Abstract
INTRODUCTION
Dysregulation of the hypothalamic-pituitary-adrenal axis (HPA-axis) is common in individuals who experience chronic psychological stress, as well as individuals with chronic pain. Changes in cortisol availability in the presence of a chronic stressor such as pain may influence the sympathetic-adrenal-medullary (SAM) system, which contributes to cardiovascular responses to stress and also exhibits altered responsiveness in the presence of pain. The purpose of this study was to investigate the relationship between HPA activity during the cortisol awakening response and cardiovascular reactivity during exposure to an acute psychological stressor in individuals with chronic neck pain.
METHODS
Area under the curve (AUC) of the salivary cortisol awakening response was assessed in 41 individuals with chronic neck pain aged 19–80 years (22 men, 23 women). Slopes representing the change in mean arterial pressure and heart rate during a baseline quiet sitting condition, a low stress condition with mental concentration, and a high stress condition combining mental concentration with social evaluative threat were calculated for each individual as an index of cardiovascular responsiveness to the acute stressor. Cardiovascular responses were regressed on cortisol awakening AUC and pain duration, adjusting for age and sex.
RESULTS
Greater mean arterial pressure (β = -0.33, p=0.02) and heart rate responses (β =-0.41, p = 0.007) to the acute psychological stressor were associated with lower cortisol awakening responses after adjusting for age and sex. Individuals with a shorter duration of chronic pain also demonstrated a larger increase in mean arterial pressure during the laboratory stressor (β =−0.39, p =0.01), but there was no relationship between pain duration and changes in heart rate (p = 0.25).
CONCLUSIONS
Individuals with a shorter duration of chronic neck pain who demonstrate heightened cardiovascular responsiveness to an acute psychological stressor also exhibit lower cortisol awakening response. These results are consistent with time-dependent adaptations across the two major stress systems in the presence of chronic pain.
INTRODUCTION
Chronic neck pain and psychological stress
The annual prevalence of neck pain has been reported to be between 30 and 50% of the population, with 10–20% of these individuals reporting interference in daily activities (Hogg-Johnson 2008). Chronic neck pain is defined as pain occurring between the superior nuchal line and the spine of the scapula for a duration longer than that expected for normal tissue healing (Guzman 2009). Typically, a pain condition is considered chronic after 3–6 months of continuous or recurrent symptoms (Sluka 2009). Between 50 and 75% of individuals who experience an acute episode of neck pain go on to develop persistent symptoms lasting more than 6 months, regardless of the mechanism of injury (Manchikanti 2009). Although neck pain continues to be a source of significant burden to both the individual and the health care system (Ferrari 2003), there is limited information on mechanisms that contribute to the development and maintenance of pain chronicity.
The most consistent risk factors for the development of chronic neck pain include psychological factors such as anxiety, depression, and perceived stress (Linton 2000, Cote 2008, Ariens 2001). Additionally, psychological distress is a prevalent comorbid condition for many chronic pain disorders, including neck pain (Nieto 2011, Sjors 2011). Psychological factors may not only contribute to the initial development of chronic pain, but may also facilitate its chronicity given that maladaptive responses to psychological stressors contribute to a poor prognosis for recovery from pain (Linton 2000). Consistent reports of an association between psychological stress and chronic pain from both cross sectional and longitudinal studies suggest that better understanding the mechanisms underlying this relationship may improve the prevention and treatment of stress-related chronic pain disorders.
Pain as a stressor
It is widely accepted that pain itself can serve as a stressor, eliciting physiologic responses from multiple systems, including the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) system. In response to an acute nociceptive stimulus, the HPA axis increases cortisol secretion which facilitates glucose metabolism, suppresses non-vital organ system functions, and inhibits pain. This response is consistent with an adaptive “fight or flight” response and ultimately allows the individual to continue performing vital functions in the presence of pain (Heim 2000). When the system continues to experience an ongoing nociceptive stimulus however, this heightened cortisol secretory response can become attenuated due to negative feedback regulation of the HPA axis. In this manner, chronic pain may lead to diminished cortisol levels over time and impede the system’s ability to modulate further increases in pain (Ehlert 2001, Tsigos 2002, Generaal 2014).
Much like the HPA axis, when an individual experiences stress in the form of an acute nociceptive stimulus, activation of the SAM system is accompanied by heightened arousal, increased blood pressure and heart rate, and vasodilation in the extremities. Simultaneously, descending non-opioid pain inhibitory pathways are activated that lead to acute analgesia (Mayer 2009). This response is also an important contributor to the “fight or flight” response, allowing increased limb function in the presence of pain. Although the effects of acute pain are well described in the literature with respect to cardiovascular responsiveness (Mayer 2009, Huang 2013), changes in the presence of prolonged nociceptive stimuli are not as well understood. However, some evidence suggests that individuals with a variety of chronic pain disorders demonstrate dysregulation of the sympathetic nervous system (Perry 1989, Stein 2004, Boneva 2007, Torpy 2000). One proposed mechanism for this relationship is that heightened sympathetic activation may perpetuate the pain state through sensitization of peripheral nociceptors to inflammation, which is mediated by norepinephrine and other inflammatory mediators (Janig 2006).
Psychological stressors
Multiple investigations have observed similar effects of psychological stressors on HPA and SAM systems as described above for nociceptive stimuli (reviewed in Hannibal 2014). For example, exposure to acute psychological stressors involving social evaluative threat evokes a transient increase in cardiac autonomic responses, followed by a delayed and more prolonged rise in cortisol (Dickerson 2004, Bosch 2009). Importantly, autonomic responses to an acute psychological stressor have been shown to predict subsequent elevations in cortisol (Bosch 2009, Uchino 1995), indicating coordinated activation of the two major stress systems. However, it is not known whether these acute stress reactions are associated with chronic dyregulation of the HPA axis among individuals with chronic neck pain. For these individuals, it seems reasonable to speculate that exaggerated autonomic responses to repeated psychological stressors and noxious stimuli throughout the day may be associated with over-activation and eventual suppression of HPA axis activity (Crofford 2002) to further perpetuate the pain state (Generaal 2014).
The HPA and SAM systems are often studied independently of each other in the context of pain; however, given evidence that both systems adapt to the presence of prolonged stressful stimuli, it is worthwhile to examine how these systems and their resulting functions interrelate in patients with chronic pain. Therefore the purpose of this study was to investigate associations between cardiovascular reactivity in response to an acute psychological stressor and chronic alterations in cortisol awakening response (CAR) among individuals with chronic neck pain. Consistent with our hypothesis that exaggerated cardiovascular responses to psychological stressors may be associated with activity-dependent HPA suppression in a system already burdened by chronic pain, we hypothesize an inverse relationship between acute cardiovascular responses to the stressor and CAR.
METHODS
Participants
Forty-five adults (23 women, 22 men) with chronic neck pain were recruited from health care clinics and the local community through physician referral and electronic, print, and radio advertisements. Individuals were considered to have chronic, interfering neck pain if they reported pain between the superior nuchal line and the spine of the scapula for at least 3 months duration, and reported >10% neck pain related disability on the Neck Disability Index. This cutoff was selected to ensure that all participants experienced at least mild interference with daily activities based on previously established disability levels (Vernon 1991) despite varying intensities of neck pain. Women 35–50 years of age were excluded due to possible confounding effects of perimenopausal hormone fluctuations on vasomotor function (Mishra 2012), which generally peak in the fourth decade of life but can begin earlier for more than half of women in late reproductive age (Hale 2014). Male participants were age matched to women. Women under 35 years of age were included if they had regular menstrual cycles (26–31 days apart, confirmed by tracking onset of menses over two consecutive cycles) and were not using hormonal contraceptives. Pre-menopausal women were tested in the early follicular (low estrogen) phase of the menstrual cycle (6–10 days after onset of menses), as CAR has been shown to be elevated during ovulation (Wolfram 2011). Post-menopausal women over 50 years of age were enrolled if they reported having no menstrual cycle during the previous year, and were not taking hormone supplement medications. Individuals with major psychological or cardiovascular disorders, and those taking medications known to affect these systems (e.g. anti-depressants, beta-blockers) were also excluded. All participants provided signed informed consent for study procedures approved by the Colorado Institutional Review Board with additional protections for the use of deception during the stress manipulation.
Salivary cortisol collection
Prior to the experimental session, participants were provided with four 10 mL collection vials housed in a MEMS cap container (Behavioral Medicine Core Laboratory, National Jewish Health, CO) that recorded a time stamp upon opening and closing of the container. This allowed for verification of adherence to instructed collection times for the salivary CAR. Participants were instructed to drool into a numerically labeled collection vial through a straw immediately upon waking and every 15 minutes thereafter, for a total of four separate saliva samples collected on the morning of the experimental session. Participants were instructed not to eat, drink, chew gum, or brush their teeth until after the last morning sample was collected. Samples were stored in the participant’s home freezer until returned to the laboratory in an ice container on the day of the experiment, after which all samples were stored at -80 degrees C until later analyzed in duplicate for cortisol concentrations using a commercially available enzyme immunoassay (Salimetrics LLC, State College, PA; sensitivity <0.007 ug/dL, intra-assay coefficient of variation = 3.65%.
Acute psychological stressor
Prior to the experimental session, participants were asked to refrain from participating in vigorous exercise or using non-prescription analgesic medications within 24 hours of testing, and consuming a major meal or caffeine within 1 hour of testing. All experimental sessions were scheduled between 1300 and 1600 hours to control for any diurnal fluctuations in stress response outcomes across participants. At the beginning of the experimental session, participants completed a medical history questionnaire to assess the duration of neck pain, the intensity of symptoms on the day of testing using a 10 cm visual analogue scale (VAS), and trait anxiety levels using the Speilberger Trait Anxiety (STAI-T) Index. The STAI-T provides an index of generalized anxiety, including traits of nervousness and fear-based worry that remain relatively stable despite fluctuating contextual factors. It is scored using a 4-point Likert scale ranging from 20–80 points, with higher scores indicating greater trait anxiety (Speilberger 1983).
The mental concentration task consisted of a computerized version of the Operation Span (OpSpan) test, which requires participants to solve arithmetic problems while memorizing sequential words lists and selecting answers with a computer mouse (Conway 2005). The OpSpan test required approximately 5 minutes to complete and was repeated for each participant under low stress and high stress conditions, separated by at least 15 minutes. Mean arterial blood pressure (MAP) and heart rate (HR) were monitored using an automated device (Coulbourn Instruments, PA) in a resting seated position prior to the start of the experiment and immediately upon completion of the low and high stress conditions.
In the low stress condition, participants were told that their performance would not be monitored during a “practice trial” with no requirements for speed or accuracy, and individuals were given positive feedback by a familiar tester regardless of their performance. Prior to the high stress condition, participants were told that they would be videotaped and were instructed to perform the test as fast and accurately as possible. Additionally, a monetary reward was offered for high scores. This condition was administered by an authoritative and unfamiliar tester who did not provide any positive feedback. Participants were naive to the purpose of the stress manipulation prior to the experiment, and were fully debriefed immediately upon completion of the high stress condition.
Data analysis
The CAR measurement is often used as an index of basal HPA activation, with typical CAR levels in healthy individuals increasing by approximately 50% within the first 30 minutes of waking, regardless of morning routine (Wust 2000). CAR was measured as area under the curve (AUC) of the four samples collected within the first 45 minutes of wakening. Cortisol AUC was calculated with respect to ground in nmol/L over the 45 minute period using a widely applied formula: (Pruessner 2003). As indices of autonomic cardiovascular responses to the acute stressor, a best-fit linear analysis (e.g., slope) of MAP and HR were applied for each individual to quantify the magnitude of change between resting baseline, low stress, and high stress conditions. Changes in salivary cortisol across collection time points, and changes in MAP and HR across stress conditions were assessed with Repeated Measures Analysis of Variance (RMANOVA) with Tukey HSD post-hoc adjustments as appropriate.
Two multivariate regression models were performed using CAR levels and pain chronicity (e.g., duration of neck pain) as independent predictor variables. The dependent variable was the slope of MAP in the first regression, and the slope of HR in the second regression. Pain chronicity was included in the model as a surrogate measure of time-dependent adaptive changes. Given their previously reported relationship with cortisol, age (Almeida 2009) and sex (Kirschbaum 1992) were included in both regression models as a priori covariates. Secondary Pearson correlation (r) analyses were used to explore bivariate relationships between variables that were not investigated in the primary regression models. Significance levels were set at an alpha of 0.05. All statistical analyses were performed in SAS version 9.3 (SAS v 9.3, SAS Institute Inc. Cary, NC USA). Results are reported as mean (SD) in the text and figures.
RESULTS
Participant characteristics
A total of 164 saliva samples were collected and analyzed; participants who did not provide all 4 morning saliva samples were excluded from analysis (2 men, 2 women). The remaining 41 participants ranged from 19 to 80 years of age (43.5(18.7) years), with an average body mass index of 24.9(4.2) kg/m2. The intensity of neck pain as measured by the VAS on the day of testing was mild on average (2.56 (2.01) cm), with ratings ranging from 0 cm (no pain) to 7.5 cm (severe) on a 10 cm scale. Neck Disability Index scores indicated mild, bordering on moderate, levels of neck-related disability on average (29.6 (14.8)%), with scores ranging from 12 (mild disability) to 70 (complete disability)%. The self-reported duration of neck pain ranged from 6 months to 30 years, with an average of 10.0 (7.9) years. Trait anxiety scores (STAI-T) were 32.9(8.6) on average, with scores ranging from 22 to 58 points.
Cortisol awakening response and acute cardiovascular responses
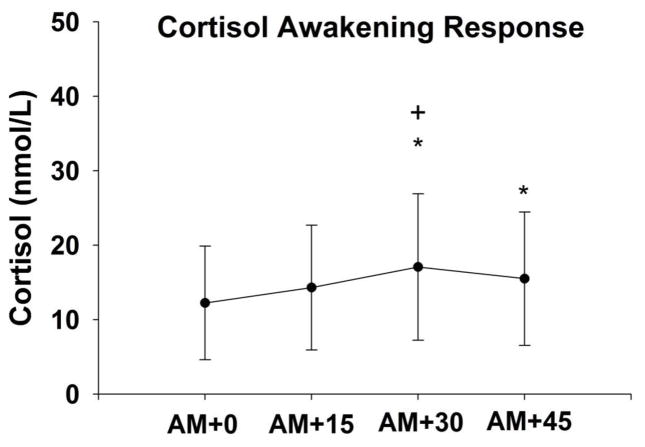
There was a significant increase in salivary cortisol levels across the four collection time points (F=7.1, df=163, p<0.001), consistent with the cortisol awakening response. A 39.5% increase in cortisol occurred 30 minutes after waking (Figure 1). Additionally, there was a significant increase in MAP across the three experimental conditions (F=49.1, df=122, p<0.001), with an average slope of 4.7(3.6) mmHg. However changes in HR were more variable and did not demonstrate a consistent increase (F=0.01, df=122, p=0.98), with an average slope of -0.06(2.9) across conditions (Figure 2).
Figure 1.
Mean (SD) salivary cortisol levels upon wakening (AM+0) and every 15 minutes thereafter (AM+15, AM+30, AM+45). The cortisol awakening response (CAR) was measured as the area under the curve for each participant. * p<0.05 compared to AM+0; + p<0.05 compared to AM+15
Figure 2.
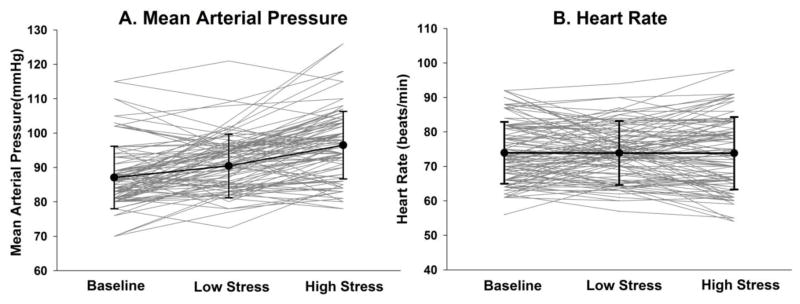
Individual (grey) and group mean (SD) (black) responses to the acute psychological stressor. On average, mean arterial pressure (A) increased across baseline, low stress, and high stress conditions (p<0.001), whereas heart rate (B) did not (p=0.98). Individual responses to the stressor were measured as the slope of the line across conditions.
Associations between CAR and acute cardiovascular responses to the stressor
There were no significant relationships between pain intensity on the day of testing and either CAR (r = 0.07, p = 0.65) or cardiovascular responses to the stressor (r = 0.19, p = 0.19 for HR, and r = 0.01, p = 0.96 for MAP). Multivariate regression analyses demonstrated that CAR and pain duration were significantly associated with MAP responses to stress after adjusting for age and sex (F = 3.08, p = 0.03), with both CAR and pain duration being inversely related to MAP slope (β = -0.33, p = 0.02, and β = -0.39, p = 0.01 respectively). The overall adjusted model for HR responses was not significant (F=2.19, p=0.08); there was a significant inverse association between CAR and HR slope (β=-0.41, p=0.007), however, pain duration was not significantly related to HR responses in this model (β=-0.16, p=0.25). There were no significant age or sex effects observed in the adjusted models (p>0.20). Furthermore, there were no significant associations between self-reported trait anxiety and any of the primary physiological outcomes. For illustrative purposes, bivariate CAR associations with pain intensity and acute cardiovascular responses to the stressor are shown in Figure 3.
Figure 3.
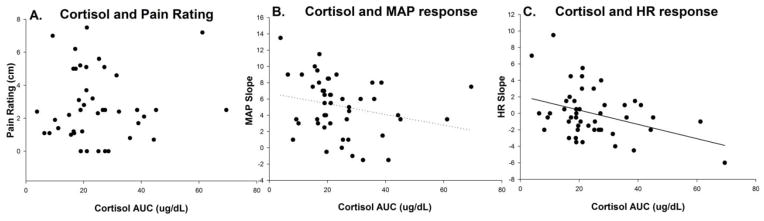
Scatter plots showing bivariate associations between cortisol area under the curve (AUC) (x-axis) and pain intensity on the day of testing (A), mean arterial pressure (MAP) slope (B), and heart rate (HR) slope (C) on the y-axes, respectively. Significant beta weights were found between cortisol AUC and cardiovascular responses to the stressor in the adjusted multivariate regression.
DISCUSSION
This study examined the association between cardiovascular responses to an acute psychological stressor and CAR levels in a chronic pain population. Results indicated that heightened MAP responses to the stressor were associated with a lower cortisol awakening response and a shorter duration of self-reported chronic pain. These findings are consistent with the hypothesis that elevated autonomic responses to psychological stressors, particularly in the earlier stages of a persistent pain syndrome, may be related to chronic suppression of HPA activity. This observation suggests a potential model for stress-related pain chronicity that can be explored in future experimental studies.
Association between CAR and acute cardiovascular responses to psychological stress
Few studies have investigated the relationship between HPA axis activity and cardiovascular reactivity among individuals with chronic pain. Animal models of repeated acute stress have demonstrated that the HPA response becomes attenuated over time, whereas the catecholamine response increases (Thiagarajan 1989). This finding of divergent adaptations in the two major stress systems is consistent with the inverse relation between CAR and cardiovascular responses observed in the present study. However, habituation or sensitization of the stress response for each of these systems likely depends on the type and duration of the stressor. Examining a repeated stress paradigm in humans, Schommer et al. (2003) found that cortisol and heart rate responses habituated with repeated applications of the stressor, whereas the norepinephrine response did not change. Interestingly, although responsiveness did not change, basal levels of norepinephrine increased with repeated exposure to the stressor which may support differential regulation of basal and acute reactivity of the major stress systems.
Dysregulation of HPA function has been well documented among individuals with chronic pain, for whom noxious stimuli may serve as a repetitive stressor causing long-term adaptive changes (Hannibal 2014). Among individuals with chronic neck pain, the present results indicate that heightened autonomic responses to an acute psychological stressor are associated with reduced HPA activity, as indexed by the CAR. It has previously been shown in healthy individuals that autonomic responses to an acute psychological stressor predict the subsequent rise in cortisol levels (Bosch 2009, Uchino 1995), indicating a link between cardiovascular responses and HPA activation.
Together, these findings suggest that when superimposed on a system already burdened by persistent pain, elevated autonomic responses to relatively minor daily stressors may be related to chronic suppression of HPA function, ultimately reducing its analgesic effects and perpetuating the pain state. Suppression of HPA function may occur through negative feedback regulation, cortisol depletion, changes in receptor sensitivity, or other related mechanisms (Crofford 2002). This model of stress-related pain chronicity is consistent with findings from the present investigation; however, experimental studies are needed to verify causal relationships and mechanisms underlying the observed association between acute cardiovascular responses to stress and chronic adaptations in HPA function.
Previous studies indicate that the inverse association between CAR and acute cardiovascular responses to psychological stress may be explained by interactions between the HPA and SAM systems at multiple central and/or peripheral sites during exposure to acute psychological stressors in the presence of persistent pain. For example, many studies implicate activation of higher brain centers such as the amygdala, hippocampus, and hypothalamus by emotional or fear based stressors (Tsigos 2002). Specifically, the central nucleus of the amygdala has been shown to be involved in both cardiovascular and endocrine responses to psychological stress (Van de Kar 1999). Although cortical activation was not examined in the present study, pain has previously been interpreted as a fear-based threat that can activate amygdalar pathways affecting downstream regulation of cortisol and catecholamine release (Lloyd 2014). This interaction is thought to be mediated by descending circuits involving the paraventricular nucleus (PVN) of the hypothalamus, based on studies showing that lesions of the PVN reduce corticosterone secretion in response to stress (Richardson 1990). Projections of catecholamine pathways are not as well characterized, but are thought to originate either through medullary connections to the paraventricular nucleus, or from the locus coeruleus in the brainstem (Van de Kar 1999). Interestingly, the locus coeruleus is also known to be activated by noxious stimuli (Szabadi 2012).
Association between pain chronicity and acute cardiovascular responses to psychological stress
Pain duration was included in the regression models as a surrogate measure of time-dependent adaptations that may occur with persistent pain. Somewhat surprisingly, we found that blood pressure but not heart rate responses to the psychological stressor were inversely associated with pain chronicity. Importantly, this association was independent of age. Previous studies have shown that transient increases in blood pressure allow for a sympathetically mediated hypoalgesic response to an acute pain stimulus (Sacco 2013). Inhibition of pain is thought to be an integral part of the “fight or flight” response, which is facilitated by the additional release of serotonin and noradrenaline. However, when pain becomes chronic, the relationship between blood pressure and pain appears to be reversed. For example, an association between elevated blood pressure and higher pain sensitivity has been observed in individuals with chronic back or orofacial pain (Sacco 2013, Maixner 1997). This time-dependent adaptation is thought to be due to changes in the baroreceptor reflex caused by a reduction in baroreceptor sensitivity to persistent pain. Decreased baroreceptor sensitivity attenuates the hypoalgesic effect of SAM activation, resulting in increased pain perception (Maixner 1997). Thus, individuals may engage sympathetically mediated pain inhibitory mechanisms more readily during exposure to psychological stressors in the earlier stages of chronic pain. These findings indicate that pain chronicity is an important determinate of cardiovascular responses to psychological stress. Interestingly, CAR and blood pressure responses to the acute stressor were related to the duration but not the intensity of neck pain. Although the lack of association for pain intensity may be explained by small discrepancies in times of measurement for CAR (morning of experiment), pain intensity (afternoon just prior to experiment), and cardiovascular responses (afternoon during experiment), it seems likely that adaptations in physiological stress responses are more strongly related to the chronicity of symptoms, rather than the magnitude of neck pain which exhibits large day-to-day variability (Balter 2013).
Study Limitations
The present study accounted for several variables that have not been well controlled in previous investigations of the relationship between CAR levels and cardiovascular responses to psychological stress in humans, including age, sex, diurnal rhythms, and perimenopausal status. However, our results should be interpreted in the context of several limitations. First, causal relationships cannot be established through correlation analyses, and proposed hypotheses regarding interactions across these complex systems remain speculative until verified by experimental investigations. Although our findings are consistent with the hypothesis that elevated autonomic responses to acute stressors are related to chronic suppression of HPA activity, it is equally plausible that reduced HPA activity in the presence of chronic pain may ultimately lead to an adaptive increase in autonomic responses to psychological stress. The relationship between these systems may also be mediated through concurrent activation of higher brain centers, in addition to direct interactions in the periphery. Future research is needed to delineate central and peripheral mechanisms contributing to observed interactions between the HPA and SAM systems in response to psychological stress in chronic pain populations, as well as in healthy individuals without a history of pain. Investigation of pain-free individuals is needed to determine whether the observed associations are specific adaptations to a chronic pain state.
A second limitation is that cardiovascular responses do not provide a direct measure of SAM activation, as they are influenced by inputs from multiple systems (Buijs 2013). This may partially explain discrepancies in heart rate and blood responses to the acute stressor and their association with pain duration. Future studies would benefit from more direct measures of sympathetic function (e.g., epinephrine and norepinephrine) to clarify the relation between acute SAM activation and chronic adaptations in HPA function.
Finally, salivary cortisol was collected only on a single day. Recent studies have suggested that cortisol secretion may have large day-to-day variations, which can be influenced by factors such as the sleep-wake cycle, and weekday versus weekend collection times (Karlamangla 2013). As such, HPA activity may have been more accurately captured by sampling CAR over multiple days. Similarly, HPA activity was measured at a different time point (morning of the experiment) and in response to a different stressor (awakening) compared to measurement of cardiovascular responses. This was done to reflect chronic adaptations in HPA function, and does not provide information on concurrent responses of the two stress systems to the same acute stressor as has been done in previous studies (Bosch 2009, Uchino 1995).
In summary, the present findings demonstrate that individual cardiovascular responses to an acute psychological stressor are inversely associated with the cortisol awakening response in patients with chronic neck pain. In addition, those experiencing a shorter duration of pain also demonstrated more pronounced blood pressure responses to the stressor. Taken together, these results support time-dependent adaptations in interactions between the two major stress response systems in the presence of persistent pain that may inform our understanding of how psychological stress contributes to the maintenance of pain.
Highlights.
We measured cardiovascular and cortisol awakening response in individuals with neck pain
Greater mean arterial pressure was associated with lower cortisol awakening response
Shorter duration of pain was related to higher mean arterial pressure response
Acknowledgments
This research was supported by NIH R01 AR056704 awarded to KSM, and a Promotion of Doctoral Studies Scholarship from the Foundation for Physical Therapy awarded to BS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Hogg-Johnson S, van der Velde G, Carroll LJ, holm LW, Cassidy JD, Guzman J, Coe P, Haldeman S, Ammendolia C, Carragee E, Hurwitz E, Nordin M, Peloso P. Bone and joint decade 2000–2010 Task force on neck pain and its associated disorders. The burden and determinants of neck pain in the general population: results of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Spine. 2008;33:39–51. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]
- Guzman J, Haldeman S, Carroll LJ, Carragee EJ, Hurwitz EL, Peloso P, Nordin M, Cassidy JD, Holm LW, Cote P, van der Velde G, Hogg-Johnson S. Clinical practice implications of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders: from concepts and findings to recommendations. J Manipulative physiol ther. 2009;32(2):227–43. doi: 10.1016/j.jmpt.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Sluka K. Mechanisms and management of pain for physical therapists. IASP press; Seattle WA: 2009. p. 7. [Google Scholar]
- Manchikanti L, Singh V, Data S, Cohen SP, Hirsch JA American Society of Interventional Pain Physicians. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12(4):35–70. [PubMed] [Google Scholar]
- Ferrari R, Russel AS. Regional musculoskeletal conditions: neck pain. Best Pract Res Clin Rheumatol. 2003;17(1):57–70. doi: 10.1016/s1521-6942(02)00097-9. [DOI] [PubMed] [Google Scholar]
- Linton SJ. A review of psychological risk factors in back and neck pain. Spine. 2000;25(9):1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- Cote P, van der Velde G, Cassidy JD, Carroll LJ, Hogg-Johnson S, Holm LW, Carragee EJ, Hdeman S, Nordin M, Hurwitz EL, Guzman J, Peloso PM. The burden and determinants of neck pain in workers: results of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Spine. 2008;33(4 Suppl):S60–74. doi: 10.1097/BRS.0b013e3181643ee4. [DOI] [PubMed] [Google Scholar]
- Ariëns GA, van Mechelen W, Bongers PM, Bouter LM, van der Wal G. Psychosocial risk factors for neck pain: a systematic review. Am J Ind Med. 2001;39(2):180–193. doi: 10.1002/1097-0274(200102)39:2<180::aid-ajim1005>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nieto R, Miro J, Huguet A, saldana C. Are coping and catastrophising independently related to disabiliyt and depression in patients with whiplash associated disorder? Disabil Rehabil. 2011;33(5):389–98. doi: 10.3109/09638288.2010.491576. [DOI] [PubMed] [Google Scholar]
- Sjors A, Larsson B, Persson AL, Gerdle B. An increased resonse to experimental muscle pain is related to psychological status in women with chronic non-traumatic neck-shoulder pain. BMC Musculoskelet Disord. 2011;12:230. doi: 10.1186/1471-2474-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57(1–3):141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Generaal E, Vogelzangs N, Macfarlane G, Geenen R, Smit J, Penninx B, Dekker J. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: partly masked by depressive and anxiety disorders. BMC Musculoskeletal Disorders. 2014;15(277) doi: 10.1186/1471-2474-15-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E, Bushnell M. Functional Pain Syndromes. IASP Press; Seattle WA: 2009. p. 275. [Google Scholar]
- Huang CJ, Webb HE, Zourdos MC, Acevedo EO. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013;7;4:314. doi: 10.3389/fphys.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry F, Heller PH, Kamiya J, Levine JD. Altered autonomic function in patients with arthritic or with crhonic orofacial pain. Pain. 1989;39:77–84. doi: 10.1016/0304-3959(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Stein PK, Domitrovich PP, Ambrose K, Lyden A, Fine M, Gracely RH, Clauw DJ. Sex effects on heart rate variability in fibromyalgia and Gulf War illness. Arthritis Rheum. 2004;51:700–8. doi: 10.1002/art.20687. [DOI] [PubMed] [Google Scholar]
- Boneva RS, Decker MJ, Maloney EM, Lin JM, Jones JF. Helgason HG Heim CM Rye DB Reeves WC. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population based study. Auton Neurosci. 2007;68:1707–16. doi: 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia. Arthritis and rheumatism. 2000;43(4):872–880. doi: 10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Janig W, Levine JD. Autonomic-neuroendocrine-immune responses in acute and chronic pain. In: McMahon SB, Koltzenburg M, editors. Wall and MElzack’s textbook of pain. 5. Edinburgh: Elsevier; 2006. pp. 205–218. [Google Scholar]
- Hannibal KE, Bishop MD. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys Ther. 2014 doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJ, Helmerhorst EJ, Edwards KM. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med. 2009;71(8):877–85. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Malarkey W, Glaser R. Individual differences in cardiac sympathetic control predict endocrine and immune responses to acute psychological stress. J Pers Soc Psychol. 1995;69(4):736–43. doi: 10.1037//0022-3514.69.4.736. [DOI] [PubMed] [Google Scholar]
- Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manip Physiol Ther. 1991;14(7):409–415. [PubMed] [Google Scholar]
- Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ. 2012:344e402. doi: 10.1136/bmj.e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale GE, Robertson DM, Burger HG. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. 2014;142:121–131. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Wolfram M, Bellingrath S, Kudielka BM. The cortisol awakening response (CAR) across the menstrual cycle. Psychoneuroendocrinology. 2011;36(6):905–912. doi: 10.1016/j.psyneuen.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. PsychonBull Rev. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represents measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, STawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender. Psychology and aging. 2009;24(4):819–27. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Thiagarajan AB, Gleiter CH, Mefford IN, Eskay RL, Nutt DJ. Effect of single and repeated electroconvulsive shock on the hypothalamic-pituitary-adrenal axis and plasma catecholamines in rats. Psychopharmacology. 1989;97(4):548–52. doi: 10.1007/BF00439562. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Crofford LJ. The hypothalamic-pituitary-adrenal axis in the pathogenesis of rheumatic disease. Endocirinol Metab Clin North Am. 2002;31(1):1–13. doi: 10.1016/s0889-8529(01)00004-4. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Frontiers in Neuroendocrinology. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Findlay G, Roberts N, Nurmikko T. Illness behavior in patients with chronic low back pain and activation of the affective circuitry of the brain. Psychosom Med. 2014;76(6):413–21. doi: 10.1097/PSY.0000000000000076. [DOI] [PubMed] [Google Scholar]
- Richardson Morton KD, Van de Kar lD, Brownfiedl MS, Lorens SA, Napier TC, Urban JH. Stress-induced renin and corticosterone secretion is mediated by catecholaminergic nerve terminals in the hypothalamic paraventricular nucleus. Neuroendocrinology. 1990;51:320–327. doi: 10.1159/000125356. [DOI] [PubMed] [Google Scholar]
- Szabadi E. Modulation of physiological reflexes by pain: role of the locus coeruleus. Front Integr Neurosci. 2012;6:94. doi: 10.3389/fnint.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, Pioli S, Magnano A, Spagnoli F, Giuri PG, Fiaccadori E, Caiazza A. The relationship between blood pressure and pain. Journ Clin Hypertens. 2013;15(8):600–605. doi: 10.1111/jch.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Kincaid S, Sigurdsson A, Harris MB. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59(5):503–11. doi: 10.1097/00006842-199709000-00007. [DOI] [PubMed] [Google Scholar]
- Balter JE, Molner JL, Kohrt WM, Maluf KS. Mechanical pain sensitivity and the severity of chronic neck pain and disability are not modulated across the menstrual cycle. J Pain. 2013;14(11):1450–9. doi: 10.1016/j.jpain.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM. The autonomic nervous system: a balancing act. Handb Clin Neurol. 2013;117:1–11. doi: 10.1016/B978-0-444-53491-0.00001-8. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawkis RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences--findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38(11):2585–97. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]