e.g. printing inks, insecticides, synthetic rubbers) or glycerols (cements, structured lipids, lubricants) for industrial purposes (Dashiell, 1989).
A current priority in anti‐HIV microbicide development is the identification and validation of non‐ARV compounds that would prevent cross‐over resistance to clinical agents used therapeutically against HIV. Candidate non‐ARV anti‐HIV microbicides must meet stringent requirements such as safety, efficacy and affordability metrics (Shattock and Rosenberg, 2012). Though previous experiments in cell culture have indicated some potential adverse effects associated with CV‐N treatment (Kouokam et al., 2011), studies in nonhuman primate models have not shown similar deleterious effects (Brichacek et al., 2013). CV‐N has also been repeatedly shown to be effective in both vaginal and rectal challenge models of SHIV in macaques (Lagenaur et al., 2011; Li et al., 2011; Tsai et al., 2003, 2004). One of the difficulties in fully evaluating CV‐N as a microbicide has been the high cost of production. CV‐N produced in soya bean seeds addresses this critical requirement, and soya beans should be further evaluated as a production system to produce other suitable candidate microbicides for further preclinical evaluation and possibly, clinical testing in humans.
Experimental procedures
Vector construction
The 306‐bp fragment corresponding to cv‐n gene coding region (GenBank accession number: L48551.1) was amplified from the vector pET30b‐CVN by polymerase chain reaction (PCR). The resulting 318‐bp sequence was cloned into the sites HindIII and EcoRI, from the vector pβcong1 (Cunha et al., 2011a,b), which contains the α'‐subunit from β‐conglycinin promoter and signal peptide to generate the vector pβCong1CVN (Figure 1). The pAC321 selective vector contains the mutated acetohydroxy acid synthase (ahas) gene, which confers tolerance to the herbicide imazapyr (Aragão et al., 2000).
Producing genetically engineered soya bean plants
Vectors pβCong1CVN and pAC321 were co‐bombarded (ratio 1 : 1) into somatic embryonic axes from mature soya bean seeds of cultivar BR16 (EMBRAPA, Brazil), as previously described (Rech et al., 2008). Putative transgenic plantlets were kept in a greenhouse to set seeds.
Preliminary identification of putative transgenic soya bean plants
For testing ahas and cv‐n gene presence, DNA from putative T0 plants was isolated from leaf discs (Doyle and Doyle, 1987), and PCR analyses were conducted as previously described (Cunha et al., 2011a,b; Rech et al., 2008). PCR analyses of seed‐derived progeny T1, considered each seed an individual event. A small piece (1–2 mm2) from the cotyledon was removed, without damaging the embryos, allowing the isolation of genomic DNA and further germination of the putative transgenic events.
Northern blot analysis
Transgenic T3 seeds harvested 2, 4, 6 and 8 weeks after pollination were analysed for the presence of CV‐N primary transcripts. Total RNA from each immature seed (200 mg) was isolated using a total RNA Purification System® (Invitrogen, Life Technologies, Grand Island, NY) according to the manufacturer's protocol. Genomic DNA was eliminated by sample digestion with 2 U of DNase 1® (Ambion, Life Technologies, Grand Island, NY) for 10 min at 37 °C. Two 32P‐labelled probes (at 106 c.p.m./mL) were used to detect primary transcripts of the cv‐n gene and the internal control corresponding to the endogenous elongation factor gene. Both probes, with 318 and 560 bp, respectively, were obtained by PCR as previously outlined (Li et al., 2006). Autoradiograms were obtained by exposing the membranes to BioMax MS film (Kodak, Rochester, NY).
Localization of rCV‐N in soya bean seeds
For the ultrastructural immunocytochemistry analysis of rCV‐N in cotyledons of transgenic plant lines, mature T3 transgenic seeds were used and the analyses were conducted as previously described (Cunha et al., 2011a,b).
Quantification of rCV‐N in soya bean transgenic seeds (ELISA)
Total soluble protein (TSP) extracts from soya bean leaves, stems, flowers, roots and seeds (each seed weighed approximately 200 mg) were obtained by homogenizing 1.5 g of each organ in 10 mL PBS buffer (10 mM Na2HPO4, 1.7 mM NaH2PO4, 140 mM NaCl, 2.7 mM KCl, pH 7.2). Samples were homogenized on ice with three 30s pulses on the lowest setting of a Polytron PT (Kinematica, Bohemia, NY) and immediately frozen at −80 °C. Samples were thawed on ice and centrifuged at 12 000 g for 10 min at 4 °C, and the aqueous supernatant was collected. The supernatant was filtered with a Millipore 0.22‐μm polyethersulfone disc filter under sterile conditions. Protein concentrations were determined using Pierce BCA Protein Assay Reagent (Quantum Scientific, VWR Inc., West Chester, PA) following manufacturer's instructions. ELISA was performed to determine the rCV‐N concentration in the TSP extracts from T1 seeds as described in ELISA section below. Seeds presenting higher expression levels were chosen for further cultivation in greenhouse.
Extraction and purification of rCV‐N
Lyophilized soya bean seeds were crushed to a fine powder (8 g) and extracted with 100 mL extraction buffer (50 mM PBS, 0.02% NaN3, 1 : 100 dilution of protease inhibitor cocktail, Sigma‐Aldrich) at 4 °C for 120 h, before being filtered (20–25 μm Whatman filter paper). The insoluble fraction was resuspended in 50 mL distilled, deionized water (ddH2O) and centrifuged (16 000 g, 1 h at 4 °C). The pellet was lyophilized, resuspended in 100 mL ddH2O and precipitated with 67% ethanol (−20 °C overnight) before centrifugation (9000 g, 1 h, 4 °C). The supernatant was concentrated to 5 mL, lyophilized and reconstituted in ddH2O before being precipitated with 50% ethanol and centrifuged as before. The resulting pellet was resuspended in 1 mL ddH2O and separated by reverse phase‐HPLC (Microsorb C‐18 column, 5 μm, 10 mm × 250 mm, Rainin Instrument Co., Columbus, OH) with a linear gradient of 0–100% acetonitrile with 0.5% trifluoroacetic acid (TFA) for 60 min at 1.5 mL/min. Collected fractions were dried, resuspended in ddH20 and analysed. Pure rCV‐N eluted at 60%–64% acetonitrile.
SDS‐PAGE and Western blot analysis
Samples were resolved on precast NuPAGE® 4%–10% Bis‐Tris polyacrylamide gels in MES SDS running buffer (Life Technologies, Grand Island, NY) under reducing conditions according to the manufacturer's instructions. Proteins were visualized by Coomassie Blue staining or transferred to 0.2 μm PVDF membrane (Invitrogen, Grand Island, NY) for standard Western blotting: primary antibody was polyclonal rabbit anti‐CV‐N antibodies (Molecular Targets Laboratory, CCR, NCI, NIH, Bethesda, MD) used at 1 : 1500 dilution (overnight incubation), and secondary antibody was peroxidase‐conjugated goat anti‐rabbit antibody (Thermo Fisher Scientific Inc.) used at 1:2000 for 1 h. Western blot was detected using the SignalFire ECL reagent system (Cell Signaling Technology Inc., Boston, MA).
ELISA studies of the binding of rCV‐N to the HIV envelope glycoprotein gp120
Recombinant HIV‐1IIIB gp120 (100 ng/well, ImmunoDiagnostics, Woburn, MA) coated on a 96‐well plate, was blocked with 3% nonfat dry milk (Bio‐Rad) for 2 h and washed thrice with PBST before being incubated with serial dilutions of rCV‐N or control CV‐N (produced in E. coli) for 2 h. Wells were washed with PBST, incubated for 1 h with a 1:1000 dilution of polyclonal rabbit anti‐CV‐N antibody (NIH) and washed as before. Wells were incubated with peroxidase‐conjugated goat anti‐rabbit secondary antibody (Thermo Fisher Scientific Inc., Rockville, MD) at a 1 : 1000 dilution for 1 h. After washing, detection was carried out with the TMB 2‐component Microwell peroxidase substrate kit (KPL Inc., Gaithersburg, MD) according to the manufacturer's protocol, and absorbance was read at 450 nm.
N‐Terminal protein sequencing, amino acid analysis and mass spectrometry
N‐terminal amino acid sequencing and amino acid analysis were performed (on an Applied Biosystems 494 sequencer (sequential Edman degradation) and a Beckman 6300 automated amino acid analyser, respectively. LC‐MS was performed on an Agilent 1100 system (Zorbax 300SB‐C18 column, 5 μm, 4.6 mm × 150 mm, Agilent Technologies, Santa Clara, CA) with a linear gradient of 0–100% acetonitrile with 5% (v/v) acetic acid in the mobile phase. Corresponding peaks were analysed by electrospray ionization mass spectrometry.
Anti‐HIV activity assays
To assess the anti‐HIV activity of rCV‐N samples, a 2,3‐bis‐[2‐methoxy‐4‐nitro‐5‐sulfophenyl]‐2H‐tetrazolium‐5‐carboxanilide inner salt (XTT)‐tetrazolium‐based assay was used to determine the ability of rCV‐N to protect the T‐lymphoblastic cell line CEM‐SS from a T‐tropic laboratory‐adapted strain of HIV (HIV‐1RF) as described previously (Gulakowski et al., 1991; Mori et al., 1998). Additional anti‐HIV studies in CEM‐SS cell using the same methodology with both HIV‐1RF and HIV‐1IIIB were performed at ImQuest BioSciences LLC. (Frederick, MD).
Soya bean seeds microscale downstream processing
A microscale processing of soya bean seeds was conducted aiming to simulate the downstream process routinely used in industry on a large scale. Thirty seeds were processed to separate oil and protein fractions, and analytical procedures to determine oil levels and ureatic activity were conducted as previously described (AOCS, 1980a; Erickson, 1995). The analysis was carried out following good laboratory practices (ITAL/NBR ISO/IEC 17025:2005).
Conflict of Interest
Drs. O'Keefe and McMahon are listed inventors on patents owned by the US government on the antiviral protein cyanovirin‐N.
Supporting information
Figure S1 LC‐MS (electrospray ionization) spectrum and deconvoluted ion set for the purified rCV‐N showing the expected molecular weight of 11 009 Da.
Figure S2 Dot‐blot of oil and protein extracts from non‐transgenic and transgenic T3 seeds submitted to standard defat processing.
Table S1 GC‐MS of fatty acids produced by soybean seeds (NEGC) and soybean seeds producing CV‐N (CV‐N).
Acknowledgements
The authors are grateful to Luis C. Lemos for technical assistance and to Ana C.M.M. Gomes for transmission electron microscopy assistance, MS. Warley W. Almeida for green house seed support, Marly C.F. Coelho for high‐throughput ELISA CV‐N detection assays, Maggie Garvey for the technical performance of the CEM‐SS/HIV‐1 in vitro cell‐based assay and Lauren H. Krumpe for assistance in manuscript preparation. The authors thank Dr. Amilcar Tanuri, University Federal of Rio de Janeiro, for discussion on CV‐N assays in soya bean seeds and Dr. José Mandarino, Embrapa Soybean, for information on soya bean seeds processing. The authors also thank the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (USA) for the kind gift of XTT (NSC# 601519) for use in antiviral assays. This study was supported by funds from Empresa Brasileira de Pesquisa Agropecuária (Embrapa), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio a Pesquisa do Distrito Federal (FAP‐DF), NIH Intramural AIDS Targeted Antiviral Program and the Intramural Program of the Center for Cancer Research at the National Cancer Institute (B.O., K.R., J.W., J.M.). This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health under contract no. NO1‐CO1‐12400 (C.S.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- American Oil Chemists Society (1980a) Urease Activity. Official Method Ba 9‐58. Champagne, IL, USA: American Oil Chemists Society. [Google Scholar]
- Aragão, F.J.L. , Sarokin, L. , Vianna, G.R. and Rech, E.L. (2000) Selection of transgenic meristematic cells utilizing a herbicidal molecule results in the recovery of fertile transgenic soybean [Glycine max (L.) Merril] plants at a high frequency. Theor. Appl. Genet. 101, 1–6. [Google Scholar]
- Boyd, M.R. , Gustafson, K.R. , McMahon, J.B. , Shoemaker, R.H. , O'Keefe, B.R. , Mori, T. , Gulakowski, T.R.J. , Wu, L. , Rivera, M.I. , Laurencot, C.M. , Currens, M.J. , Cardellina, J.H. II , Buckheit, R.W. Jr , Nara, P.L. , Pannell, L.K. , Sowder, R.C. and Henderson, L.E. (1997) Discovery of cyanovirin‐N, a novel human immunodeficiency virus‐inactivating protein that binds viral surface envelope glycoprotein gp120; potential applications to microbicide development. Antimicrob. Agents Chemother. 41, 1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichacek, B. , Lagenaur, L.A. , Lee, P.P. , Venzon, D. and Hamer, D.H. (2013) In vivo evaluation of safety and toxicity of a Lactobacillus jensenii producing modified Cyanovirin‐N in a rhesus macaque vaginal challenge model. PLoS ONE, 8, e78817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa, V. , Stieh, D. , Mamhood, N. , Hu, Q. , Fletcher, P. and Shattock, R.J. (2009) Cyanovirin‐N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J. Gen. Virol. 90, 234–243. [DOI] [PubMed] [Google Scholar]
- Cavazzoni, J. , Volk, T. , Bugbee, B. and Dougher, T. (1999) Phasic temperature and photoperiod control for soybean using a modified CROPGRO model. Life Support Biosph Sci. 6, 273–278. [PubMed] [Google Scholar]
- Cunha, N.B. , Murad, A.M. , Cipriano, T.M. , Araújo, A.C.G. , Aragão, F.J.L. , Leite, A. , Vianna, G.R. , McPhee, T.R. , Souza, G.H.M.F. , Waters, M.J. and Rech, E.L. (2011a) Expression of functional recombinant human growth hormone in transgenic soybean seeds. Transgenic Res. 20, 811–826. [DOI] [PubMed] [Google Scholar]
- Cunha, N.B. , Murad, A.M. , Ramos, G.L. , Maranhão, A.Q. , Brígido, M.M. , Araújo, A.C.G. , Lacorte, C. , Aragão, F.J.L. , Covas, D.T. , Fontes, A.M. , Souza, G.H.M.F. , Vianna, G.R. and Rech, E.L. (2011b) Accumulation of functional recombinant human coagulation factor IX in transgenic soybean seeds. Transgenic Res. 20, 841–855. [DOI] [PubMed] [Google Scholar]
- Dashiell, G.L. (1989) Lecithin and food processing applications In ACOS Monograph (Szuhaj B.F., ed.), pp. 16 Champaign, IL: American Oil Chemists Society. [Google Scholar]
- Doyle, J.J. and Doyle, J.L. (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15. [Google Scholar]
- Drake, P.M.W. , Madeira, L.D. , Szeto, T.H. and Ma, J.K.C. (2013) Transformation of Althaea officinalis L. by Agrobacterium rhizogenes for the production of transgenic roots expressing the anti‐HIV microbicide cyanovirin‐N. Transgenic Res. 22, 1225–1229. [DOI] [PubMed] [Google Scholar]
- Elghabi, Z. , Karcher, D. , Zhou, F. , Ruf, S. and Bock, R. (2011) Optimization of the expression of the HIV fusion inhibitor cyanovirin‐N from the tobacco plastid genome. Plant Biotechnol. J. 9, 599–608. [DOI] [PubMed] [Google Scholar]
- Erickson, D.R. (1995) Overview of modern soybean processing and links between processes Chapter 5. Practical Handbook of Soybean Processing and Utilization. Amer Oil Chemists Society (AOCS), 6th edn (Erickson D.R., ed.), pp. 56–64. AOCS, 2710 S. Boulder, Urbana, IL 61802?6996, USA. [Google Scholar]
- Essex, M. (1996) Retroviral vaccines: challenges for the developing world. AIDS Res. Hum. Retroviruses 12, 361–363. [DOI] [PubMed] [Google Scholar]
- Friend, D.R. and Kiser, P.F. (2013) Assessment of topical microbicides to prevent HIV‐1 transmission: concepts, testing, lessons learned. Antiviral Res. 99, 391–400. [DOI] [PubMed] [Google Scholar]
- Gartner, S.P. , Markovits, D.M. , Kaplan, M.H. , Gallor, R.C. and Popovic, M. (1986) The role of mononuclear phagocytes in HTLV‐III/LAV infection. Science, 233, 215–219. [DOI] [PubMed] [Google Scholar]
- Gulakowski, R.J. , McMahon, J.B. , Staley, P.G. , Moran, R.A. and Boyd, M.R. (1991) A semiautomated multiparameter approach for anti‐HIV drug screening. J. Virol. Methods, 33, 87–100. [DOI] [PubMed] [Google Scholar]
- Hohl, I. , Robinson, D.G. , Chrispeels, M.J. and Hinz, G. (1996) Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J. Cell Sci. 109, 2539–2550. [DOI] [PubMed] [Google Scholar]
- Imoto, Y. , Yamada, T. , Kitamura, K. and Kanazawa, A. (2008) Spatial and temporal control of transcription of the soybean beta‐conglycinin alpha subunit gene is conferred by its proximal promoter region and accounts for the unequal distribution of the protein during embryogenesis. Genes Genet Syst. 83, 469–476. [DOI] [PubMed] [Google Scholar]
- Kantolic, A.G. and Slafer, G.A. (2007) Development and seed number in indeterminate soybean as affected by timing and duration of exposure to long photoperiods after flowering. Ann. Bot. 99, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W.S. and Krishnan, H.B. (2004) Expression of an 11 kDa methionine‐rich delta‐zein in transgenic soybean results in the formation of two types of novel protein bodies in transitional cells situated between the vascular tissue and storage parenchyma cells. Plant Biotechnol. J. 2, 199–210. [DOI] [PubMed] [Google Scholar]
- Kouokam, J.C. , Huskens, D. , Schols, D. , Johannemann, A. , Riedell, S.K. , Walter, W. , Walker, J.M. , Matoba, N. , O'Keefe, B.R. and Palmer, K.E. (2011) Investigation of griffithsin's interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS ONE, 6, e22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladin, B.F. , Tierney, M.L. , Meinke, D.W. , Hosangadi, P. , Veith, M. and Beachy, R.N. (1987) Developmental regulation of β‐conglycinin in soybean axes and cotyledons. Plant Physiol. 84, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur, L.A. , Sanders‐Beer, B.E. , Brichacek, B. , Pal, R. , Liu, X. , Liu, Y. , Yu, R. , Venzon, D. , Lee, P.P. and Hamer, D.H. (2011) Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 4, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , O'Leary, J.J. , Huang, Y. , Huner, N.A. , Jevnikar, A. and Ma, S. (2006) Expression of cholera toxin B subunit and the B chain of human insulin as a fusion protein in transgenic tobacco plants. Plant Cell Rep. 25, 417–424. [DOI] [PubMed] [Google Scholar]
- Li, M. , Patton, D.L. , Cosgrove‐Sweeney, Y. , Ratner, D. , Rohan, L.C. , Cole, A.M. , Tarwater, P.M. , Gupta, P. and Ramratnam, B. (2011) Incorporation of the HIV‐1 microbicide cyanovirin‐N in a food product. J. Acquir. Immune Defic. Syndr. 58, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, T. , Pannell, L.K. , Shoemaker, R.H. , Wu, L. , McMahon, J.B. and Boyd, M.R. (1998) Recombinant production of cyanovirin‐N, a potent HIV (human immunodeficiency virus)–inactivating protein derived from a cultured cyanobacterium . Protein Expr. Purif. 12, 151–158. [DOI] [PubMed] [Google Scholar]
- Mori, T. , Barrientos, L.G. , Han, Z. , Gronenborn, A.M. , Turpin, J.A. and Boyd, M.R. (2002) Functional homologs of cyanovirin‐N amenable to mass production in prokaryotic and eukaryotic hosts. Protein Expr. Purif. 26, 42–49. [DOI] [PubMed] [Google Scholar]
- Mori, T. , Saruta, Y. , Fukuda, T. , Prak, K. , Ishimoto, M. , Maruyama, N. and Utsumi, S. (2009) Vacuolar sorting behaviors of 11S globulins in plant cells. Biosci. Biotechnol. Biochem. 73, 53–60. [DOI] [PubMed] [Google Scholar]
- Muntz, K. (1998) Deposition of storage proteins. Plant Mol. Biol. 38, 77–99. [PubMed] [Google Scholar]
- O'Keefe, B. , Vojdani, F. , Buffa, V. , Shattock, R.J. , Montefiori, D.C. , Bakke, J. , Mirsalise, J. , d'Andreae, A.‐L. , Hume, S.D. , Bratcherf, B. , Saucedo, C.J. , McMahon, J.B. , Pogue, G.P. and Palmer, K.E. (2009) Scaleable manufacture of HIV‐1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl Acad. Sci. USA, 106, 6099–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech, E.L. , Vianna, G.R. and Aragao, F.J. (2008) High‐efficiency transformation by biolistics of soybean, common bean and cotton transgenic plants. Nat. Protoc. 3, 410–418. [DOI] [PubMed] [Google Scholar]
- Robinson, D.G. , Oliviusson, P. and Hinz, G. (2005) Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic, 6, 615–625. [DOI] [PubMed] [Google Scholar]
- Sexton, A. , Drake, P.M. , Mahmood, N. , Harman, S.J. , Shattock, R.J. and Ma, J.K.C. (2006) Transgenic plant production of cyanovirin‐N, an HIV microbicide. FASEB J. 20, 356–358. [DOI] [PubMed] [Google Scholar]
- Shattock, R.J. and Rosenberg, Z. (2012) Microbicides: topical prevention against HIV. Cold Spring Harb. Perspect Med. 2, a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa, F. , Takagi, H. , Hirose, S. and Wakasa, Y. (2007) Endosperm tissue is good production platform for artificial recombinant proteins in transgenic rice. Plant Biotechnol. J. 5, 84–92. [DOI] [PubMed] [Google Scholar]
- Tsai, C.‐C. , Emau, P. , Jinag, Y. , Tian, B. , Morton, W.R. , Gustafson, K.R. and Boyd, M.R. (2003) Cyanovirin‐N Gel as a Topical Microbicide Prevents Rectal Transmission of SHIV89.6P in Macaques. AIDS Res. Hum. Retroviruses, 19, 535–541. [DOI] [PubMed] [Google Scholar]
- Tsai, C.C. , Emau, P. , Jiang, Y. , Agy, M.B. , Shattock, R.J. , Schmidt, A. , Morton, W.R. , Gustafson, K.R. and Boyd, M.R. (2004) Cyanovirin‐N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retroviruses, 20, 11–18. [DOI] [PubMed] [Google Scholar]
- UNAIDS . (2013) Joint United Nations Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic 2013. UNAIDS/JC2502/1/E.
- Vitale, A. and Raikhel, N.V. (1999) What do proteins need to reach different vacuoles? Trends Plant Sci. 4, 149–155. [DOI] [PubMed] [Google Scholar]
- Watanabe, S. , Hideshima, R. , Xia, Z. , Tsubokura, Y. , Sato, S. , Nakamoto, Y. , Yamanaka, N. , Takahashi, R. , Ishimoto, M. , Anai, T. , Tabata, S. and Harada, K. (2009) Map‐based cloning of the gene associated with the soybean maturity locus E3. Genetics, 182, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, K.A. , Rightmire, B.R. , Chen, J.C. and Tanwilson, A.L. (1986) Differential proteolysis of glycinin and beta‐conglycinin polypeptides during soybean germination and seedling growth. Plant Physiol. 82, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, F.Q. , Fan, C.M. , Zhang, X.M. and Fu, Y.F. (2013) The phytochrome gene family in soybean and a dominant negative effect of a soybean PHYA transgene on endogenous Arabidopsis PHYA. Plant Cell Rep. 32, 1879–1890. [DOI] [PubMed] [Google Scholar]
- Xiong, S. , Fan, J. and Kitazato, K. (2010) The antiviral protein cyanovirin‐N: the current state of its production and applications. Appl Microbiol Biot. 86, 805–812. [DOI] [PubMed] [Google Scholar]
- Yamada, Y. , Nishizawa, K. , Yokoo, M. , Zhao, H. , Onishi, K. , Teraishi, M. , Utsumi, S. , Ishimoto, M. and Yoshikawa, M. (2008) Anti‐hypertensive activity of genetically modified soybean seeds accumulating novokinin. Peptides, 29, 331–337. [DOI] [PubMed] [Google Scholar]
- Yoo, B.Y. and Chrispeels, M.J. (1980) The origin of protein bodies in developing soybean cotyledons ‐ a proposal. Protoplasma, 103, 201–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 LC‐MS (electrospray ionization) spectrum and deconvoluted ion set for the purified rCV‐N showing the expected molecular weight of 11 009 Da.
Figure S2 Dot‐blot of oil and protein extracts from non‐transgenic and transgenic T3 seeds submitted to standard defat processing.
Table S1 GC‐MS of fatty acids produced by soybean seeds (NEGC) and soybean seeds producing CV‐N (CV‐N).


